S,S-Dimethyl Dithiocarbonate: A
Convenient Reagent for the Synthesis of
Symmetrical and Unsymmetrical Ureas
Man-kit Leung,* Jun-Liang Lai, King-Hang Lau,Hsiao-hua Yu, and Hsiang-Ju Hsiao Department of Chemistry, National Taiwan University,
Taipei, Taiwan, Republic of China Received December 28, 1995
The condensation of primary amines with phosgene or isocyanates is a classic method for the synthesis of organic ureas.1 However, due to their high toxicity and
reactivity, phosgene and isocyanates are difficult to handle in the laboratory. Although several substitutes for phosgene such as triphosgene and carbonyldiimid-azole have been developed during the last few decades,2,3
these reagents are themselves prepared from phosgene. Alternative methods involving drastic reaction conditions, such as direct reactions of amines with dialkyl carbonates at high temperature4 and the reaction of amines with
N,N′-diphenylurea in the presence of Et3N in refluxing
DMF,5have been reported previously. Our efforts are
therefore directed toward the development of mild re-agents that can be used instead of phosgene or its derivatives in urea synthesis. Since S,S-dimethyl dithio-carbonate (DMDTC) is structurally similar to phosgene and can be prepared from methanol, carbon disulfide, and dimethyl sulfate by a two-step sequence,6DMDTC
was deemed an appropriate candidate for our investiga-tion. Although dimethyl sulfate is a suspected human carcinogen, the substance is relatively nonvolatile (bp 188 °C) and can be handled safely with care in the laboratory.
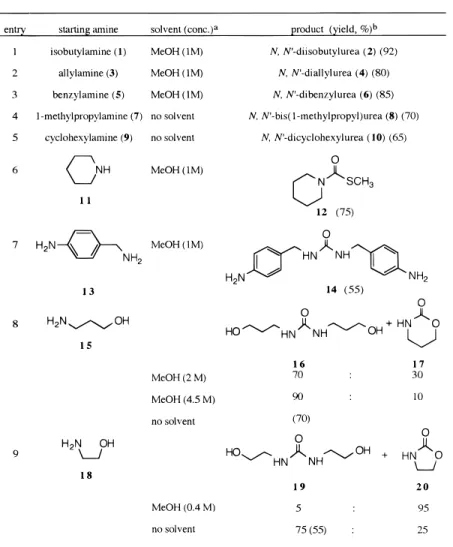
To explore the feasibility of using DMDTC in urea synthesis, we first reacted DMDTC with 2 equiv of primary alkyl amines 1, 3, and 5 using methanol or ethanol as the solvent at 60 °C and obtained, respectively, the desired symmetrical ureas 2, 4, and 6 as the only products (Table 1). No incorporation of methanol or ethanol into the products occurred according to the 1H
NMR analyses. While the above procedure is quite successful for primary alkylamines, we discovered that DMDTC is relatively sensitive to steric environment and the nucleophilicity of the amino group. Thus,R -substi-tuted amines 7 and 9 react with DMDTC at much slower rates. Furthermore, reaction of tert-butylamine with DMDTC does not proceed under the same conditions. Although piperidine (11) is considered to be a good nucleophile, it only reacts with DMDTC at a moderate rate, affording thiocarbamate 12 as the product. At-tempts to extend our procedure to less nucleophilic aromatic amines such as aniline were unsuccessful, resulting in recovery of the starting materials. From the
above observations, we concluded that DMDTC is a very selective reagent toward amines. By taking advantage of its selectivity, we successfully obtained the bisaniline 14 directly from DMDTC and 4-aminobenzylamine (13) without the need for protection and deprotection proce-dures. Furthermore, heating DMDTC with 2 equiv of 3-aminopropanol (15) in methanol afforded a mixture of bis(3-hydroxypropyl)urea 16 and the cyclic urethane 17 in a ratio of 7:3. More interesting is the fact that 16 is a highly crystalline compound which was readily purified by recrystallization from chloroform. Nevertheless, be-cause of the proximity of the amino group and the hydroxy group, preparation of bis(2-hydroxyethyl)urea (19) in methanol was less successful, rendering oxazoli-done (20) as the major product. Since the product ratio of the symmetrical urea to the cyclic carbamate is dependent on the reaction concentration of the corre-sponding amino alcohol, we carried out the synthesis at higher concentration, obtaining the desired sym-metrical bis(hydroxyalkyl)ureas 16 and 19 in satisfactory yields.
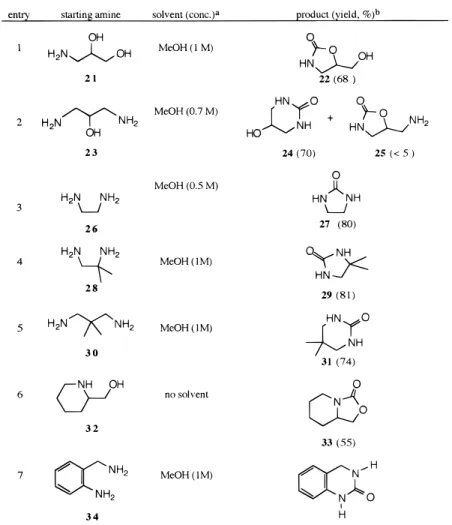
Further experiments with DMDTC (Table 2) revealed that aliphatic amines bearing a hydroxy or an amino substituent at the β or γ position react in dilute solution to provide predominantly cyclic ureas or car-bamates. In particular, reaction of (( )-3-aminopropane-1,2-diol (21) with DMDTC afforded exclusively 5-(hydroxymethyl)oxazolidin-2-one (22), the kinetically favored isomer. On the other hand, 1,3-diamino-2-propanol (23) reacted under the same conditions to give 24 as the major product. Also formed was 5-(aminom-ethyl)oxazolidin-2-one (25) as a minor component. We attribute the regioselectivity of the cyclization to the greater nucleophilicity of the amino group, which favors the six-membered ring closure over oxazolidone forma-tion.
In an effort to ascertain the scope of DMDTC applica-tion to the synthesis of unsymmetrical ureas, we exam-ined the possibility of preparing N-alkyl-S-methyl thio-carbamate by mono aminolysis of DMDTC. First, benzylamine was allowed to react with excess DMDTC (1.6 molar equiv), affording N-benzyl-S-methyl thiocar-bamate (36) and dibenzylurea (6) in a ratio of 1:30. This result implies that the formation of dibenzylurea (6) at the second stage of the reaction is faster than N-benzyl-S-methyl thiocarbamate (36) formation from DMDTC. To
prevent thiocarbamate 36 from converting to dibenzyl-urea, we carried out the reaction under basic conditions, in the course of which 36 should be deprotonated im-mediately after being formed. Since the anion 37 is relatively stable toward nucleophilic substitution at ambient temperature and will not react further to give (1) (a) Hegarty, A. F. In Comprehensive Organic Chemistry; Barton,
D., Ollis, W. D., Eds.; Pergamon Press: New York, 1979; Vol. 2, p 1067. (b) Sandler, S. R.; Karo, W. Organic Functional Group Preparations; Academic Press: New York, 1971; Vol. 2, p 135.
(2) Staab, H. A. Angew. Chem., Int. Ed. Engl. 1962, 1, 531. (3) Eckert, H.; Forster, B. Angew. Chem., Int. Ed. Engl. 1987, 26, 894.
(4) Flyes, T. M.; James, T. D.; Pryhitka, A.; Zojsji, M. J. Org. Chem. 1993, 58, 7456.
(5) Ramadas, K.; Srinivasan N. Org. Prep. Proc. 1993, 25, 600. (6) (a) Degani, I.; Fochi, R.; Regondi, V. Synthesis 1980, 375. (b) Degani, I.; Fochi, R.; Regondi, V. Synthesis 1981, 149.
S0022-3263(95)02282-1 CCC: $12.00 © 1996 American Chemical Society
Downloaded by NATIONAL TAIWAN UNIV on July 29, 2009
dibenzylurea (6), quenching of 37 led to the thiocarbam-ate 36 in high yield. Further condensation of 36 with
tetrahydrofurfurylamine furnished the unsymmetrical urea 38 (Table 3). This synthetic strategy was extended to the preparation of bisureas, a new class of guest-host molecules that has been developed recently for molecular recognition,7 by using bisthiocarbamate 39 as the key
intermediate.
The foregoing methodology offers a new approach to the synthesis of symmetrical and unsymmetrical ureas. It is particularly attractive for the preparation of hy-droxy- and amino-substituted ureas that are not easy to obtain by conventional methods. In addition, unsym-metrical ureas have been prepared from DMDTC and two different amines through a two-step sequence. This method is especially useful when the corresponding isocyanates are unavailable.
Experimental Section
Materials. Amines (Aldrich, Janssan, Tokyo Kasei) were
commercially available and used as received. S,S-Dimethyl dithiocarbonate (DMDTC) was prepared according to the pro-cedures reported in the following section. Carbon disulfide is highly toxic, while benzene and dimethyl sulfate are suspected human carcinogens; these reagents should be handled in a properly ventilated fume hood, and rubber gloves should be worn.
Preparation of S,S-Dimethyl Dithiocarbonate (DM-DTC). To a suspension of granulated KOH (21.5 g, 0.33 mol)
in anhydrous Et2O and benzene (1:1, 140 mL) was added
methanol (10.5 g, 0.33 mmol), followed by dropwise addition of a solution of CS2(24.7 g, 0.33 mol) in benzene (15 mL) at 0 °C.
The reaction mixture was kept at 0 °C for 5 h, and Me2SO4(41
g, 0.33 mol) was added. The reaction was allowed to react at ambient temperature for 20 h. The organic layer then was decanted, washed sequentially with dilute HCl solution and saturated NaCl solution, dried over anhydrous Na2SO4, and
(7) (a) Nishizawa, S.; Bu¨ hlmann, P.; Iwao, M.; Umezawa, Y. Tetrahedron Lett. 1995, 36, 6483. (b) Fan, E.; Van Arman, S. A.; Kincaid, S.; Hamilton, A. D. J. Am. Chem. Soc. 1993, 115, 369. (c) Albert, J. S.; Hamilton, A. D. Tetrahedron Lett. 1993, 34, 7363.
Table 1. Preparation of Symmetrical Ureas from Condensation of Various Amines with DMDTC
2RNH2+(MeS)
2CO f RNHCONHR
aInitial concentration of the starting amine.bIsolated yield.
Downloaded by NATIONAL TAIWAN UNIV on July 29, 2009
concentrated using simple distillation to afford crude O,S-dimethyl dithiocarbonate (39 g). The oily crude product was then subjected to thermal rearrangement at 100-110 °C, using tetrabutylammonium iodide (3 mol %) as a catalyst. After being heated for 18 h, the yellowish crude oil was fractionally distilled under reduced pressure to give DMDTC as a colorless oil (25 g, 58%): bp 60-62 °C (20 mmHg) (lit.6bp 58-59 °C, 16 Torr);1H NMR (200 MHz, CDCl3) δ 2.43 (s, 6H) (lit.6 1H NMR (CCl4) δ
2.40 (s, 6H));13C NMR (75 MHz, CDCl
3) δ 189.8, 12.7.
Typical Synthetic Procedures for Symmetrical Ureas.
N,N′-Bis(isobutyl)urea (2) (Method A). To a stirred solution
of isobutylamine (1) (0.18 g, 2.6 mmol) in methanol (1.5 mL) was added DMDTC (0.16 g, 1.3 mmol). The mixture was heated at 60 °C for 24 h. The released malodorous methyl sulfide by product was absorbed and oxidized by NaOCl solution. When the reaction was complete, the reaction mixture was concen-trated under reduced pressure, providing a crude solid which was further purified by recrystallization from methanol-H2O (1:1) to give 2 as colorless crystals (92%): mp 130-132 °C (lit.
8 128-130 °C); 1H NMR (200 MHz, CDCl 3) δ 5.54 (broad triplet, 2H), 2.91 (t, J)6.1 Hz, 4H), 1.72-1.56 (m, 2H), 0.84 (d, J) 6.6 Hz, 12 H);13C NMR (75 MHz, CDCl 3) δ 159.4, 47.7, 29.0,
20.1; IR (KBr) cm-13347, 1623, 1572; MS (EI, 70 eV) m/e 172
(M+
, 100), 157 (M+
-CH3, 20), 129 (M
+
-C3H7, 25), 72 (29), 58 (81); HRMS calcd for C9H20N2O 172.1576, obsd 172.1573. Anal.
Calcd for C9H20N2O: C, 62.75; H, 11.70; N: 16.26. Found: C,
62.49; H, 11.67; N; 16.21.
N,N′-Diallylurea (4): colorless crystals; mp 93-95 °C (lit.
9
mp 92-94 °C).
N,N′-Dibenzylurea (6). Recrystallization from CHCl3
-hexane yielded 6 as colorless crystals: mp 166-168 °C (lit.10 mp 167-170 °C).
N,N′-Cyclohexylurea (10). Recrystallization from MeOH
yielded 10 as colorless crystals: mp 230-231 °C (lit.
1bmp 229
-230 °C from MeOH).
S-Methyl 1-Piperidinecarbothioate (12). Flash chroma-tography on silica gel, using MeOH-CH2Cl2(1:7) as the eluent, afforded 12 as a colorless oil: 1H NMR (200 MHz, CDCl
3) δ 3.52 -3.40 (m, 4H), 2.32 (s, 3H), 1.70-1.49 (m, 6H);
13C NMR (50 MHz,
CDCl3) δ 167.1, 45.7 (broad peak), 25.6, 24.4, 12.8; IR (neat,
NaCl) cm-1
1734; MS (EI, 70 eV) m/e 159 (M+
, 28), 112 (M+
-CH3S, 81), 69 (100); HRMS (EI, 70 eV) calcd for C7H13NOS
159.0719, obsd 159.0722.
N,N′-Bis(4-aminobenzyl)urea (14). Flash chromatography
on silica gel, using MeOH-CHCl3(1:20) as the eluent afforded
14 as colorless crystals: mp 200-202 °C; 1H NMR (200 MHz, DMSO-d6) δ 6.90 (d, J)8.2 Hz, 4H), 6.50 (d, J)8.2 Hz, 4H), 6.04 (broad triplet, 5.6 Hz, 2H), 4.90 (bs, 4H), 4.02 (d, J)5.6 Hz, 4H);13C NMR (50 MHz, DMSO-d 6) δ 158.2, 147.4, 128.2, 127.8, 113.9, 43.0; IR (KBr) cm-13416, 3318, 1606, 1560; MS
(EI, 70 eV) m/e 270 (M+, 40), 177 (32), 164 (61), 121 (100), 106
(45), 94 (20); HRMS (EI, 70 eV) calcd for C15H18N4O 270.1480,
obsd 270.1479. Anal. Calcd for C15H18N4O: C, 66.64; H, 6.71;
N, 20.72. Found: C, 66.34; H, 6.67; N, 20.59.
N,N′-Bis(2-hydroxyethyl)urea (19) (Method B). Excess
ethanolamine and DMDTC were mixed and heated at 60 °C for 15 h. The released malodorous methyl sulfide by product was absorbed and oxidized by NaOCl solution. When the reaction was complete, the unreacted ethanolamine was removed under reduced pressure by simple distillation, providing a crude (8) Franz, R. A.; Applegath, F.; Morriss, F. V.; Baiocchi, F. J. Org.
Chem. 1961, 26, 3306.
(9) Laufer, D. A.; Al-Farhan, E. J. Org. Chem. 1991, 56, 891. (10) Pihuleac, J.; Bauer, L. Synthesis 1989, 61. Table 2. Condensation of Various Diamines or Amino Alcohols with DMDTC
aInitial concentration of the starting amine.bIsolated yield.
Downloaded by NATIONAL TAIWAN UNIV on July 29, 2009
mixture of oxazolidone and bis(2-hydroxyethyl)urea which was further purified by recrystallization from methanol-ethyl ace-tate (1:4.5) to give 19 as colorless crystals: mp 82-84 °C;
1H
NMR (200 MHz, DMSO-d6) δ 5.96 (broad triplet, 2H), 4.62 (bs,
2H), 3.33 (bs, 4H), 3.02 (q, J)6 Hz, 4H);
13C NMR (75 MHz,
DMSO-d6) δ 158.4, 60.8, 42.1; IR (KBr) cm-1
3343, 1621; MS (EI, 70 eV) m/e 149 (M+
+H, 5), 148 (M
+, 2), 118 (100), 117
(60), 70 (40); HRMS (EI, 70 eV) calcd for C5H12N2O3148.0848,
obsd 148.0842. Anal. Calcd for C5H12N2O3: C, 40.53; H, 8.17;
N, 18.91. Found: C, 40.08; H, 8.16; N, 18.78.
N,N′-Bis(3-hydroxypropyl)urea (16). Recrystallization from
MeOH-CHCl3provided 16 as colorless crystals: mp 83-86 °C (lit.4mp 93-94 °C from CH3CN);1H NMR (200 MHz,
DMSO-d6) δ 5.82 (t, J)6.0 Hz, 2H), 4.00-4.50 (bs, 2H), 3.38 (t, J) 6.0 Hz, 4H), 3.00 (q, J)6 Hz, 4H), 1.48 (quintet, J)6 Hz, 4H) (lit.4 1H NMR (90 MHz, D 2O) δ 3.5 (t, J)7.0 Hz, 4H), 3.1 (q, J )7 Hz, 4H), 1.6 (m, 4H)); 13C NMR (50 MHz, DMSO-d 6) δ 158.5, 58.4, 36.4, 33.2 (lit.4 13C NMR (62.89 MHz, D 2O) δ 160.7, 59.2, 36.7, 31.8); IR (KBr) cm-13339 (br), 1613, 1584; MS (EI, 70 eV) m/e 176 (M+, 20), 146 (65), 132 (90), 102 (40), 74 (100), 57 (91);
HRMS (EI, 70 eV) calcd for C7H16N2O3176.1161, obsd 176.1155.
N,N′-Bis(1-methylpropyl)urea (8). Recrystallization from
CHCl3-hexane yielded 8 as colorless crystals: mp 131-132.5 °C (lit.8mp 135 °C);1H NMR (300 MHz, CDCl
3) δ 4.30 (bs, 2H),
3.62 (m, 2H), 1.48 (m, 4H), 1.07 (d, J)6.7 Hz, 6H), 0.87 (t, J) 7.4 Hz, 6H);13C NMR (75 MHz, CDCl
3) δ 157.6, 47.3, 30.3, 21.0,
10.3; IR (KBr) cm-13339 (br), 1613, 1584; MS (EI, 70 eV) m/e
172 (M+, 12), 143 (M+
-C2H5, 63), 72 (20), 58 (100); HRMS (EI, 70 eV) calcd for C9H20N2O 172.1576, obsd 172.1573. Anal. Calcd
for C9H20N2O: C, 62.74; H, 11.70; N, 16.26. Found: C, 62.49;
H, 11.57; N, 16.39.
Typical Synthetic Procedures for Cyclic Ureas and Carbamates. 5-(Hydroxymethyl)oxazolidin-2-one (22). To
a stirred solution of 3-aminopropane-1,2-diol (21) in methanol was added DMDTC. After being heated at 60 °C for 24 h, the reaction mixture was concentrated under reduced pressure, providing a crude oil which was further purified by flash chromatography, using MeOH-CH2Cl2(1:10) as the eluent to give 22 as colorless oil: 1H NMR (200 MHz, DMSO-d
6) δ 7.38 (bs, 1H), 5.06 (t, J)5.6 Hz, 1H), 4.45-4.57 (m, 1H), 3.33-3.58 (m, 3H), 3.21 (dd, J)8.4, 6.6 Hz, 1H) (lit. 9 1H NMR (CD 3OD) δ 4.8 (bs, 2H), 4.6 (m, 1H), 3.25-3.80 (m, 4H));13C NMR (50 MHz, DMSO-d6) δ 159.4, 76.4, 62.3, 41.6 (lit.11 13C NMR (CD3OD) δ
78.5, 63.5, 42.9); IR (KBr) cm-13320 (br), 3297, 1731; MS (EI, 20 eV) m/e 118 (M+ +H, 5), 100 (M + -OH, 24), 86 (M + -CH2 -OH, 100); HRMS (EI, 70 eV) calcd for C4H7NO3117.0426, obsd
117.0420.
Tetrahydro-5-hydroxy-2(1H)-pyrimidinone (24).
Recrys-tallization of the crude product from MeOH-acetone afforded
24 as colorless crystals: mp 210-213 °C;1H NMR (200 MHz, DMSO-d6) δ 6.16 (bs, 2H), 5.07 (d, J)4 Hz, 1H), 3.79-3.82 (m, 1H), 3.05-3.20 (m, 2H), 2.80-3.00 (m, 2H); 13C NMR (50 MHz, DMSO-d6) δ 156.1, 60.4, 46.2; IR (KBr) cm-1 3344, 3297, 3217, 1645; MS (EI, 20 eV) m/e 116 (M+
, 90), 88 (30), 59 (100); HRMS (EI, 70 eV) calcd for C4H8N2O2116.0586, obsd 116.0583. Anal.
Calcd for C4H8N2O2: C, 41.37; H, 6.94; N, 24.13. Found: C,
41.03; H, 6.84; N, 23.99.
4,4-Dimethyl-2-imidazolidinone (29). Recrystallization from CH2Cl2-hexane (4:1) afforded 29 as colorless crystals: mp 139-141 °C; 1H NMR (200 MHz, CDCl 3) δ 5.63 (bs, 2H), 3.20 (s, 2H), 1.33 (s, 6H);13C NMR (50 MHz, CDCl 3) δ 163.5. 54.9, 53.8, 28.1; IR (KBr) cm-1
3281, 1704, 1688; MS (EI, 70 eV) m/e 114 (M+
, 10), 99 (100), 56 (30); HRMS (EI, 70 eV) calcd for C5H10N2O 114.0793, obsd 114.0787. Anal. Calcd for
C5H10N2O: C, 52.61; H, 8.83; N, 24.54. Found: C, 52.28; H, 8.81;
N: 24.42.
Tetrahydro-5,5-dimethyl-2(1H)-pyrimidinone (31).
Re-crystallization of 31 from CH2Cl2-hexane afforded colorless crystals: mp 254-256 °C (lit. 12255 -257 °C); 1H NMR (200 MHz, CDCl3) δ 5.19 (bs, 2H), 2.94 (d, J)2.2 Hz, 4H), 1.05 (s, 6H); 13C NMR (50 MHz, CDCl 3) δ 157.0, 52.3, 28.3, 24.7; IR (KBr) cm-1
3228, 1697; MS (EI, 70 eV) m/e 128 (M+
, 70), 113 (M+
-CH3), 56 (100); HRMS (EI, 70 eV) calcd for C6H12N2O 128.0950,
obsd 128.0950. Anal. Calcd for C6H12N2O: C, 56.23; H, 9.44;
N, 21.86. Found: C, 55.96; H, 9.40; N, 21.76.
Hexahydro-3H-oxazolo[3,4-a]pyridin-3-one (33). Flash
chromatography on silica gel, using MeOH-CH2Cl2 as the (11) Cardillo, G.; Orena, M.; Sandri, S.; Tomasini, C. Tetrahedron 1987, 43, 2505.
(12) Skinner, G. S., Hall, R. H.; Susi, P. V. J. Am. Chem. Soc. 1957, 79, 3786.
Table 3. Preparation of Unsymmetrical Ureas from DMDTC
R1NHLi+(MeS) 2CO98 (1) LDA (2) H+ R1NHCOSMe98 R2NH2 R1NHCONHR2
aOverall isolated yield.
Downloaded by NATIONAL TAIWAN UNIV on July 29, 2009
eluent, yielded 33 as a colorless oil: 1H NMR (200 MHz, CDCl 3) δ 4.38 (t, 8.1 Hz, 1H), 3.88-3.96 (m, 2H), 3.50-3.70 (m, 1H), 2.90-2.70 (m, 1H), 1.78-1.98 (m, 2H), 1.66-1.75 (m, 1H), 1.20 -1.55 (m, 3H) (lit.13 1H NMR (neat) δ 4.8 (m, 1H), 4.2 (m, 3H), 3.25 (m, 1H), 2.1 (m, 6H));13C NMR (50 MHz, CDCl 3) δ 156.8, 67.9, 54.2, 41.1, 30.2, 24.0, 22.3; IR (neat, NaCl) cm-11739; MS
(EI, 70 eV) m/e 141 (M+
, 100), 126 (20), 97(30), 83 (95), 69 (40), 55 (55); HRMS (EI, 70 eV) calcd to C7H11NO2141.0790, found
141.0799.
3,4-Dihydro-2(1H)-quinazolinone (35). Recrystallization
of 35 from MeOH-CH2Cl2led to colorless crystals: mp 222 -223 °C;1H NMR (200 MHz, DMSO-d 6) δ 8.98 (bs, 1H), 7.03 -7.14 (m, 2H), 6.78-6.87 (m, 3H), 4.28 (s, 2H) (lit. 14 1H NMR (DMSO-d6) δ 9.08 (bs, 1H), 6.80-7.50 (m, 5H), 4.40 (s, 2H)); 13C NMR (50 MHz, DMSO-d6) δ 154.8, 138.3, 127.8, 125.9, 121.1, 118.3, 113.7, 42.7; IR (KBr) cm-13248, 1718; MS (EI, 70 eV) m/e 148 (M+, 100), 147 (M+ -H, 90), 104 (20); HRMS (EI, 70 ev) calcd for C8H8N2O 148.0637, obsd 148.0638. Anal. Calcd
for C8H8N2O: C, 64.85; H, 5.54; N: 18.91. Found: C, 64.68; H:
5.42; N: 18.90.
Typical Synthetic Procedures for S-methyl N-Alkylthio-carbamates. S-Methyl N-Benzylthiocarbamate (36). To a
solution of benzylamine (0.93 g, 0.87 mmol) and diisopropyl-amine (0.89 g, 8.8 mmol) in THF (20 mL) at -78 °C under nitrogen atmosphere was added n-BuLi (1.6 M, 10.9 mL, 17.5 mmol) in hexane. After addition, the solution was stirred at -78 °C for 0.5 h, followed by addition of a solution of DMDTC (1.07 g, 8.8 mmol). The solution was then allowed to react at room temperature for 20 h. The reaction was quenched by pouring it into a mixture of ice-dilute HCl solution. The crude solid was dissolved into EtOAc, washed with aqueous Na2CO3and brine,
dried over anhydrous MgSO4, concentrated under reduced
pressure, and recrystallized from hexane to provide 36 as colorless crystals (0.95 g, 62%): mp 76-79 °C; 1H NMR (300 MHz, CDCl3) δ 7.20-7.34 (m, 5H), 5.67 (bs, 1H), 4.45 (d, J)6 Hz, 2H), 2.35 (s, 3H);13C NMR (75 MHz, CDCl 3) δ 167.9, 137.7, 128.45, 127.4, 127.3, 45.1, 12.2; IR (KBr) cm-13305, 1639; MS
(EI, 70 eV) 181 (M+, 30), 133 (30), 91 (100); HRMS (EI, 70 eV)
calcd for C9H11NOS 181.0562, obsd 181.0559. Anal. Calcd for
C9H11NOS: C, 59.64; H, 6.12; N, 7.73. Found: C, 59.65; H, 6.12;
N, 7.78.
S-Methyl N,N′-(p-Xylylene)bis(thiocarbamate) (39). Re-crystallization from EtOAc-hexane yielded 39 as colorless crystals: mp 201-203 °C; 1H NMR (200 MHz, DMSO-d 6) δ 8.64 (broad triplet, J)6.0 Hz, 2H), 7.17 (s, 4H), 4.25 (d, J)6.0 Hz, 4H), 2.20 (s, 6H);13C NMR (50 MHz, DMSO-d 6) δ 166.4, 137.8, 127.3, 43.7, 11.6; IR (KBr) cm-1 3244, 1629; MS (EI, 70 eV) 284 (M+, 1), 236 (10), 193 (46), 188 (16), 180 (37), 146 (100), 91 (48);
HRMS (EI, 70 eV) calcd for C12H16N2O2S2 284.0653, obsd
284.0652. Anal. Calcd for C12H16N2O2S2: C, 50.70; H, 5.68; N,
9.86. Found: C, 51.30; H, 5.74; N, 9.70.
Typical Synthetic Procedures for Unsymmetrical Ureas from S-Methyl N-Alkylthiocarbamates. N-Benzyl-N′-tet-rahydrofurfurylurea (38). To a stirred solution of S-methyl N-benzylthiocarbamate (36) (0.11 g, 0.63 mmol) in methanol (2
mL) was added tetrahydrofurfurylamine (0.12 g, 1.1 mmol). After being heated at 60 °C for 24 h, the reaction mixture was concentrated under reduced pressure, providing a crude solid. Recrystallization of the solid from CHCl3-hexane gave 38 as colorless crystals (0.14 g, 88%): mp 78-80 °C; 1H NMR (300 MHz, CDCl3) δ 7.18-7.30 (m, 5H), 5.86 (broad triplet, 1H), 5.55 (broad triplet, 1H), 4.27 (d, J)6 Hz, 2H), 3.90 (m, 1H), 3.60 -3.80 (m, 2H), 3.39 (m, 1H), 3.03 (m, 1H), 1.75-1.95 (m, 3H), 1.51 (m, 1H);13C NMR (100 MHz, CDCl 3) δ 158.7, 139.3, 128.5, 127.4, 127.2, 78.8, 68.0, 44.5, 44.4 28.2, 25.9; IR (KBr) cm-13339, 1615; MS (EI, 70 eV) 234 (M+, 31), 190 (17), 164 (40), 151 (20), 106
(58), 91 (80), 71 (100); HRMS calcd for C13H18N2O2234.136, obsd
234.1369. Anal. Calcd for C13H18N2O2: C, 66.64; H, 7.74; N,
11.95. Found: C, 66.67; H, 7.75; N, 11.93.
N,N′′-(p-Xylylene)bis[N′-(3-hydroxypropyl)urea] (40).
Re-crystallization of 40 from DMSO-EtOAc afforded colorless crystals: mp 219-221 °C dec;1H NMR (200 MHz, DMSO-d6) δ 7.16 (s, 4H), 6.28 (t, J)6.0 Hz, 2H), 5.89 (t, J)6.0 Hz, 2H), 4.45 (t, J)6.0 Hz, 2H), 4.15 (d, J)6.0 Hz, 4H), 3.35-3.42 (q, J)6.0 Hz, 4H), 3.05 (q, J)6.0 Hz, 4H), 1.50 (quintet, J)6.0 Hz, 4H);13C NMR (75 MHz, DMSO-d 6) δ 158.2, 139.2, 126.9, 58.4, 42.7, 36.4, 33.2; IR 3325, 1606, 1574; FAB (NBA) 339.2 (M+
+H). Anal. Calcd for C14H22N4O4: C, 56.78; H, 7.74; N, 16.55. Found: C, 56.55; H, 7.87; N, 16.50.
N,N′′-(p-Xylylene)bis[N′-(2-methylpropyl)urea] (41).
Re-crystallization from DMSO-EtOAc afforded 41 as colorless crystals: mp 237-240 °C dec;
1H NMR (300 MHz, DMSO-d 6) δ
7.15 (s, 4H), 6.19 (broad triplet, 2H), 5.92 (broad triplet, 2H), 4.14 (d, J)6 Hz, 4H), 2.82 (t, J)6 Hz, 4H), 1.60 (m, 2H), 0.81 (d, J)6.6 Hz, 12 H); 13C NMR (50 MHz, DMSO-d6) δ 158.1, 139.2, 126.9, 46.8, 42.6, 28.6, 20.0; IR (KBr) cm-13318, 1613,
1562; FAB (NBA) 335.2 (M+
+ H). Anal. Calcd for C18H30N4O2: C, 64.64; H, 9.04; N, 16.75. Found: C, 64.36; H,
8.97; N, 16.70.
N,N′′-(p-Xylylene)bis(N′-benzylurea) (42).
Recrystalliza-tion from DMSO-EtOAc afforded 42 as colorless crystals: mp 258-261 °C dec;1H NMR (300 MHz, DMSO-d6) δ 7.20-7.33 (m, 10H), 7.17 (s, 4H), 6.39 (m, 4H), 4.21 (d, J)6 Hz, 4H), 4.19 (d, J)6 Hz, 4H); 13C NMR (50 MHz, DMSO-d 6) δ 158.0, 140.9, 139.2, 128.2, 128.1, 127.0, 126.6, 42.9, 42.7; IR (KBr) cm-1 3319, 1619; FAB (NBA) 403.2 (M+
+ H). Anal. Calcd for C24H26N4O2: C, 71.62; H, 6.51; N, 13.91. Found: C, 71.52; H,
6.50; N, 13.90.
Acknowledgment. We thank the National Science
Council of the Republic of China for the financial
support (NSC 85-2113-M-002-013 and NSC
85-2815-C002-01-010M).
Supporting Information Available: 1H and13C NMR
spectra of DMDTC and compounds 4, 6, 8, 12, 14, 16, 19, 22,
24, 29, 31, 33, 35, 36, and 38-42 (38 pages). This material is contained in libraries on microfiche, immediately follows this article in the microfilm version of the journal, and can be ordered from the ACS; see any current masthead page for ordering information.
JO9522825 (13) Hall, H. K. Jr.; El-Shekiel, A. J. Org. Chem. 1980, 45, 5325.
(14) Yoshida, T.; Kambe, N.; Murai, S.; Sonoda, N. J. Org. Chem. 1987, 52, 1611.
Downloaded by NATIONAL TAIWAN UNIV on July 29, 2009