Adsorption and Decomposition of H
2S on InP(100)
Wei-Hsiu Hung*
Synchrotron Radiation Research Center, Science-Based Industrial Park, No.1, R&D Road VI, Hsin-Chu 30077, Taiwan
Hung-Chih Chen and Che-Chen Chang
Department of Chemistry, National Taiwan UniVersity, Taipei 10764, Taiwan Jyh-Tsung Hsieh and Huey-Liang Hwang
Department of Electrical Engineering, National Tsing-Hwa UniVersity, Taiwan ReceiVed: December 4, 1998; In Final Form: February 16, 1999
The adsorption and thermal reaction of H2S on the InP(100) surface is studied by synchrotron radiation (SR)
soft X-ray photoelectron spectroscopy. In addition to molecular adsorption, H2S decomposes to form the
dissociative species of S, HS, and H on the surface at 100 K. The S atom of the sulfide species preferentially bonds to the In atom, and the H atom generated by the H2S dissociation bonds to the P atom. H2S molecules
may physisorb on the surface in the form of an icelike multilayer/cluster at low temperatures, even at low coverages. The irradiation of SR white light can induce an alternative reaction of H2S with the InP surface
to form a thicker sulfur layer than that obtained by thermal deposition. The resulting sulfur layer may provide chemical protection for the InP substrate from further reaction.
Introduction
InP is an important semiconductor material with respect to its potential application for optical and high-speed devices.1
Despite this potential, the InP devices suffer from several deficiencies such as a lack of reproducibility and drain-current drift.2,3Some of these problems are thought to be caused by
interface instability. The sulfidation of the III-V surfaces has been shown to effectively reduce the surface-state densities that cause rapid recombination velocity and Fermi-level pinning.4
Therefore, the passivation of InP via a treatment by sulfur-containing compounds has drawn much attention.
Most of the previous studies have been focused on the surface sulfidation in the aqueous sulfide-based solution (e.g., (NH4)2Sx
and Na2S). However, this sulfidation technique has the inherent
disadvantages associated with wet processing, combined with the odorous smell of the sulfide solution. The sulfide-treated surface is usually mixed with undesired oxides formed in the wet passivation. On the other hand, the sulfidation through dry passivation in a H2S ambient may produce a surface that is much
less susceptible to oxygen uptake than a clean surface.5 Dry
passivation is thus of much interest in recent years. Although in a vacuum the sulfur exposure of InP is far less than that in the liquid phase for effective passivation, studies of the passivation under vacuum conditions can provide insights into the microscopic process with which the surface passivation takes place.
In previous studies, there is a general agreement that the InP surface, treated by an aqueous sulfide solution, has an indium sulfide layer on the surface without P-S bonding remaining.3,6-9
XANES, LEED, and X-ray standing wave studies proposed that
sulfur atoms preferentially form bridge bonds with two In atoms along the [110] direction.10-13 However, recent XPS results
obtained by Chasse et al. showed three sulfur components on the (NH4)2S-treated InP(100) surface.14These components were
interpreted as being due to S bridge-bonded to In on the surface, S in subsurface P sites, and polysulfidic species (Sx). The sulfur
incorporation into the substitutional site was thus proposed to occur in the wet passivation process even at room temperature. In fact, different sulfidation procedures may result in different structural configurations on the surface in the wet passivation.12
It is still not clear how the sulfur coverage and the incorporation into the subsurface sites depend on the details of the sample preparation and annealing procedure. The dry passivation process may provide a more reliable surface with desirable reproducibility because the process is easier to control and has fewer sources of contamination. On the basis of UPS and XPS studies, it was found that H2S molecules can chemisorb and
physisorb preferentially on the In atoms of the InP surface at 140 K.15 In this study, synchrotron radiation soft X-ray
photoelectron spectroscopy (XPS) is used to further elucidate the adsorption and thermal reaction of H2S molecules on the
InP(100) surface.
The sulfur coverage is relatively low on the surface treated by sulfide solution. UV white-light illumination has been used to promote the sulfidation of InP(100), leaving one monolayer of S on the surface bonding to In.4,8In addition, UV
photosul-fidation produces a greater increase in the photoluminescence intensity and in the resistance to degradation than that obtained by conventional wet passivation. The advantage obtained from the dry passivation process has thus prompted the use of H2S
gas activated by a hot filament or in a plasma.16-18 In the
plasma-based processes, the surface is exposed to the ion bombardment. It results in surface damage that exacerbates the * Corresponding author. E-mail: whung@alpha1.srrc.gov.tw. Fax:
+886-3-5783892.
10.1021/jp984629o CCC: $18.00 © 1999 American Chemical Society Published on Web 04/21/1999
Downloaded by NATIONAL TAIWAN UNIV on July 30, 2009
carrier recombination problem. An alternative dry passivation process is the use of photodissociation of H2S on InP at low
temperatures. It may lead to photolytic cleavage of the H-S bond to produce the S-passivated surface with the desorption of hydrogen. In this study, the unmonochromatized synchrotron radiation (i.e., zeroth-order or white light) is used to stimulate photodissociation and initiate alternative reaction pathways of H2S with InP. The enhancement of passivation of the InP surface
by SR irradiation is also discussed.
Experimental Section
Experiments were performed in an ultrahigh vacuum (UHV) chamber equipped with a quadrupole mass spectrometer, a LEED, and a VG CLAM II electron energy analyzer. The XPS measurements were carried out at 6m HSGM beamline of SRRC (Synchrotron Radiation Research Center, Taiwan). The incident angle of photons was 55°from the surface normal. The emitted photoelectrons were collected with the electron analyzer normal to the sample surface. The InP(100) sample (S-doped n-type, ∼3 × 1018/cm3) was cleaned by chemical etching using sulfuric
acid and hydrogen peroxide solution followed by rinsing in deionized water. The InP sample was then mounted on the Ta plate by indium bonding to achieve good contact. The sample can be cooled to 100 K with liquid nitrogen and heated by electron beam bombardment from the back of the Ta plate. The surface temperature was measured using a K-type thermocouple spot-welded close to the sample. The sample was first cleaned by Ar ion bombardment (500 eV) at an incident angle of 45° under UHV and then annealed to∼650 K for 2 min. A 4 × 2 LEED pattern was observed, which is characteristic of an In-rich InP(100) surface. H2S gas (Metheson, 99.5% min) was
introduced onto the InP(100) surface at 100 K by background dosing through a leak valve. The H2S exposure reported in this
work ranges from 0.5 to 8.0 L.
All X-ray photoelectron spectra were taken at a sample temperature of 100 K. The P 2p and S 2p spectra were recorded at 240 eV photon energy, whereas the In 4d spectra were recorded at 180 eV photon energy. The spectra are presented here after the collected data are subtracted by a Shirley background with a third-order polynomial to each side of the peak in all fits. The spectra are numerically fitted with Gaussian broadened Lorentzian spin-orbit doublets. The peak width of each core-level component is mainly determined by the Gaussian fwhm. The spin-orbit splitting value of 2p1/2and 2p3/2is 0.86
eV for P and 1.18 eV for S. The branching ratio of 2p1/2 and
2p3/2components is 0.5 for P and S.
The VUV and soft X-ray white beam used in this study is an unmonochromatized synchrotron radiation (zeroth-order light) via a reflection from a 400 lines/mm gold-coated grating with a grazing incident angle of 3°. The SR white beam has a photon energy distribution of 0-1600 eV. The temperature increase, as monitored by the thermocouple, in the substrate during SR irradiation is negligible.
Results and Discussion
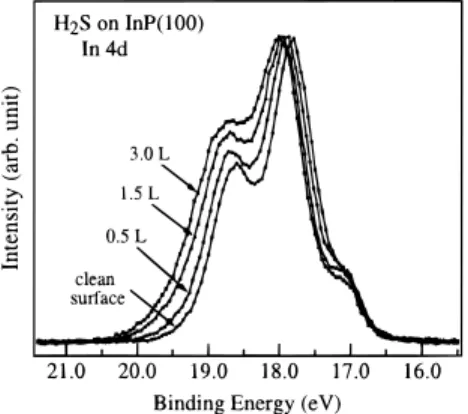
Figures 1 and 2 show the core-level spectra of In 4d and P 2p on a clean InP(100) surface exposed to various amounts of H2S. In this study, the clean InP(100) surface exhibits a 4× 2
LEED pattern with diffusive half-order streaky spots, in agreement with previous results.15,19,20This surface is believed
to be In-rich with the presence of metallic In clusters on the surface. As shown in Figure 1, the intensities of the In 4d core-level spectra are normalized to their maxima. No attempt on curve fitting was made to deconvolute these spectra because of
the complication of various In species possibly present on the surface after it is exposed to H2S. These species include In
clusters, surface In atoms, and their sulfides. A close examina-tion of Figures 1 and 2 reveals that as the H2S exposure is
increased, the In 4d and P 2p peaks become broadened and their relative intensities at the high binding energy side are increased. This indicates that when H2S adsorbs on the InP surface, new
chemical species may be formed. The new surface products have binding energies of In 4d and P 2p that are higher than that of the bulk.
The new species produced on the InP surface upon the adsorption of H2S may be decided by the bonding character of
the surface. On this surface, there is a lone pair of electrons on the P atom and an empty orbital on the In atom. The molecular H2S with its 1b1lone pair may thus preferentially bond with
the surface In atom.21The surface P atom, on the other hand,
may act as an electron donor to H+generated by the dissociation of H2S. The reaction of H2S on InP may thus result in the sulfur
species bonding to the surface In atom and the H atom bonding to the surface P atom, after H2S is dissociatively adsorbed on
the surface. The bonding of the sulfur species on In causes the
Figure 1. Soft X-ray photoelectron spectra of In 4d, collected from
the InP surface with various exposures of H2S at 100 K. The photon
energy used to collect these spectra is 180 eV. The spectra are shown after background subtraction and normalization to their intensity maxima.
Figure 2. Soft X-ray photoelectron spectra of P 2p, collected from
the InP surface with various exposures of H2S at 100 K. The photon
energy used to collect these spectra is 240 eV. The dots represent the collected data after background subtraction, the solid lines are the curve fits to the data, and the various components are shown as dash lines (SSCL: surface-shifted core level).
Downloaded by NATIONAL TAIWAN UNIV on July 30, 2009
intensity of In 4d at the high binding energy side of the core-level spectrum to increase. This proposed reaction process is in agreement with the prediction based on the enthalpy of formation of binary compounds, in which the In-S bond energy is higher than that of In-P and P-S bonds.22In an isochronal
annealing experiment, it was concluded that phosphorus hydride was more stable than indium hydride.20Other studies showed
that hydrogen preferentially etched the surface P atom on the InP surface, and then the etched surface was covered by In clusters.23,24 This indicates that hydrogen may preferentially
adsorb on and react with the surface P atom to form volatile phosphorus hydrides. A P-deficient surface was formed when phosphorus hydrides were desorbed from the surface at elevated temperatures. Results from a UPS measurement also showed that the peak intensity at Ef- 0.7 eV, due to the presence of
dangling bonds on the surface phosphorus atom, decreased with the increase of the H2S exposure on the InP surface.15It may
be noted in Figure 2 that no emission is observed in the P 2p spectra in the binding energy region of 3-6 eV higher than that of the bulk. The emission in this region is an indication of the presence of a P-S bond.6,25The adsorption of H
2S molecules
on the InP surface is similar to that of H2O, in which the O
atom also preferentially bonds with the In atom and the H atom bonds with the P atom.26
As shown in Figure 2, the intensity of the surface-shifted core level (SSCL), due to the presence of the surface P atom with the dangling bond, decreases with the increase of the H2S
exposure. The SSCL component of P 2p is shifted to higher binding energies as H2S is allowed to adsorb on InP. A good
fit of the P 2p spectra can be achieved by including two additional components with the chemical shifts of 0.3 and 0.6 eV relative to the bulk P component. These states may be attributed to phosphorus hydrides PH and PH2. This attribution
is consistent with the result from a study of the adsorption of H2S on InP(110), in which a P 2p chemical shift of 0.6 eV to
a higher binding energy was observed.21
The band-bending-induced shift is observed for S 2p on the H2S-covered InP surface, as found in the P 2p and In 4d spectra.
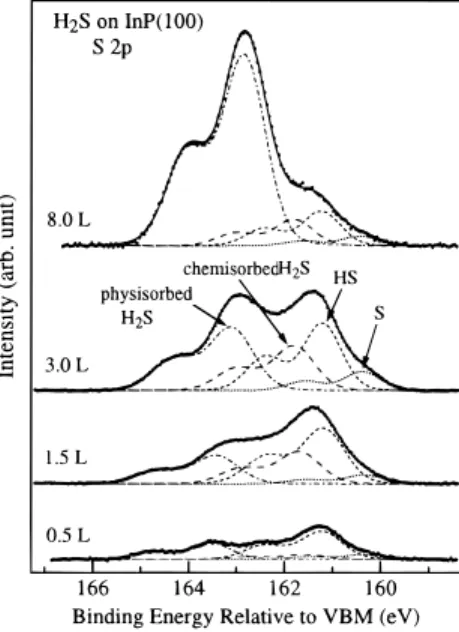
For clarity in comparison, the binding energy of S 2p is referred to as the valence band maximum (VBM) level. Figure 3 shows the S 2p core-level spectra obtained after the clean InP surface is exposed to various amounts of H2S at 100 K. It reveals that,
for all coverages studied, the adsorption of H2S on the InP
surface results in the formation of four S chemical states, with 2p3/2binding energies at 160.3, 161.2, 161.8, and 163.2 eV with
respect to VBM. The two lower binding energy states are attributed to the dissociative species of H2S, i.e., HS (161.2 eV)
and S (160.3 eV). This means that the H2S molecule decomposes
on the InP surface to form HS and S, leaving hydrogen on the surface:
In addition, the two higher binding energy states at 161.8 and 163.2 eV are attributed to H2S molecules, which chemisorb and
physisorb on the surface, respectively. This assignment will be discussed below.
The intensities of these four sulfur components increase with the increase of the H2S exposure. However, the dissociative and
chemisorbed components are saturated on the surface at an H2S
exposure of 3.0 L. This is consistent with the observation that the In 4d and P 2p spectral features are essentially unchanged at H2S exposures of more than 3.0 L. Assuming that the amount
of sulfur species is proportional to its S 2p photoelectron intensity, about 65% of the adsorbed H2S molecules on the first
layer dissociate into HS and S at saturation coverage. The S 2p3/2 component at 163.2 eV, however, increases at higher
exposures. It is thus attributed to the physisorbed H2S molecules
on the InP(100) surface at 100 K. Figure 3 also shows that the physisorption of H2S on the InP surface occurs even at low
exposure. The presence of physisorbed H2S at low coverages
implies indirectly that the adsorption behavior of H2S molecules
may be similar to that of H2O, which is known to adsorb on
the various surfaces in the form of an icelike mutilayer/cluster at low temperatures.27,28Physisorbed H
2S molecules may adsorb
through hydrogen bonding to other chemisorbed sulfur species and form a multilayer/cluster on the surface.
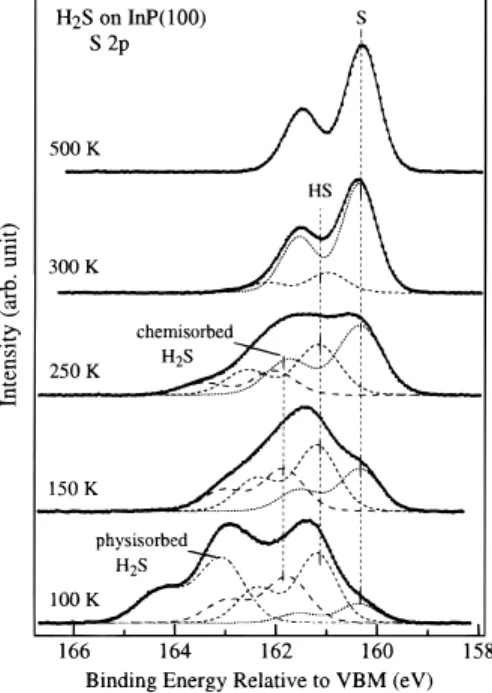
Figures 4-6 show the S 2p, P 2p, and In 4d spectra, respectively, obtained from the InP(100) surface, which is exposed to 3.0 L H2S at 100 K followed by warming to the
indicated temperatures. When the substrate is heated to 150 K, the S 2p3/2component at 163.2 eV is diminished, whereas the
main features in the In 4d and P 2p spectra remain almost unchanged. This observation supports the assignment that the S chemical state at the highest 2p3/2binding energy (163.2 eV)
in the spectra is due to the physisorbed H2S molecules. When
the surface is heated to 250 K, the intensities of S 2p3/2at 161.8
and 161.2 eV due to the presence of chemisorbed H2S and HS
species, respectively, decrease and the intensity at 160.3 eV due to the presence of the S adatom increases. This indicates that the chemisorbed H2S and HS may dissociate further at this
temperature to form the S adatom on the surface. When the surface is heated to 300 K, no chemisorbed H2S exists on the
surface. Further heating the substrate to 500 K causes all chemisorbed HS to either decompose to form an S-covered surface or desorb from the surface as molecular H2S.
Similar to that observed from a sample treated by a wet passivation process, a 1× 1 LEED pattern is observed after the surface is heated to 500 K.8,10,11,29However, in contrast with
the result obtained from a previous study of wet passivation, a change of LEED pattern from (1× 1) to (2 × 1) is not observed when the surface is further annealed to 540 K.29On the basis
of a recent STM study, it was found that the top layer of the S-passivated surface lacks a long-range order, and the (1× 1) H2S(ad)fHS
(ad), S(ad)+ H(ad)
Figure 3. Soft X-ray photoelectron spectra of S 2p, collected from
the InP surface with various exposures of H2S at 100 K. The photon
energy used to collect these spectra is 240 eV. The dots represent the collected data after background subtraction, the solid lines are the curve fits to the data, and the various components are shown in dash lines.
2
Downloaded by NATIONAL TAIWAN UNIV on July 30, 2009
LEED pattern was postulated to originate from the underlayer substrate.30However, if the (1× 1) surface order is imposed,
the bridge site is proposed to provide the most favorable energy for the S absorption, as proposed in the case of GaAs-S.31,32
In the previous XPD studies, the S atoms on the InP(100) by wet passivation were also proposed to bridge-bond to two In atoms of the first layer, which have two dangling bonds in opposite directions.9,10,13
Figure 5 shows that the intensities of phosphorus hydrides at the binding energies of 129.2 and 129.5 eV decrease when the substrate is heated from 100 to 300 K. Further heating the sample to 500 K results in the disappearance of the components of phosphorus hydrides from the surface, and the intensity of the surface P component (SSCL) is less than that observed on the clean surface. This suggests that the P atoms on the surface can be etched in the form of phosphorus hydrides through
desorption during annealing (a).
This result is in agreement with a previous study that showed that the P atom was gradually lost from the InP surface exposed to H2S at 300 K.33The gradual loss of P was thought to be
replaced with S to form the InS compound of one monolayer. It was also demonstrated that hydrogen can preferentially etch phosphorus atoms from the InP surface to form an In-rich surface with the presence of In clusters.23,24 In addition,
phosphorus hydrides (PH and PH2) may decompose at high
temperatures and the resulting H atoms may desorb from the surface in the form of molecular H2or react with neighboring
HS on the In atom to cause a recombinative desorption of H2S
(b).34,35
The chemisorbed sulfur species may desorb at elevated temperatures. As shown in Figure 6, the In 4d intensity on the high binding energy side decreases with an increase of the sample temperature. As discussed earlier, this intensity is assigned as due to the surface In atoms bonding to the sulfur species. It indicates that during the thermal annealing, the chemisorbed H2S may be molecularly desorbed from the surface
and the surface HS and H may recombine and desorb as H2S.
This argument is also supported by the result from the S 2p core-level study. Figure 4 shows that the signals associated with HS and chemisorbed and physisorbed H2S disappear at 500 K.
The S adatoms is the only sulfur species left on the InP surface after annealing. It is preferentially bonded to the In atom, which, as shown in Figure 6, has higher 4d binding energy than that of the clean surface.
The effect of SR irradiation on the reaction of H2S with InP
is then studied. Figures 7-9 show the S 2p, P 2p, and In 4d core-level spectra obtained from the InP surface after the indicated cycles of SR irradiation. A cycle of SR irradiation
Figure 4. Soft X-ray photoelectron spectra of S 2p, collected from
the InP surface exposed to 3.0 L of H2S at 100 K followed by annealing
the sample to the indicated temperatures.
Figure 5. Soft X-ray photoelectron spectra of P 2p from the InP(100)
surface exposed to 3.0 L of H2S at 100 K followed by annealing the
sample to the indicated temperatures.
Figure 6. Soft X-ray photoelectron spectra of In 4d from the
InP-(100) surface exposed to 3.0 L of H2S at 100 K and followed by
annealing the sample to indicated temperatures. The spectra are shown after background subtraction and normalization to their intensity maxima.
PH(ad), PH2(ad)98(a) PHx(g)(x e 3) 300 K
98(b) H 2(g)or H2S(g) H2S(ad)fH 2S(g) HS(ad)+ H(ad)fH 2S(g)
Downloaded by NATIONAL TAIWAN UNIV on July 30, 2009
includes the exposure of InP(100) to H2S at 100 K followed by
irradiation of SR white light before the sample is annealed to 500 K. With a slit width of 100µm and the electron current in the ring at 150 mA, the photon flux of the white beam is estimated to be about 2× 1014photons/s. The duration of the
sample exposure to SR white light is 60 s in each cycle. The irradiation area on the sample is 30-40 mm2, and the sample
current due to the emission of photoelectrons induced by SR irradiation is about 3µA.
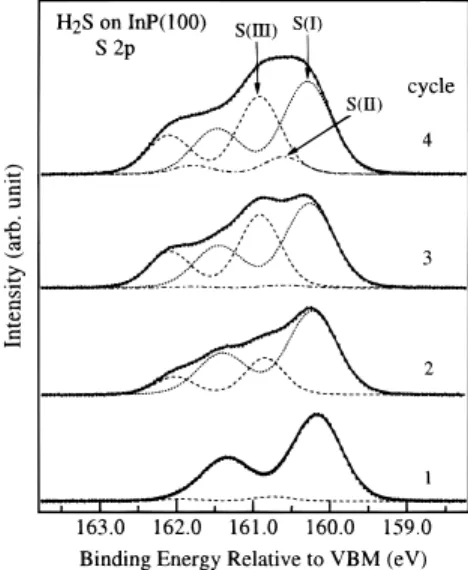
Figure 7 shows that there are three sulfur chemical states, with 2p3/2 binding energies at 160.3, 160.6, and 160.9 eV,
formed in the photoassisted sulfur deposition after cycles of SR irradiation. Similar to that obtained earlier by thermal deposition, the component observed at 160.3 eV is attributed to surface S adatoms. The component observed at 160.6 eV is attributed to surface polysulfide, which may be formed by the direct photodissociation of H2S (H2S(ad)fSx(ad)). The
compo-nent at 160.9 eV may be attributed to an S chemical state formed because of the stimulation of adsorbed H2S molecules by SR
white light to further react with the surface and subsurface P or In atoms. The SR white light may induce the photolytic cleavage of the H2S bond to generate sulfur-containing radicals. These
radicals exhibit high reactivity toward the InP surface, and a reaction to replace P in the InP subsurface may take place. This reaction, under SR irradiation, is supported by the results from other studies. A previous UPS study showed that P atoms can be replaced by S in the near-surface region during the H2S
plasma exposure at elevated temperatures (>200°C).36On the
basis of the XPD measurement, it was also suggested that the exchange of P with S occurs at the annealing temperature (350
°C), leading to the formation of the InP1-xSxpseudomorphic
layer.22The exchange between P and S atoms is
thermodynami-cally favored. Since they are similar in size, S atoms may incorporate into the InP substrate. The formation of subsurface S atoms may be prompted by the SR photon, which provides the energy necessary for the surface sulfur species to overcome the activation barrier of diffusion to move into the bulk. In fact, it was observed that when annealed to 700 K, the surface S atoms on the sulfur-passivated InP(100) surface can partially exchange with the bulk P atoms to form the P-S and In-S bonds.37
The S 2p3/2component at 160.6 eV begins to appear at higher
sulfur coverages. Figure 7 shows that the intensities of the S
chemical states at 160.3 and 160.9, both due to the reaction of H2S with the InP substrate, are nearly saturated after three
deposition cycles. They may thus constitute a passivation layer for further reaction of H2S with the InP substrate. Only the direct
photodissociation of the adsorbed H2S to form polysulfides
(160.6 eV) may take place on the surface at large numbers of deposition cycles.
The promotion of sulfidation on the InP surface by photoir-radiation is also shown in the In and P spectra after the InP surface is treated by cycles of H2S adsorption and SR irradiation.
Figure 8 shows that the intensity of In 4d on the high binding energy side is higher than that obtained by thermal deposition. This implies that a thick In sulfide layer is formed because of the incorporation of S into the subsurface under SR irradiation. As shown in Figure 9, a new chemical component for P 2p3/2
appears at 129.1 eV (higher than that of the bulk by 0.3 eV) and may be attributed to the P atoms bonding to S atoms, although its P chemical state is unknown. After three cycles, further H2S deposition and photoirradiation do not significantly
change the In 4d and P 2p spectra. The photoassisted sulfidation of the InP(100) surface causes a surface band bending by about 0.6 eV. In addition, an increase of the H2S exposure in each
cycle does not lead to an increase of the thickness of the
Figure 7. Soft X-ray photoelectron spectra of S 2p from the InP surface
exposed to 3.0 L of H2S at 100 K and followed by SR irradiation for
60 s and then annealing the sample to 500 K. S(I): surface S. S(II): polysulfide Sx. S(III): subsurface S.
Figure 8. Soft X-ray photoelectron spectra of In 4d from the InP
surface exposed to 3.0 L of H2S at 100 K and followed by SR irradiation
for 60 s and then annealing the sample to 500 K. The spectra are shown after background subtraction and normalization to their intensity maxima.
Figure 9. Soft X-ray photoelectron spectra of P 2p from the InP surface
exposed to 3.0 L of H2S at 100 K and followed by SR irradiation for
60 s and then annealing the sample to 500 K.
2
Downloaded by NATIONAL TAIWAN UNIV on July 30, 2009
passivation layer, as revealed by the near-constant signal intensities at 160.3 and 160.9 eV in the S 2p spectra, although there is an increase in the intensity for surface polysulfides (Sx).
A multilayer of Sx may thus be produced on the top of the
passivation layer of In and P sulfides by direct photodissociation. The Sxmultilayer and the InP1-xSxpassivation layer may thus
provide chemical protection for the InP surface.
Conclusion
H2S chemically reacts with the InP(100) surface at 100 K
via both molecular and dissociative adsorption, while phys-isorption of H2S may take place even at low exposures. The
reaction proceeds with the S atom preferentially bonding to the In atom of the surface, which results in the formation of three different S chemical states on the surface. On the other hand, the H atom, after H2S dissociates on the surface, is
thermody-namically more favorable for bonding with the surface P atom. Various phosphorus hydrides may then be produced. The desorption of these hydrides from the surface at elevated temperatures results in a P-deficient surface, and the resulting surface may be covered by the InS compound and In clusters. In addition, the sulfur species may also desorb from the annealed surface, through either a molecular desorption of the chemi-sorbed H2S or a recombinative desorption of the surface HS
and H.
Under SR irradiation, new chemical states may be produced because of the stimulation of the adsorbed species by SR white light to form more reactive species, possibly radicals. The SR white light also provides the energy necessary to induce the replacement of P in InP by S and to incorporate S into the InP subsurface. With cycles of H2S exposure and SR irradiation, a
passivation layer, possibly in the form of InP1-xSx, can be
produced. SR irradiation with H2S exposure may thus produce
an InP surface that is chemically protected from further reaction. Only direct photodissociation of the adsorbed H2S can take place
on this passivated surface to form polysulfides.
Acknowledgment. This work was supported by SRRC and National Science Council under Grant No. NSC88-2113-M-213-003. We also express our appreciation to the beamline group at SRRC for their instrumental support.
References and Notes
(1) Wieder, H. H. J. Vac. Sci. Technol. 1981, 18, 827.
(2) Klopfenstein, P.; Bastide, G.; Rouzeyre, M.; Gendry, M.; Durand, J. J. Appl. Phys. 1988, 63, 150.
(3) Maeda, F.; Watanabe, Y.; Oshima, M. Appl. Phys. Lett. 1993, 62, 297.
(4) Ashby, C. I. H.; Zavadil, K. R.; Howard, A. J.; Hammons, B. E.
Appl. Phys. Lett. 1994, 64, 2388.
(5) Kawanishi, H.; Sugimoto, Y.; Akita, A. J. Appl. Phys. 1991, 70, 805.
(6) Wilmsen, C. W.; Geib, K. M.; Shin, J.; Iyer, R.; Lile, L.; Pouch, J. J. J. Vac. Sci. Technol. B 1989, 7, 851.
(7) Lau, W. M.; Jin, S.; Wu, X. W.; Ingrey, S. J. Vac. Sci. Technol. A
1991, 9, 994.
(8) Tao, Y.; Yelon, A.; Sacher, E.; Lu, Z. H.; Graham, M. J. Appl.
Phys. Lett. 1992, 60, 2669.
(9) Fukuda, Y.; Suzuki, Y.; Sanada, N. J. Appl. Phys. 1994, 76, 3059. (10) Lu, Z. H.; Graham, M. J.; Feng, X. H.; Yang, B. X. Appl. Phys.
Lett. 1992, 60, 2773.
(11) Warren, O. L.; Anderson, G. W.; Hanf, M. C.; Griffiths, K.; Norton, P. R. Phys. ReV. B 1995, 52, 2959.
(12) Mitchell, C. E. J.; Hill, I. G.; McLean, A. B.; Lu, Z. H. Prog. Surf.
Sci. 1995, 50, 325.
(13) Sugiyama, M.; Maeyama, S.; Oshima, M. J. Vac. Sci. Technol. A
1996, 14, 1812.
(14) Chasse, T.; Chasse, A.; Peisert, H.; Streubel, P. Appl. Phys. A 1997,
65, 543.
(15) Shiomura, M.; Moller, P. J.; Nerlov, J.; Christensen, S. V.; Guo, Q.; Sanada, N.; Fukuda, Y. Appl. Surf. Sci. 1997, 121/122, 237.
(16) Tiedje, T.; Colbow, K. M.; Rogers, D.; Fu, Z.; Eberhardt, W. J.
Vac. Sci. Technol. B 1989, 7, 837.
(17) Yablonovitch, E.; Gmitter, T. J.; Bagley, B. G. Appl. Phys. Lett.
1990, 57, 2241.
(18) Herman, J. S.; Terry, F. L., Jr. Appl. Phys. Lett. 1992, 60, 716. (19) Weiss, W.; Hornsyein, R.; Schmeisser, D.; Gopel, W. J. Vac. Sci.
Technol. B 1990, 8, 715.
(20) Allinger, Th.; Persch, V.; Schaefer, J. A. Proceedings of the SPIE,
Physical Concept of Materials for NoVel Optoelectronic DeVice Appications I; SPIE: Bellingham, WA, 1990; Vol. 1361, p 935.
(21) Dudzik, E.; Muller, C.; McGovern, I. T.; Lloyd, D. R.; Patchett, A.; Zahn, D. R. T.; Johal, T.; McGrath, R. Surf. Sci. 1995, 344, 1.
(22) Gallet, D.; Hollinger, G. Appl. Phys. Lett. 1993, 62, 982. (23) Steitz, F. G.; Allingger, Th.; Polyakov, V.; Woll, J.; Goldmann, A.; Erfurth, W.; Lapeyre, G. J.; Schaefer, J. A. Appl. Surf. Sci. 1996, 104/
105, 169.
(24) Woll, J.; Allingger, Th.; Polyakov, V.; Schaefer, J. A.; Goldmann, A.; Erfurth, W. Surf. Sci. 1994, 315, 293.
(25) Kwok, R. W. M.; Lau, W. M. J. Vac. Sci. Technol. 1992, 10, 2515. (26) Unplublished results that H2O adsorbs dissociatively and
molecu-larly on the InP(100) surface at 100 K.
(27) Hung, W. H.; Schwartz, J.; Bernasek, S. L. Surf. Sci. 1991, 248, 332.
(28) Thiel, P. A.; Madey, T. E. Surf. Sci. Rep. 1987, 3, 211. (29) Ogiwara, H.; Fan, J. F.; Nannichi, Y.; Sugahara, H.; Oshima, M.
Jpn. J. Appl. Phys., Part 2 1991, 30, L322.
(30) Qin, X. R.; Lu, Z. H.; Shapter, J. G.; Coatsworth, L. L.; Griffiths, K.; Norton, P. R. J. Vac. Sci. Technol. A 1998, 16, 163.
(31) Jin, J.-M.; Dharma-wardana, M. W. C.; Lockwood, D. J.; Aers, G. C.; Lu, Z. H.; Lewis, L. J. Phys. ReV. Lett. 1995, 75, 878.
(32) Omori, S.; Ishii, H.; Nihei, Y. Surf. Sci. 1997, 381, 165. (33) Barbouth, N.; Berthier, Y.; Oudar, J.; Moison, J. M.; Bensousson, M. J. Electrochem. Soc. 1989, 133, 1663.
(34) Nooney, M. G.; Liberman, V.; Matrtin, M. J. Vac. Sci. Technol. A
1995, 13, 1837.
(35) Conrad, S.; Mullins, D. R.; Xin, Q.-S.; Zhu, X. Y. Surf. Sci. 1997,
382, 79.
(36) Nelson, A. J.; Frigo, S. P.; Rosenberg, R. J. Vac. Sci. Technol. A
1993, 11, 1022.
(37) Tian, Z.; Dharma-wardana, M. W. C.; Lu, Z. H.; Cao, R.; Lewis, L. J. Phys. ReV. B 1997, 55, 5376.
Downloaded by NATIONAL TAIWAN UNIV on July 30, 2009