Pergamon
Deep-Sea Research 1, Copyright © 1994 Elsevier Science Vol. 41, No. 1, pp. 101-112, 1994 LtdPrinted in Great Britain. All rights reserved 0967-0637/94 $6.00 + 0.00
Water column distribution of 23°Th and 232Th in the Black Sea
CHIH-AN HUH,* JAMES M.
KELLEY,tJAMES W. MURRAy,: and CHING-LING
WEI§
(Received 2 June 1992; in revised form 21 December 1992; accepted 22 December 1992)
Abstract--Profiles of 23{~ and 232Th at a station in the western central Black Sea were determined using a highly sensitive mass spectrometry method. Compared with most open ocean and coastal waters, concentrations of "dissolved" (<0.4/~m) 23°Th and 232Th in the Black Sea are significantly higher, primarily due to lower scavenging rate in this predominantly anoxic environment. Above the anoxic zone (0-95 m) about 42% of 23°Th and 57% of 232Th are in the particulate form, compared with 9 and 21%, respectively, in the anoxic zone. The distribution indicates that a sizable fraction of particulate Th is associated with Mn-containing particles at the O2-H2 S interface just above the anoxic zone, which is released into solution when such particles are transported across the redox boundary and are dissolved in the anoxic zone. From the partitioning and isotopic composition of Th between dissolved and particulate phases, it is estimated that approximately 20- 40% of the dissolved 23°Th in the water column is terrigenous. The residence time of dissolved Th in the Black Sea water column is 43-48 years, compared with 6-20 years for the same depth range elsewhere in the world oceans.
I N T R O D U C T I O N
THE Black Sea is by far the largest anoxic basin in the world. It is an ideal environment for studying the geochemical cycling of elements across the oxic-anoxic interface, a subject that has long intrigued oceanographers. During the R.V. Knorr expedition to the Black Sea in June 1988, the water column at Sta. BS3-2 (42°50'N, 32°00'E) in the central western basin (Fig. 1) was sampled, with especially detailed resolution across the O2-H2S transition zone, for the analyses of isotopes of uranium and thorium, 21°pb and 21°po. This constitutes the most comprehensive study of scavenging processes in the water column of anoxic environments using natural decay-series isotopes. The data on uranium isotopes, 23 4Th, 210 Pb and 210 Po have been reported previously (WEI, 1990; WEI and MURRAY, 1991; WEI and MURRAY, in press). The newly acquired
230Th
and232Th
data are integrated with the previous 234Th data and discussed in this paper.234Th (T1/2
= 24.1 days) and a major portion of23°Th (T1/2
= 7 5 , 2 0 0 years) are produced in seawater from the decay of238U
and234U,
respectively, whereas 232Th (T1/2 = 1.4 × 101° years) in the ocean is derived from continents via fluvial and aeolian pathways. If Th*College of Oceanic and Atmospheric Sciences, Oregon State University, OR 97331, U.S.A. tBattelle Pacific Northwest Laboratories, Richland, WA 99352, U.S.A.
$School of Oceanography, University of Washington, Seattle, WA 98195, U.S.A. §Institute of Oceanography, National Taiwan University, Taipei, Taiwan, R.O.C.
1()2 ( ' . - A H t m ,'t al. 4 6 ° N U S / S R. I q~ [ ] I
'1
4 4 a 4 2 ° 4 0 ° R O M A N ULGARIA .j ., f - i 2 6 ° E 2 8 ° F i g . l. • BS3-2 T U R K E Y 3 0 ° 3 2 ° 3 4 ° 3 6 ° 3 8 ° Location o f t h e s a m p l i n g s t a t i o n (BS3-2) in t h e B l a c k Sea. 4 0 ° 4 2 °isotopes are used as tracers to study chemical scavenging processes in the oceanic water column in a comprehensive manner, it is necessary to combine these three Th isotopes. This is because
234Th is too
short-lived to be applicable to the deep ocean where scavenging rates are usually slow. Conversely, 23°Th is too long-lived to be useful as a sensitive tracer for studying chemical scavenging in the surface ocean where scavenging rates are typically fast. However,23°Th
has an additional continental source from the surface. In order to resolve the two components of 23°Th, it is necessary to invoke232Th,
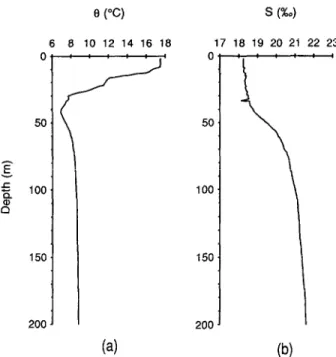
the only nonradioge- nic isotope of Th, which is derived only from lands. If Th isotopes are used as proxies to study the cycling of trace metals, it is especially important to include 232Th which serves as a necessary link between most trace metals and radiogenic Th isotopes ( H u n and BACON, 1985).Figure 2 shows profiles of salinity and potential temperature at the time of the cruise. Salinity increased from 18.2%o at the surface to 22.3%o at the bottom, with the halocline layer centered at c a 50 m. Potential temperature decreased from 17.5°C at surface to a
minimum of 7°C at 45 m and then increased to 9°C throughout the rest of the water column. These structures produce a layer of high stability at the base of the oxic zone which has important bearings on metal scavenging and particle cycling, as discussed later.
Figure 3 shows profiles of oxygen and sulfide. Dissolved oxygen is high in the surface with a photosynthetically-produced maximum at 10 m. Oxygen then decreases rapidly to less than 10 # M at 55 m and less than 5 # M between 55 and 95 m. Sulfide first becomes detectable at about 95 m and increases almost linearly with depth. Based on the distribution of these two redox-sensitive species, the water column can be viewed as a
23Drh and 23% in the Black Sea 103 6 8 10 12 14 16 18 17 18 19 20 21 22 23 100 150 200 J
(a)
(b)
Fig. 2. Profiles of (a) potential temperature and (b) salinity at BS3-2.
Oxygen and sulfide (PM)
E
5 100 % cl 150 200104 ( . - A . H u . et al.
three layered system, with the oxic zone from the surface to 55 m, the "suboxic" zone from 55 to 95 m, and the anoxic zone below 95 m (MURRAY e t a l . , 1989).
METHODS
Water samples were collected using 30-1 Niskin bottles mounted on a CTD rossette. These bottles were outfitted with Teflon-coated stainless steel springs. Nitrogen gas was used to pressure filter (at 12 psi) seawater through 0.4-pro Nuclepore filters. T h e filtrates were drawn into 1-1 polyethylene bottles, and immediately spiked with 1 dpm 229Th and acidified to p H - 2 using double distilled HCi. The filters were rinsed with about 15 ml of deionized water to remove sea salt and stored in Petri dishes.
In the laboratory, after the addition and equilibration of 1 mg Fe carrier, concentrated U L T R E X N H 4 O H was added to the water samples to raise the p H to r-7 and effect the adsorption and coprecipitation of Th isotopes with Fe(OH)3. Following a 24-h equili- bration in a water bath of 60°C, the precipitates were collected by centrifugation, washed with D.I. water by resuspension, and re-centrifuged. The coprecipitation and washing procedure is a major step to remove alkali- and alkaline earth samples, which constitute the major cations in seawater. After dissolving the "salt-free" precipitates in 2 ml of 8 N HC1, the samples were ready for the subsequent ion exchange procedures to purify Th.
T h r e e successive ion-exchange columns were used to separate literally the entire periodic table of elements from Th. All columns are loaded with anion exchange resin (AG1 × 8, 100-200 mesh) and washed sequentially with D.I. water, 8 N HCI, and D.I. water again. The columns were converted to required forms just prior to use. The first column (Bio-Rad 10-ml polypropylene column) was loaded with 2 ml of resin (wet volume) and conditioned with 8 N HCl. The second column was identical to the first one, except that it is conditioned with 8 N HNO3. The third and final column was a 1-ml "micro-column" packed with 0.2 ml resin and pre-conditioned with 8 N H N O 3, The 0.2-ml capacity column was fabricated from a 2-ml polyethylene Pasteur pipet by cutting the top third of the pipet bulb and trimming 20 mm from the tip. The tip was fitted with a 4 mm diameter frit (1/16 in. thickness, 35 p m pore size).
The 2 ml sample solution (in 8 N HCI) was loaded to the first column and the eluate containing Th was collected with a 10 ml Teflon beaker. After all sample solution had passed through, the column was washed with 5 ml of 8 N HCI which was also collected in the same beaker. The eluate was then evaporated to dryness. The first column retained and separated the Fe carrier, and most other metals in the periodic table, from Th. Elements eluted off the column along with Th were any remaining alkali- and alkaline earth metals, and a few other trace metals in seawater such as AI, Sc, and rare earths.
After the eluate from the first column was evaporated to incipient dryness, the sample was transposed to nitric form with 2 ml of 8 N HNO3. The solution was then charged to the second column. Th was retained on the column, whereas metals not separated from Th in the first column passed through the second column. Following the passage of the sample solution, the column was washed with 10 ml of 8 N H N O 3. The Th was eluted off the column with two 2-ml increments of 8 N HC1, which was collected in the original Teflon beaker and evaporated to dryness.
Normally the sample was rather clean at this point. However, it was necessary to perform a final micro-column operation to ensure purification and facilitate the prep- aration of sample source for mass spectrometry. This was done by dissolving the dry-down
23°Th and 232al"h in the Black Sea 105 spot from the previous step by - 0 . 5 ml of 8 N HNO 3 and passing the solution through the micro-column. After washing the column with 1 ml of 8 N HNO3, Th was eluted off the column with 0.5 ml of 1 N HC1. The eluate was dripped directly from the column tip onto a Teflon pad which was mounted on a glass pedestal. The pad was 1/16 in. thick, 3/4 in. diameter, with a conical depression in the center to hold 0.5 ml of solution.
The filter samples were transferred to Teflon beakers and spiked with I dpm 229Th and 1 mg Fe carrier in the laboratory. The polycarbonate matrices were decomposed by soaking in NH4OH with gentle heating to evaporate NH4OH. Then a mixture of HNO3, HCI and HF was used to digest the particulates. After total dissolution of the filter samples, the chemical procedures were the same as described above for seawater samples.
In handling and processing the samples, clean-room practices should be stringently observed to avoid contamination and minimize blank levels. The sample bottles and all labware were pre-cleaned by acid-leaching. The chemical procedures were always per- formed in a laminar-flow hood. The acids and NH4OH used were of ULTREX grade or double distilled.
The Th isotopic analysis was performed at Battelle Pacific Northwest Laboratory using a three-stage mass spectrometer. Detailed procedures for source preparation and loading will be reported separately (HuH et al., in preparation).
R E S U L T S A N D D I S C U S S I O N
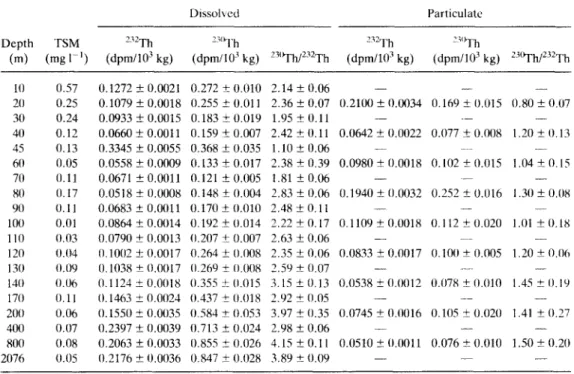
Concentrations of 23°Th, 232Th and the 23°Th/232Th activity ratio in the dissolved and particulate forms are listed in Table 1, and the profiles are shown in Figs 4 and 5. Where appropriate in the following discussion, the notations 23°Zhd, 232Thd, 23°Th_ and 232Th_ dissolved z'I'h, particulate °Th and particulate will be used to represent dissolved 23°Th, " 23 • 23 v . ~" 232Th, respectively. Note that the terms "dissolved" and particulate used in this paper are only operationally defined, as based on filtration using 0.4-#m membrane filters.
Dissolved 23°Th and 232Th
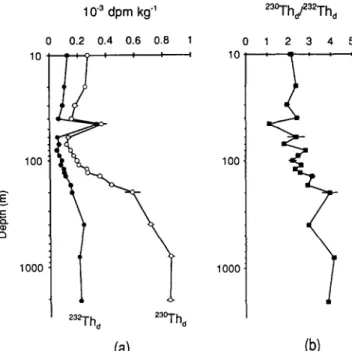
Profiles o f 23°Zhd a n d 232Thd are strikingly similar (Fig. 4). Both show the following features: (i) a decrease, by a factor of more than two, from the surface to a broad minimum centered around 70 m; (ii) a distinct subsurface peak at 45 m superimposed on the above trend; and (iii) a sharp increase with depth in the mid-water column from 70 to 400 m (for 232Th) o r 800 m (for 23°Zh).
Concentrations o f 232Th d in the water column vary between 5 x 10 -5 and 3.3 × 10 -4 dpm kg -1, averaging 1.5 x 10 -4 dpm kg -x. These values are significantly higher than 232Thd in most other deep ocean and coastal waters reported recently (e.g. HuH and BACON, 1985; Hurt and BEASLEY, 1987; NOZAKI and YAMADA, 1987; H u n et al., 1989; CtaUN, 1989). However, compared with 232Th data reported three decades ago for 11 locations in the Black Sea (LAZAREV e t al., 1961), data presented here are one to two orders
of magnitude lower. As discussed in HUH et al. (1989), we believe the earlier measure- ments are erroneously high due to sampling and analytical contamination.
If 23°Th d concentrations at the same depths in the water column are compared, the Black Sea data at BS3-2 are very close to those in the Arctic Ocean (BACON etal., 1989), and are much higher than all available data from elsewhere (e.g. NOZAKI et al., 1981; BACON and ANDERSON, 1982; NOZAKI and NAKANISHI, 1985; Hurt and BEASLEY, 1987; MURNANE et al.,
1 O0 ( ".-A. Ht:~i et al.
Table 1. Distribution o f total suspended matter ( TSM) and concentrations of23°Th and e V-' Th in dissolved atut particulate forrns in the water column o f Sta. BS3-2 (42°50'N, 32°00'E) in the central western Black Sea. The listed standard deviations (_+ 1 o) are based on propagated error f r o m counting statistics, including ± I 6'!4, uncertainty
f r o m the 22'~Th .spike activity
Dissolved Particulate Depth T S M 232Th 23~1'h 232Th 23'~1"h (m) ( m g 1-1) (dpm/103 kg) (dpm/103 kg) 23°Th/232Th (dpm/103 kg) (dpm/103 kg) 23°Th/232Th 10 0.57 0.1272 --- 0.0021 0.272 ± 0.010 2.14 __+ 0.06 . . . . 20 0.25 0 . 1 0 7 9 + 0 . 0 0 t 8 0.255-+0.011 2.36__+0.07 0.2100_+0.0034 0.169_+0.015 0.80_+0.07 30 0.24 0.0933__+0.0015 0.183_+0.019 1.95_+(I.11 . . . . . 40 0.12 0.0660_+0.0011 0.159_+0.007 2.42_+(I.11 0.0642_+0.0t122 0.077_+0.008 1.20_+0.13 45 0.13 0.3345 _+ 0.0(155 0.368 __+ (t.035 1.10 _+ 0.06 - - 6(I 0.05 0.0558 _+ 0.0009 0.133 _+ 0.017 2.38 _+ 0.39 0.0980 ± 0.0018 0.102 _+ 0.015 1.04 _+ 0.15 70 0.11 0.0671 _+ 0.0011 0.121 _+ 0.005 1.81 _+ 0.06 . . . . 80 0.17 0.0518 _+ (I.0008 0.148 _+ (t.004 2.83 _+ 0.06 0.1940 _+ (I.0032 0.252 _+ 0.016 1.30 _+ 0.08 90 0.11 0.0683_+0.0011 0.170_+0.010 2.48_+0.11 . . . . 100 0.01 0.0864_+0.0014 0.192_+0.014 2 . 2 2 + 0 . 1 7 0.1109_+0.0018 0.112_+0.020 1.01_+0.18 110 (/.03 0.0790 ± 0.0013 (I.207 ± 0.007 2.63 _+ 0.06 . . . . 120 0.04 (I.10(12_+(t.0(t17 0.264±__0.008 2.35__+0.(/6 (/.0833__+0.0017 0 . 1 0 0 ± 0 . 0 0 5 1.20-+0.(16 13(1 0.09 (1.1(t38 -+ 0.0017 (I.269 +__ 0.008 2.59 -+ 0.07 . . . .
14(I (I.06 0.1124 -+ 0.0(118 (I.355 -+ 0.015 3.15 + 0.13 0.(/538 __+ 0.(t012 (I.(/78 ± 0.010 1.45 ± 0.19 17(/ 0.11 (I.1463 + (I.0(124 (1.437 -+ (t.(118 2.92 -+ (I.05 . . . .
20(1 0.06 (/.1550 _+ 0.0(135 (1.584 __+ 0.053 3.97 -+ (I.35 0.0745 _+ (/.(1(/16 0.105 ± 0.02(I 1.41 _+ (/.27 400 (I.(/7 0.2397 _+ 0.0039 (/.713 _+ 0.024 2.98 _+ 0.06 - - - - - - 80(/ (I.08 0.2063_+0.0033 0.855_+0.026 4.15_+0.11 (/.0510_+(I.(t011 0.076_+0.010 1 . 5 0 + - 0 . 2 0 2076 0.05 (/.2176 _+ 0.0036 0.847 _+ (/.(t28 3.89 _+ ( I . ( 1 9 . . . .
1990; CLE6C et al., 1991). Mechanisms leading to the similar 23°Thd concentrations in the Arctic Ocean and Black Sea are conceivably different in these two settings. The high 23~>I'h o concentrations in the Arctic Ocean can be attributed to extremely low particle flux and hence low scavenging rates (BACON et al., 1989). In the Black Sea, the flux of particles is relatively high, while the production rate of 23°Th is atypically low due to the low uranium concentration (3.3 ppb in typical seawater vs 1.3-2.1 ppb in Black Sea water; WH and MURRAY, 1991). The higher-than-usual 23°Thd concentrations in Black Sea are related to the redox chemistry in this special environment (discussed later).
Except at 45 m, the 23°Tho/e32Th 0 activity ratio increases with depth by a factor of 2 (Fig. 4), from 2 to 4, indicating the increased contribution of radiogenic e3t~-I'h with depth (discussed later). The anomalously low 23°Thj232Tho at 45 m (1.1) is similar to the
23 232 230
°ThJ Thp ratio in the upper water column (see later), which, coupled with high - Tho and 2~ZTho concentrations at that depth clearly indicate the effect of particles. As noted earlier, at BS3-2, 45 m is the depth of the highest density gradient (hence static stability) in the water column. Such a correlation (between 232Th maximum and density gradient) has also been noted elsewhere (HuH and BACON, 1985; CntJN, 1989) and it alludes to the possible effect of less than 0.4-~m or colloidal-sized particles in the "dissolved" fraction (HoNEVMAN and SANTSCm, 1991). It is interesting to note that the concentration of filtered particles in the oxic zone is characterized by a pronounced decrease from 0.57 mg 1-1 at 10 m to 0.04 mg 1 -l at 55 m (WEI and MURRAY, 1991), suggesting the production of <0.4-,tm particles at the expense of >0.4-/xm particles and remineralization of particles. This
23°Zh and 232Th in the Black Sea
107
E 100 r~ 100 1000 1 0 .3 d p m k g "1 0 0.2 0.4 0.6 0.8 1 10 232Th d 23°Th d(a)
23°Thd/232Th d 0 1 2 3 4 5 10 - : ; : 100 1000(b)
Fig. 4. Profiles of (a) dissolved 23°Th and 232Th and (b) dissolved 23°Th/232Th ratio at BS3-2. The depth axis is a log scale.
0 0.3 10 10 .3 d p m k g 1 o.1 0.2 I I r ~ 1000
23~Thp
(a)
23OTh/32Th p 0 0.5 1 1.5 2 10 , , , ' 1 O0 1000(b)
230 232Fig. 5. Profiles of (a) particulate Th and Th and (b) particulate 23°Th/232Th ratio at BS3-2. The depth axis is a log scale.
108 C.-A. Hu~ et al.
disaggregation/remineralization process may be microbially mediated
(CHo
and AZAM. 1988). Reduced particle size and an established strong pycnocline will retard particulate settling and water mixing, thereby maintaining a layer of increased concentration of "dissolved" matter in the region of high density gradient. The secondary phosphate maximum (CoDmPOTI et al., 1991) and subsurface maxima of NO 3 +NO,, - and in situ fluorescence (KARL and KNAUER, t991) found near 45 m at BS3-2 may be related to this speculated process. This hypothesis is consistent with the model prediction of CLEG6 and WmTFlELD (1990) which showed that the highest disaggregation and remineralization rates occur at a subsurface depth.The decrease with depth of dissolved 23t~Fh and 232Th in the upper water column reflects input at surface of continental detritus via aeolian (HAosAL1HO~3LU et al., 1991) and/or riverine (LEWIS and LANDIN6, 1991) pathways• Based on ancillary data, an attempt is made to estimate the fraction of 232Th in freshly delivered alumino-silicates that is soluble on contact with surface seawater• Although there is no data on the flux of continental materials at the site, a first order approximation can be made by assuming that it is similar to the deposition rate of bottom sediments. At an adjacent station (BS4-9), BARNES and COCHRAN (1991) estimated, based on 21°Pb stratigraphy, an apparent sedimentation rate of 40 cm ky -1. In order to convert this apparent sedimentation rate to mass accumulation rate, we need to know the porosity of wet sediments and the density of dry sediments• Although porosity was not directly determined on the BS4-9 core, it is reasonable to assume a value of 85-90% as estimated for the upper 10 cm of other cores in the deep Black Sea (Ross and DEGENS, 1974; CALVERT etal., 1987; C r u s l u s and ANDERSON, 1991). As for dry sediment density, a value of 2.5 g cm -3 is assumed. From these, a mass accumulation rate of 0.01-0.015 g cm-2 y J is obtained. Multiplying the mass accumulation rate by the concentration of 232Th, which averages 1.0 dpm g - t with little variation in the upper 10 cm (BARNES and COCHRAN, 1991), results in 0.01-0.015 dpm cm -2 y i as the total flux of e32Th. The question then follows is: what is the flux of d~ssolved - -Th to the surface water'? This can be estimated from the inventory and residence time of dissolved e3eTh in the surface water based on the steady-state assumption. The inventory of dissolved 23ZTh in the upper 40 m is 0.00042 dpm c m - e The residence time of dissolved 23ZTh in surface water should be the same as that of 234Th in theory, which in the upper 40 m is about 100 days (WEI and MURRAY, 1991). Thus, the dissolution flux of 232Th from terrigenous material is estimated to be 1.5 × 10 3 dpm cm 2 y--l, which accounts for 9-13% of the total 232Th delivered to the surface Black Sea. Considering the uncertainties involved in the calculation, the result seems to be fairly reasonable; it is not inconsistent with the percentages of leachable A1 in aeolian dusts (5-15%) determined from seawater leaching experiments by MARING and DUCE (1987).
Radiogenic vs terrigenous contribution of dissolved 23ffFh
T h e r e are two sources of 23°Wh d in seawater: radiogenic and terrigenous. The radiogenic component is produced from the decay of 234U in seawater; and the terrigenous com- ponent is derived from leaching by seawater of particles of continental origin, as discussed earlier. Because 23eTh d has only the continental source, the terrigenous c o m p o n e n t of 23°Wh d can be estimated from the concentration of 232Thd and the 23°Th/232Th ratio in continental detritus, i.e.
2:~r'h and 232Th in the Black Sea 109
% of 2~>'l-h d derived from continental detritus
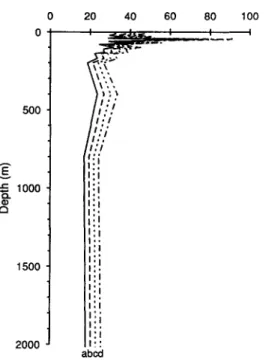
500 E 1000 Q . 03 a 1500 2000 20 40 60 I . . 1 I:. I;I :i
,il
Ii:"
|:1 :!I
Iii I: i I't 80 100 I I abcdFig. 6. Variation with depth of the terrigenous component of dissolved 23°Zh as a fraction of total dissolved 230111 at each depth. The four profiles are calculated by varying the 23°Th~32Th ratio of
the source material from (a) 0.7 to (d) 1.0.
The radiogenic component of
23°Zhd can
then be calculated by subtracting the terrigenous component from the measured, total 23°Th:(230Thd)radiogenic ---- 230Th d - (23°Zhd)terrigenous.
The question then is the 23ffFh/E32Th ratio in the source material. This ratio can be reasonably constrained by the following considerations. The 23°Zhj232Th ratio in surface sediments, which is 1.47 + 0.15 (n -- 5) in the top 10 cm of BS4-9, is identical to those (1.45 + 0.05; n = 3) in suspended particles in the anoxic zone. Because these values carry a considerable signal of radiogenic 23°Th, they should be considered as upper limits for
23°Th/232Th in continental detritus. The lowest particulate 23°Zh/E32Th ratio observed in the oxic zone is 0.80 + 0.07 (Table 1). If0.8 is taken as the 23°Th/232Th ratio in continental detritus, it is calculated that the terrigenous component
of
232°Thd decreases from - 4 0 % near the surface to - 2 0 % below 800 m. Figure 6 shows that the result does not change dramatically if the ratio is varied between 0.7 and 1, which we believe should bracket the real 23°Th/E32Th ratio in freshly delivered continental material.Particulate
23(~
and232Th
Due to a mishap in the chemical analysis, half of the filter samples, which were processed in a batch, were lost. Despite the poor sample resolution in the particulate Th profile, there
I10 C.-A. HuH et al
'Fable 2. Water column inventories o f 23~Th and 23eTh at Sta. BS3-2
hwcntory (dpm m :)
230Th 2:~2Th
Depth
range Dissolved Particulate Dissolved Particulate
0-55 m (oxic) 13. l 7.2 7.2 ~.2 55-95 m (suboxic) 6.3 6.7 3.2 5.6 95-2076 rn (anoxic) 1560 16(1 410 110
still exist some phenomena that can be discerned. The available data show a general correspondence between the concentrations of particulate Th and total suspended matter, and suggest the existence of near surface maxima and subsurface maxima around 80 m (Table 1 and Fig. 5). It is important to note that the subsurface maxima of particulate 23°Th and 23~Fh appear to coincide with the maxima of particulate Mn (LEwis and LANDING, 1991) and particulate 234Th
(WEI
and MURRAY, 1991) found at 70-80 m. Below 80 m, there exists a strong positive correlation (r = 0.99) between particulate 23°Th and particulate Mn: 23°Thp = 0.021 Mnp + 0.078; n = 6. Because the samples for both measurements were collected from the same station at the same time, the coherence provides evidence of scavenging of dissolved Th by Mn-containing particles at the redox boundary and recycling of Th along with Mn in the anoxic deep water.In the oxic and suboxic zones, 232Thp is higher than 232Th d whereas 23°Thp is fairly comparable t o 23°Th d. The trend is clearly reversed in the anoxic zone where both isotopes show much lower concentration in the particulate form than in the dissolved form. This is similar to the observation made in Saanich Inlet (CnuN and Hun, 1988), and reflects the fact that, as an A-type metal, Th is not efficiently scavenged by sulfides.
Inventories of dissolved and particulate 23°Th and 23~Fh in the oxic (0-55 m), suboxic (55-95 m), and anoxic (95-2076 m) zones are estimated, as given in Table 2. The data show that in the oxic and sub-oxic zones combined, 42% of 23°Th and 57% of 232Th are in the particulate form. By comparison, only 9% of 23°Th and 21% of 232Th are in the particulate form in the anoxic zone. We believe the major carrier phase of particulate Th is Mn- oxyhydroxides in the suboxic zone and alumino-silicates in the oxic and anoxic zones.
T h e 23°Thp/232Thp activity ratio increases from 0.8 near surface to 1.5 at depth, which is in parallel with the increase of 23°Thd/232Thd from 2 to 4. The correspondence between these two ratios is consistent with the thinking that the adsorption of Th by suspended particles is a reversible process ( B A c o N and ANDERSON, 1982; MOORE and HUNTER, 1985).
Fluxes and residence times of dissolved Th isotopes
The residence times of dissolved Th below the euphotic zone are normally too long to be derived from the 238U-234Th disequilibrium. The problem can be overcome using the
234U-23°Th disequilibrium. Because only the radiogenic component of 23°Thd is relevant to this application, the terrigenous component must be subtracted from the total e3°Tho. Assuming that the 23°Th/e3ZTh ratio in terrigenous particles is 0.7-1, the inventory of radiogenic 23°Th d in the water column can be calculated to be 1160-1290 dpm m -2 using the method discussed earlier. The production rate of Z3°Th in the water column, based on
23°Zh and 232Th in the Black Sea 111
the detailed 234U data (WEI and MURRAY, 1991), is 26.8 dpm m -2 y-a. Thus, the residence time of dissolved 23°Zh in the water column (0-2076 m) is 43-48 years, compared with 6-20 years calculated from data for the same depth range at other open-ocean sites (NOZAKI e t al., 1981, 1987; BACON and ANDERSON, 1982; NOZAKI and NAKANISI-II, 1985; COCI-IRAN et al., 1987; CLEGG et al., 1991).
Acknowledgements--We thank the assistance of Barbara Paul in sampling at sea and Lee Bond in mass
spectrometric analysis. The first author wishes to thank the NORCUS (Northwest College and University Association for Science) program for making possible his research appointment at the Battelle Pacific Northwest Laboratory in 1991. Two anonymous reviewers provided helpful comments on our manuscript. This work was supported by NSF grants OCE-8614400 (JWM), OCE-8916087 and OCE-9115530 (CAH). This is University of Washington Contribution no. 1955.
R E F E R E N C E S
BACON M. P. and R. F. ANDERSON (1982) Distribution of thorium isotopes between dissolved and particulate forms in the deep sea. Journal of Geophysical Research, 87 (C3), 2045-2056.
BACON M. P., C.-A. HUH and R. M. MOORE (1989) Vertical profiles of some natural radionuclides over the Alpha Ridge, Arctic Ocean. Earth and Planetary Science Letters, 95, 15-22.
BARNES C. E. and J. K. COCHRAN (1991) Geochemistry of uranium in Black Sea sediments. Deep-Sea Research,
38, Suppl. 2, $1227-$1254.
CALVERT S. E., J. S. VOGEL and J. R. SOUTHON (1987) Carbon accumulation rates and the origin of the Holocene sapropel in the Black Sea. Geology, 15,918-921.
Cno B. C. and F. AZAM (1988) Major role of bacteria in biogeochemical fluxes in the ocean's interior. Nature,
332,441-443.
CHUN Y. (1989) Geochemistry of thorium in natural water systems. M.S. Thesis, Oregon State University, Corvallis, Oregon, 98 pp.
CHUN Y. and C.-A. HtJri (1988) The behavior of 23°Th and 232Th in Saanich Inlet. LOS, 69, 1240-1241.
CLEGG S. L. and M. WHITF1ELD (1990) A generalized model for the scavenging of trace metals in the open ocean-- I. Particle cycling. Deep-Sea Research, 37, 809-832.
CLEGG S. L., M. P. BACON and M. WHITFIELD (1991) Application of a generalized scavenging model to thorium isotope and particle data at equatorial and high latitude sites in the Pacific Ocean. Journal of Geophysical Research, 96 ( C l l ) , 20655-20670.
COCHRAN J. K., H. D. LIVINGSTON, D. J. HIRSCHBERG and L. D. SURPRENANT (1987) Natural and anthropogenic radionuclide distributions in the northwest Atlantic Ocean. Earth and Planetary Science Letters, 84, 135-
152.
CODISPOTI L. A., G. E. FRIEDERICH, J. W. MURRAY and C. M. SAr, AMOTO (1991) Chemical variability in the Black Sea: implications of continuous vertical profiles that penetrated the oxic/anoxic interface. Deep-Sea Research, 38, Suppl. 2, $691-$710.
Causlus J. and R. F. ANDERSON (1991) Immobility of 21°pb in Black Sea sediments. Geochimica et Cosmochi- mica Acta, 55,327-333.
HACISALIHO~LU G., T. I. BALKA§, S. G. TUNCEL, D. H. HERMAN, I. OLMEZ and G. TUNCEL (1991) Trace element composition of the Black Sea aerosol. Deep-Sea Research, 38, Suppl. 2, $1255-$1266.
HONEYMAN B. D. and P. H. SANTSCHI (1991) Coupling adsorption and particle aggregation: laboratory studies of "colloidal pumping" using 59Fe-labeled hematite. Environmental Science and Technology, 25, 1739-1747.
HvH C.-A. and M. P. BACON (1985) Thorium-232 in the eastern Caribbean Sea. Nature, 316,718-721.
H u n C.-A. and T. M. BEASLEY (1987) Profiles of dissolved and particulate thorium isotopes in the water column of coastal Southern California. Earth and Planetary Science Letters, 85, 1-10.
HUH C.-A., W. S. MOORE and D. C. KADKO (1989) Oceanic23ETh: a reconnaissance and implications of global distribution from manganese nodules. Geochimica et Cosmochimica Acta, 53, 1357-1366.
H u n C.-A., J. M. KELLEY and T. C. TAPAS (in preparation) Determination of thorium isotopes in seawater samples by mass spectrometry.
KARL D. M. and G. A. KNAUER (1991) Microbial production and particle flux in the upper 350 m of the Black Sea.
112 C.-A. HuH el al.
LAZAREV K. F., D. S. NIKOLAEV and S. M. GRASHCttENKO (1961) Concentration of thorium isotopes in sea water. Radiokhimiya, III, 623~535.
LEwis B. L. and W. M. LANDING (1991) The biogeochemistry of manganese and iron in the Black Sea. Deep-Sea Research, 38, Suppl. 2. $773-$803.
MARING H. B. and R. A. DUCE (1987) The impact of atmospheric aerosols on trace metal chemistry. Earth and Planetary Science Letters, 84, 381-392.
MOORE R. M. and K. A. HUNTER (1985) Thorium adsorption in the ocean: rcversibility and distribution amongst particle sizes. Geochimica et Cosmochimica Acta, 49, 2253-2257.
MURNANE R. J., J. L. SARMIENTO and M. P. BACON (1990) Thorium isotopes, particle cycling models, and inverse calculations of model rate constants. Journal of Geophysical Research, 95 (C9), 16195-16206.
MURRAY J. W., H. W. JANNASCH, H. HONJO, R. F. ANDERSON, W. S. REEBURGH, Z. TOP, G. E. FRIEDERICH, L. A. CODISPOTI and E. IZDAR (1989) Unexpected changes in the oxic/anoxic interface in the Black Sea. Nature, 338, 411-413.
NOZAK1 Y., Y. HOR1BE and T. TSUSOTA ( 1981 ) The water column distributions of thorium isotopes in the western North Pacific. Earth and Planetary Science Letters, 54,203-216.
NOZAKI Y. and T. NAKANISHI (1985) 231Pa and 23°Th profiles in the open ocean water column. Deep-Sea Research, 32, 1209-1985.
NOZAKI Y. and M. YAMADA (1987) Thorium and protactinium isotope distributions in waters of the Japan Sea. Deep-Sea Research, 34, 1417-1430.
NOZAK~ Y., H.-S. YANG and M. YAMADA (1987) Scavenging of thorium in the ocean. Journal of Geophysical Research, 92 (C1), 772-778.
Ross D. A. and E. T. DEGENS (1974) Recent sediments of the Black Sea. In: The Black Sea--geology, chemistry and biology, E. T. DEGENS and D. A. Ross, editors, American Association of Petroleum Geologists, Tulsa, OK, pp. 183-199.
WEI C.-L. (1990) Studies of marine scavenging by naturally occurring radionuclides. Ph.D. Thesis, University of Washington, Seattle, 199 pp.
WEI C.-L. and J. W. MURRAY (1991 )234Thp238U disequilibria in the Black Sea. Deep-Sea Research, 38, Suppl. 2, $855-$873.
WEt C.-L. and J. W. MURRAY (in press) The behavior of scavenging isotopes in marine anoxic environments: lead-210 and polonium-210 in the water column of the Black Sea. Geochimica et Cosmochimica Acta.