Localization, Transport, and Uptake of
D
-Aspartate
in the Rat Adrenal and Pituitary Glands
Jen-Ai Lee,*
,† Zhiqun Long,*
,‡ Noriyuki Nimura,‡ Takeshi Iwatsubo,*
Kazuhiro Imai,*
,1and Hiroshi Homma*
,‡
*Graduate School of Pharmaceutical Sciences, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan; †Department of Pharmaceutical Analysis, School of Pharmacy, Taipei Medical University, 250 Wu-Hsing Street,
Taipei 110, Taiwan; and ‡School of Pharmaceutical Sciences, Kitasato University, 5-9-1 Shirokane, Minato-ku, Tokyo 108-8641, Japan
Received August 10, 2000, and in revised form October 2, 2000; published online December 19, 2000
Large amounts ofD-aspartate (D-Asp) are present in
the rat adrenal and pituitary glands.D-Asp is thought
to be synthesized in the mammalian body and also accumulates in various tissues following intraperito-neal or intravenous administration. This report exam-ines the origins ofD-Asp in the adrenal and pituitary glands. We administeredD-Asp to male rats
intraperi-toneally and immunolocalized this exogenousD-Asp in
adrenal and pituitary tissue, using an anti-D-Asp
anti-serum which was previously developed in our labora-tory. D-Asp levels in the rat adrenal gland have been
shown to undergo a transient increase at 3 weeks of age and to decrease rapidly thereafter. We found that in the adrenal gland, exogenous D-Asp administered
intraperitoneally was incorporated into the same re-gion of the adrenal cortex in which endogenousD-Asp
was present. By Northern and Western blot analysis and immunohistochemistry of glutamate (Glu) porter, we also found that expression of the Glu trans-porter (GLAST), which has an affinity forD-Asp, tran-siently increased at 3 weeks of age and that localiza-tion patterns of the Glu transporter within the tissue were almost coincident with those of endogenous D -Asp. These observations suggest thatD-Asp in the
ad-renal cortex of 3-week-old male rats is primarily ac-quired by uptake from the vascular system. We have previously shown thatD-Asp is specifically localized in prolactin (PRL)-containing cells in the anterior lobe of the adult rat pituitary gland. Here we report that in the pituitary gland, exogenousD-Asp accumulated in endothelial cells, but not in PRL-containing cells. Northern and Western blot analysis and
immunohisto-chemistry of Glu transporter revealed that develop-mental changes in the Glu transporter (GLAST) ex-pression did not correlate with tissue levels ofD-Asp
and that the Glu transporter was not expressed in PRL-containing cells. These observations suggest that, in contrast to the adrenal gland, most of theD-Asp in
the pituitary gland of adult male rats originates inside the gland itself. © 2001 Academic Press
Key Words: D-aspartate; D-amino acids;
gluta-mate transporter; adrenal gland; pituitary gland; immunohistochemistry.
Recent investigations have demonstrated the
in-volvement of
D-aspartate (
D-Asp)
2in a variety of
bio-logical activities in the mammalian body.
D-Asp
sup-presses melatonin secretion in cultured rat
pinealo-cytes (1) and isolated rat pineal gland (2), presumably
via activation of the glutamate (Glu) receptor (mGlu
R3) (3–5), and increases testosterone production in
iso-lated rat Leydig cells by stimulating the expression of
steroidogenic acute regulatory protein (StAR) (6, 7).
This stimulation of StAR expression is apparently Glu
receptor-independent and requires
D-Asp to be taken
up by the cells (6, 7). Moreover, it has been
demon-strated that
D-Asp is actually synthesized in
mamma-lian cells (8). These lines of evidence suggest that
D-Asp
1To whom correspondence should be addressed. Fax:
⫹81-3-5841-4885. E-mail: kimai@mal.f.u-tokyo.ac.jp.
2
Abbreviations used: D-Asp, D-aspartate; Glu, glutamate; PRL, prolactin; IR, immunoreactivity; ZF, zona fasciculata; ZR, zona re-ticularis; ZG, zona glomerulosa; Star, steroidogenic acute regulatory protein; Ser, serine; SD, Sprague–Dawley; PBS, phosphate-buffered saline; NA, nonadrenaline; A, adrenaline; Mops, 3-[N-morpho-lino]propanesulfonic acid; G3PDH, glyceraldehy3-phosphate de-hydrogenase; ECL, enhanced chemiluminescence.
242 0003-9861/01 $35.00
Copyright © 2001 by Academic Press All rights of reproduction in any form reserved.
plays an important role as a messenger in mammalian
endocrine and neuroendocrine tissues (9).
D
-Asp is synthesized in rat pheochromocytoma
(PC12) cells (8), and a serine (Ser)-specific racemase
which was recently cloned from rat brain is assumed to
be involved in the synthesis of
D-Ser (10, 11), another
D-amino acid which is found in abundance in
mam-mals. It therefore appears likely that
D-Asp is also
synthesized in the mammalian body, although the
pre-cise synthetic route has yet to be elucidated. On the
other hand, when
D-Asp is administered to rats either
intraperitoneally (12) or intravenously (13), it
accumu-lates via the vascular system in various tissues,
includ-ing testis and the pineal, pituitary, and adrenal glands.
It therefore remains to be determined whether
D-Asp is
synthesized in almost every organ, or is produced by a
restricted range of tissues and subsequently
accumu-lated in other tissues via the vascular system.
The
D-Asp content of a number of tissues has been
shown to change markedly during development.
D-Asp
levels are transiently increased during the
develop-ment of human (14) and rat brain (15), rat retina (16)
and adrenal gland (17), and chicken embryonic brain
(16). In previous reports (18 –22), we used an anti-
D-Asp antibody to study developmental changes in
D-Asp
localization within various rat tissues. As development
proceeds,
D-Asp appears and becomes localized in
spe-cific types of cells within these tissues, and both the
tissue localization and the intracellular distribution of
D
-Asp change during development. However, the origin
of the
D-Asp observed in these tissues remains
un-known. In addition, it is unclear how the levels and
localization of
D-Asp within the tissues are regulated.
It has been reported that the localization pattern of
D
-Asp is the inverse of that of
D-Asp oxidase in several
rat tissues:
D-Asp content is low in cells in which
D-Asp
oxidase activity is high and vice versa (9, 23–25).
How-ever,
D-Asp is not detectable in some tissues which lack
D-Asp oxidase activity, so tissue levels of
D-Asp are
apparently determined not only by degradative
D-Asp
oxidase activity but also by other factors, possibly
in-cluding regulation of endogenous synthesis and/or
up-take from the vascular system.
Rat adrenal and pituitary glands apparently contain
very low levels of
D-Asp oxidase activity (9).
D-Asp
levels in the adrenal gland transiently increase at 3
weeks of age, decrease thereafter, and remain at an
adult level after 8 weeks of age (17). In contrast, the
D
-Asp level in the pituitary gland continues to increase
gradually from 1 to 8 weeks of age (15, 17). In
3-week-old rats,
D-Asp is predominantly localized in most
re-gions of the adrenal cortex, whereas in 8-week-old rats
it is localized primarily in the adrenal medulla (19). In
the pituitary gland,
D-Asp is localized in prolactin
(PRL)-containing cells or some other very closely
re-lated type of cells in the anterior lobe of the gland (22).
In this report,
D-Asp which was administered
intra-peritoneally to 3-week-old and 8-week-old rats was
in-corporated into various tissues via the vascular
sys-tem. We subsequently localized this exogenous
D-Asp
within the adrenal and pituitary glands by
immuno-histochemistry and compared the localization patterns
of exogenous and endogenous
D-Asp.
It seems likely that
D-Asp is incorporated into cells
via the
L-Glu transporter, which has an affinity for
D-Asp in addition to
L-Glu and
L-Asp (26 –28). We
there-fore also analyzed the localization of the Glu
trans-porter and developmental changes in its expression in
the adrenal and pituitary glands. We then compared
these results with the localization of endogenous
D-Asp
and developmental changes in the
D-Asp content of
these tissues in order to examine the origins of
D-Asp in
the rat adrenal and pituitary glands.
MATERIALS AND METHODSChemicals. D,L-Asp was purchased from Sigma Chemical Co. (St.
Louis, MO). Sodium pentobarbital was from Abbott Laboratories (IL). Glutaraldehyde and paraformaldehyde used in immunohisto-chemical studies were obtained from EM Science (PA) and sodium cacodylate was from TAAB Laboratories (Reading, UK). FITC-con-jugated goat anti-rabbit IgG antibody was obtained from Organon Teknika (Durham, NC, UK), and mouse monoclonal anti-rat PRL antibody (IgG1 fraction) were obtained from QED Bioscienc Inc. (USA). Texas red-conjugated goat anti-mouse IgG (H⫹ L) antibody, were obtained from Jackson ImmunoResearch (West Grove, PA). The Dako PAP Kit was purchased from DAKO (Denmark). [␣-32
P]dCTP (110 TBq/mmol) was a product of Amersham Pharmacia Biotech Inc. (Piscataway, NJ). Oligonucleotide primers were prepared by Sawady Technology Inc. (Tokyo, Japan). Restriction enzymes and Taq DNA polymerase were purchased from TAKARA (Kyoto, Japan). Other chemicals were of the highest grade available.
Animals. Male Sprague–Dawley (SD) rats (specific pathogen free) purchased from Charles River Japan Inc. (Kanagawa, Japan) were kept in a constant 12-h light/12-h dark cycle (lights on at 7:00
AM) with free access to food and water.
Determination of theD-Asp content of rat adrenal and pituitary
gland by HPLC. D-Asp in saline (1.0mol/g body weight, approx. 0.5 ml, neutralized) or saline as control was administered to 8-week-old male SD rats by intraperitoneal (ip) injection (12). Fifteen min or 5 h after ip injection, the rats were anesthetized with diethyl ether and sacrificed by exsanguination from the abdominal aorta. Asp levels in the adrenal and pituitary glands were determined by HPLC with a Pirkle-type chiral stationary phase and fluorometric detection as described previously (29).D-Asp was administered to two rats and
Asp levels in the glands were represented as average⫾ half range. Immunohistochemistry. Rats were anesthetized by ip injection of sodium pentobarbital solution (50 mg/kg body weight). After 1–2 min of transcardial perfusion with Ringer’s solution, animals were fixed by perfusion with fixative solution (2.5% glutaraldehyde, 2% para-formaldehyde, 0.1 M cacodylate, pH 7.4) at a rate of 10 ml/min for 20 min. The tissues were removed and postfixed in the fixative solution for 2 h at 4°C and cryoprotected in 10, 15, and 20% sucrose in PBS before being frozen in embedding medium (OCT Compound, Miles Laboratories, Naperville, IL). Cryostat tissue sections (10m thick-ness) were mounted on poly(L)-lysine-treated slides (Matsunami Glass Ind., Japan) and air-dried. Sections were pretreated for 20 min with 0.5% NaBH4in PBS to inactivate residual glutaraldehyde and
30 min and incubated overnight at 25°C with anti-D-Asp antibody prepared in this laboratory (at a dilution of between 1:30 and 1:1000 in PBS containing 10% calf serum and 0.1% sodium azide) (18, 20). Immunoreactivity was visualized by the peroxidase–antiperoxidase method using the PAP complex (DAKO). The sections were counter-stained with hematoxylin. Preabsorption of the antibody with a liquid phase conjugate of glutaraldehyde and D-Asp (1 mM) abol-ished D-Asp immunoreactivity in all the cases described in this study.
For staining with anti-Glu transporter antisera, animals were fixed by perfusion with 4% paraformaldehyde solution in PBS, pH 7.4. Then the immunostaining of GLAST was carried out as that of
D-Asp described above. The sections were probed with 1:50 anti-GLAST antiserum (CovalAb, France), and visualized by the peroxi-dase–antiperoxidase method or fluorescent secondary antibodies (FITC-conjugated anti-rabbit IgG antibodies). We also determined the localization of GLAST with the anti-GLAST antiserum which was kindly donated by Prof. M. Watanabe (Univ. Hokkaido, School of Medicine). There are no differences between the localization deter-mined by these two anti-GLAST antisera.
Localization of exogenousD-Asp, which was administered ip and accumulated abundantly in the tissues, was determined using a much higher dilution of the anti-D-Asp antiserum than was used to detect endogenous D-Asp. The antiserum dilution used to detect exogenous D-Asp did not detect any endogenous D-Asp in control animals injected with saline.
The noradrenaline (NA)- and adrenaline (A)-storing cells of the adrenal medulla were distinguished as described in a previous report (19). After identification of NA cells by fixation of tissue sections in 50% Karnovisky solution, the sections were further probed with anti-D-Asp antiserum.
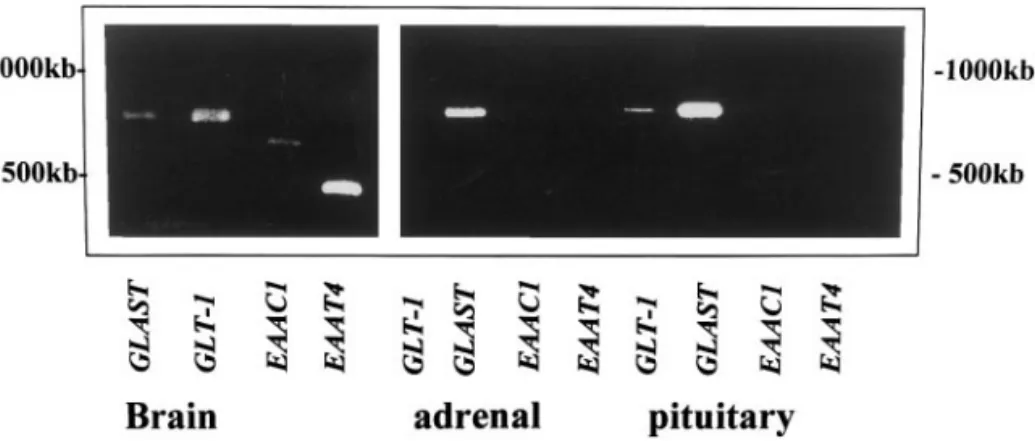
Reverse transcription–polymerase chain reaction (RT-PCR) analy-sis. Total RNA was extracted from isolated rat glands with Isogen reagent (Wako Chemical Ind., Osaka, Japan), a monophasic solution of phenol and guanidine isothiocyanate. One microgram of total RNA was transcribed into cDNA followed by PCR amplification using a TaKaRa RNA PCR kit (AMV) Ver. 2.1 (TaKaRa, Kyoto, Japan). The primers used were based on published sequences: GLT-1: sense: GGGAAGAAGAACGACGAGGTG (bases 466 – 486), antisense, AC-CTCCATCCAGGATGACCCCATTC (bases 1242–1266) (30); GLAST: sense: TCGTGCAGGTGACTGCCGCAG (bases 482–502), antisense: CTGTCCAAAATTCAGGTCAAAG (bases 1254 –1275) (31); EAAC1: sense: GACGCCATGTTGGATCTGATCAGGAA (bases 418 – 443), anti-sense: GCTTCATAGAGCGCAGTGCCGTCCAT (bases 1096 –1121) (32); EAAT4: sense: CGAGTGGTAACAAGGACGAT (bases 757–776), antisense: GTGTGTTACCCCTCATCTAC (bases 1211–1230) (33). The PCR products amplified by these primer pairs were 801, 794, 704, and 474 base pairs long, respectively.
Northern blot analysis. The rat Glu transporter cDNA fragments described above were cloned into the pT7Blue-2 T-vector (Novagen, Madison, WI). The resulting inserts were extracted and purified using a QIAEX II kit (QIAGEN Inc., Valencia, CA), and labeled with 1.85 MBq of [␣-32
P]dCTP (109
dpm/mg) using a DNA labeling kit (Ready To Go, Amersham Pharmacia Biotech Inc., NJ), according to the respective manufacturers’ instructions. Labeled probes were sep-arated from free nucleotides with G50 spin columns (ProbeQuant, Amersham Pharmacia Biotech). mRNA was extracted from adrenal or pituitary glands with the QuickPrep Micro mRNA purification kit (Amersham Pharmacia Biotech). Approximately 5g of mRNA was separated by electrophoresis on 1.0% agarose/18% formaldehyde/ Mops gels and transferred onto Hybond N⫹nylon membranes (Am-ersham Pharmacia Biotech). After prehybridization for 1 h at 65°C, filters were hybridized with the labeled probes (Glu transporter cDNA or rat glyceraldehyde-3-phosphate dehydrogenase (G3PDH) cDNA) at 1–2⫻ 106dpm/cm2for 2 h at 65°C in hybridization buffer
(Rapid hyb buffer, Amersham Pharmacia Biotech). Following hybrid-ization, membranes were rinsed with 2⫻ SSPE, 0.1% SDS, washed
in 0.1⫻ SSPE, 0.1% SDS, and then exposed to Kodak X-OMAT AR films at⫺80°C for 1–2 days. Intensities of autoradiographic bands were estimated by densitometric scanning.
Western blot analysis. A total membrane fraction of rat pituitary or adrenal gland was prepared as follows with a slight modification of the method of Yamada et al. (34). Male SD rats (1, 3, 8, and 13 weeks of age) were anesthetized with diethyl ether and sacrificed by exsanguination from the abdominal aorta. The pituitary and adrenal glands were dissected and homogenized separately in 2 ml SME buffer (20 mM Mops–Tris (pH 7.0) containing 0.3 M sucrose, 5 mM EDTA, 5g/ml pepstatin A, 0.1 mM phenylmethylsulfonyl fluoride, and 4g/ml aprotinin). The homogenate was centrifuged at 900g for 10 min, and the supernatant was centrifuged at 100,000g for 30 min. The resulting pellet was suspended in the same buffer and the protein content was determined using a BioRad protein assay with bovine serum albumin as standard. Proteins (50g per lane) were separated on 12% SDS/polyacrylamide gels and electrophoretically transferred to nitrocellulose membranes. The nitrocellulose mem-branes were blocked in 10 mM phosphate-buffered saline (PBS) containing 5% skim milk and 0.2% Tween 20 at 4°C overnight and then probed with antibodies (GLT-1 antibody 1g/ml, GLAST anti-body 0.5 g/ml) for 1 h at room temperature. These anti-rat Glu transporter antisera were kindly donated by Prof. M. Watanabe (Univ. Hokkaido, School of Medicine). The membranes were then washed, incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (at a dilution of 1:5000) for 1 h, and immunoreactive bands detected using enhanced chemiluminescence (ECL, Amer-sham Pharmacia Biotech Inc.).
RESULTS
In Vivo Uptake of
D-Asp into Rat Adrenal and
Pituitary Glands
When
D-Asp is intraperitoneally administered to
rats, it accumulates via the vascular system in various
tissues, including the testis and pituitary gland (12).
However, the precise localization of the exogenous
D-Asp within these tissues has yet to be determined.
Intravenous administration of radiolabeled
D-Asp also
resulted in the accumulation of radioactivity in these
tissues as well as the pineal and adrenal glands (13).
However, the radioactivity detected in tissues does not
necessarily represent intact
D-Asp alone, but may also
include metabolites, and the results of this study are
therefore difficult to interpret. Thus the localization of
the exogenous
D-Asp within the tissues cannot be
de-termined precisely even by autoradiography of the
tis-sue sections. In the present study, we administered
D
-Asp to rats by ip injection and localized this
exoge-nous intact
D-Asp in the tissues using anti-
D-Asp
anti-serum at a dilution which did not detect endogenous
D
-Asp in control animals. The localization patterns of
exogenous and endogenous
D-Asp were then compared,
in order to determine whether the exogenous
D-Asp
was incorporated into the same tissue regions in which
endogenous
D-Asp was found.
The
D-Asp levels in the adrenal and pituitary glands
were, respectively, 21.9
⫾ 18.7 and 13.8 ⫾ 11.8 nmol/
gland 15 min after
D-Asp injection and 36.4
⫾ 1.7 and
endogenous
D-Asp levels in the adrenal and pituitary
glands were 0.56
⫾ 0.076 and 0.49 ⫾ 0.092 nmol/gland,
respectively. The localization of exogenous
D-Asp was
examined in the adrenal gland of 3- and 8-week-old
rats, and in the pituitary gland of 8-week-old rats. The
endogenous
D-Asp concentration in rat adrenal glands
shows a transient increase at 3 weeks of age, and
markedly decreases thereafter, remaining at an adult
level after 8 weeks of age (17). In contrast, the level in
the pituitary gland continues to increase gradually
from 1 to 8 weeks of age (17).
When
D-Asp was administered intraperitoneally to
3-week-old rats, immunoreactivity (IR) to exogenous
D
-Asp in the adrenal gland appeared primarily in the
cytoplasm of cells in the zona fasciculata (ZF) and zona
reticularis (ZR) of the cortex, but was almost
undetect-able in the zona glomerulosa (ZG) (Fig. 1A). In the
adrenal medulla, exogenous
D-Asp IR was detected in
scattered, irregularly shaped groups of cells (data not
shown). This localization of exogenous
D-Asp is almost
identical to the localization of endogenous
D-Asp in the
adrenal cortex (Fig. 1A, inset) and in the adrenal
me-dulla (19).
At 8 weeks of age, IR to exogenous
D-Asp was intense
in large clusters of cells in the adrenal medulla, but
was less intense in the ZF and ZR of the cortex (Fig.
FIG. 1. In vivo uptake ofD-Asp into rat adrenal and pituitary glands.D-Asp was administered intraperitoneally to male rats (3 or 8 weeksof age) and this exogenousD-Asp was localized in the adrenal and pituitary glands 5 h after injection using anti-D-Asp antiserum. (A) Adrenal cortex of 3-week-old rat. IR to exogenousD-Asp is prominent in the ZF and ZR (ZR is not shown in this figure) of the cortex, but almost completely absent from the ZG. Anti-D-Asp antiserum was diluted 1:1500, at which concentration endogenousD-Asp was not stained. Bar, 60m. (Inset) EndogenousD-Asp in the adrenal cortex of 3-week-old rat. Localization pattern of exogenousD-Asp is almost identical to that
of endogenousD-Asp. Anti-D-Asp antiserum was diluted 1:100. Bar, 120m. (B) Adrenal medulla of 8-week-old rat. ExogenousD-Asp is evident in the adrenal medulla, not in the ZF and ZR of the cortex. Anti-D-Asp antiserum was diluted 1:300. Bar, 120m. (Inset) Endogenous
D-Asp in the adrenal medulla of 8-week-old rat. Localization of exogenousD-Asp is similar to that of endogenousD-Asp. Anti-D-Asp antiserum was diluted 1:50. Bar, 60m. (C) (a) Immunolocalization of exogenousD-Asp in the adrenal medulla of 8-week-old rat. (b) Fluorescent
photomicrograph of the section shown in (a) Noradrenaline (NA)-storing cells of the medulla are stained and adrenaline (A)-storing cells are not. The cells that are stained in b (NA cells, white arrows) are negative for exogenousD-Asp (arrows in a). (D) Anterior lobe of the pituitary gland of 8-week-old rat. ExogenousD-Asp is primarily evident in the endothelial cells of the blood vessels. Anti-D-Asp antiserum was diluted 1:1000. Bar, 60m. (Inset) EndogenousD-Asp in the anterior lobe of the pituitary gland of 8-week-old rat. Anti-D-Asp antiserum was diluted
1:300. Bar, 60m. EndogenousD-Asp is present in PRL-containing cells or some other very closely related type of cells (22). The cells positive for exogenousD-Asp differ in both morphology and distribution from those positive for endogenousD-Asp.
1B). This staining pattern is similar to that of
endog-enous
D-Asp (Fig. 1B, inset) (19). The adrenal medulla
comprises NA-storing and A-storing cells, and IR to
exogenous
D-Asp was evident in A cells (Fig. 1C), while
in some sections the IR was also associated with NA
cells (data not shown). Endogenous
D-Asp is specifically
localized to the cytoplasm of A cells in the adrenal
medulla (9, 19). In the rat adrenal medulla,
D-Asp is
presumably acquired by local synthesis in addition to
uptake, since it is produced in a clonal strain of rat
pheochromocytoma (PC12) cells, which is derived from
the rat adrenal medulla (8). The selective localization
of
D-Asp to A cells is assumed to be due to
D-Asp oxidase
activity, since
D-Asp oxidase activity is localized in the
medulla and selectively associated with NA cells (9).
In the anterior lobe of the pituitary gland of
8-week-old rats, IR to exogenous
D-Asp was primarily evident
in the endothelial cells of the blood vessels (Fig. 1D),
whereas endogenous
D-Asp was detected in
PRL-con-taining cells or some other very closely related cell type
(Fig. 1D, inset) (22). In contrast to the adrenal gland,
exogenous
D-Asp in the pituitary gland was mostly
incorporated into different cells from those which
con-tained endogenous
D-Asp. Moreover,
D-Asp oxidase is
exclusively localized in the intermediate lobe and not
detected in the anterior lobe (9).
Developmental Changes in Glu Transporter
Expression in Rat Adrenal and Pituitary Glands
D-Asp is likely to be taken up into cells by the
L-Glu
transporter, which has an affinity for
D-Asp in addition
to
L-Glu and
L-Asp (26 –28). We therefore examined
developmental changes in the expression of the Glu
transporter in the adrenal and pituitary glands and
compared the results with developmental changes in
D
-Asp concentrations in the same tissues. RT-PCR
demonstrated that GLAST is the predominant isoform
in the rat adrenal and pituitary glands, while GLT-1 is
also detected in the pituitary at a very low level (Fig.
2). In the adrenal gland, steady-state levels of GLAST
mRNA were transiently increased at 3 weeks of age
(Fig. 3A), consistent with the transient increase in
adrenal
D-Asp content at the same age. In contrast, in
the pituitary, GLAST mRNA levels remained almost
constant from 1 to 13 weeks of age (Fig. 3B). This result
is in marked contrast to developmental changes in
pituitary
D-Asp concentration, which continues to
in-crease gradually from 1 to 8 weeks of age (17). GLT-1
mRNA was not detected in the pituitary by Northern
blot, presumably due to its low level of expression (data
not shown), although it was detected at a very low level
by RT-PCR.
Western blotting was also used to detect GLAST in
the rat adrenal and pituitary glands (Figs. 3C and 3D).
The level of GLAST protein in the adrenal gland
in-creased significantly at 3 weeks of age, and several
forms of different molecular mass (glycosylated
mono-mer, dimono-mer, and glycosylated dimer) (35) were readily
discernible at that age (Fig. 3C). This result is
compa-rable with the transient increase of adrenal
D-Asp
con-tent at 3 weeks of age. In the pituitary, GLAST protein
levels were almost constant during development (Fig.
3D); this is consistent with the mRNA levels described
above and contrasts with the gradual increase in
D-Asp
concentration in the pituitary.
Localization of Glu Transporter in the Rat Adrenal
and Pituitary Glands
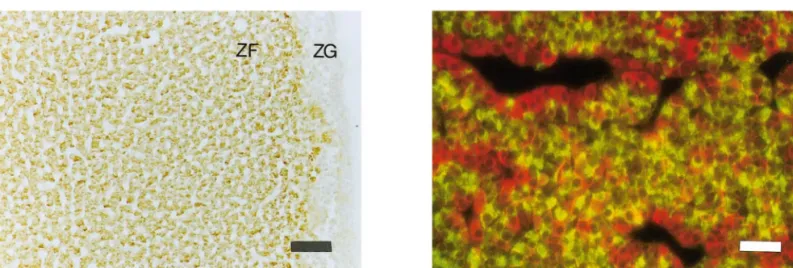
Figure 4A shows the spatial distribution of GLAST
protein in the rat adrenal gland. In 3-week-old rats,
GLAST was localized in the ZF and ZR of the adrenal
cortex, but was almost undetectable in the ZG (Fig.
4A). This result is consistent with the observation
de-scribed above, that exogenous and endogenous
D-Asp
are both localized primarily in the ZF and ZR at 3
weeks of age. In the adrenal medulla of 8-week-old
FIG. 2. RT-PCR of Glu transporter subtypes in the rat adrenal and pituitary glands. Total RNA (1g) extracted from the brain and the adrenal and pituitary glands of 8-week-old rats was transcribed into cDNA, followed by PCR amplification with primer pairs specific for GLT-1, GLAST, EAAC1, and EAAT4. The amplified products were resolved on 1.2% agarose gels and stained with ethidium bromide.rats, IR to GLAST was localized not only in A cells but
also in NA cells (data not shown). Endogenous
D-Asp is
specific to A cells in the adrenal medulla, and this
selective localization is presumably due to
D-Asp
oxi-dase activity, since the oxioxi-dase activity is selectively
associated with NA cells (9).
Double staining with anti-GLAST and anti-PRL
an-tibodies was used to examine whether GLAST is
asso-ciated with PRL-containing cells, which also contain
D
-Asp, in the pituitary gland of 8-week-old rats (Fig.
4B). PRL-positive cells (red) were only rarely
superim-posed (yellow) on GLAST-positive cells (green),
sug-gesting that PRL-containing cells do not contain the
Glu transporter.
DISCUSSION
In a previous report we demonstrated that, in the
course of primary culture of parenchymal cells from the
rat pineal gland,
D-Asp was not synthesized but was
efficiently taken up by the cells (1). Given that the
pineal gland contains a large amount of
D-Asp in vivo,
this result suggests that
D-Asp in the pineal gland is
derived from other tissue(s) and acquired by cells of the
pineal gland from the vascular system, although it does
not rule out loss of
D-Asp synthesis activity during the
primary culture of the cells. A recent report indicated
that a high concentration of
D-Asp is found in rat
tes-ticular venous blood plasma, suggesting that
D-Asp is
produced in the testis and secreted into the venous
blood (36). These lines of evidence indicate that
D-Asp
may be produced in certain specific mammalian
tis-sue(s), and subsequently accumulated in other tissues
by uptake from the vascular system. In the present
study, we examined the origins of
D-Asp in the rat
adrenal and pituitary glands.
D
-Asp concentration in the rat adrenal gland
in-creases transiently at 3 weeks of age (17). It had been
presumed that this result was brought about by
tran-sient increase of
D-Asp synthesis at 3 weeks of age in
the gland. Here we present evidence that suggests that
this endogenous
D-Asp in the adrenal cortex of
3-week-old rats is primarily acquired by uptake from the
vas-FIG. 3. Developmental changes of Glu transporter expression in the rat adrenal and pituitary glands. (A, B) Northern blot analysis of GLAST mRNA in the rat adrenal (A) and pituitary (B) glands at various ages (1, 3, 8, and 13 weeks). Analysis of mRNA from the cerebellum, cerebrum, and kidney was also carried out to provide positive and negative controls, and the blot was reprobed with G3PDH cDNA as a loading control. (C, D) Western blot analysis of GLAST in the rat adrenal (C) and pituitary (D) glands at various ages (1, 3, 8, and 13 weeks).cular system. However, the primary tissue(s) in which
D
-Asp synthesis takes place is not clear at present.
D-Asp in the adrenal medulla of 8-week-old rats is
probably metabolized by
D-Asp oxidase following
up-take from the vascular system or local synthesis, as
described under Results.
In contrast, our observations suggest that most of
the
D-Asp in the pituitary gland originates inside the
tissue, although they do not rule out the possibility
that other type(s) of Glu transporter are present in the
pituitary gland, which has an affinity for
D-Asp but has
not been cloned yet. At present, four different types of
Glu transporter (EAAT1(GLAST), EAAT2(GLT-1),
EAAT3(EAAC1), and EAAT4) have been
character-ized, while another retina-specific isoform with a low
affinity for
D-Asp (EAAT5) is also recognized (37).
Recently we found that
D-Asp is actually synthesized
in a PRL-secreting clonal strain of rat pituitary tumor
cells (GH3) and that it promotes thyrotropin-releasing
hormone (TRH)-induced PRL secretion from the cells
(38). Thus,
D-Asp in the rat anterior pituitary gland
appears to act as an autacoid in an autocrine or
para-crine fashion, whereas
D-Asp in the rat adrenal gland
might be a messenger that acts in an endocrine
fash-ion, thereby stimulating steroid production (6, 7).
D
-Asp is also found in other rat tissues, including the
cerebrum, cerebellum, and retina of newborn rats and
the spleen and thymus of adult rats, and is present in
a variety of mouse and human tissues (39, 40). The
localization of
D-Asp within these tissues has not been
examined. Furthermore, the origin of the endogenous
D
-Asp in these tissues is totally unknown.
Identifica-tion and cloning of the enzyme(s) responsible for the
synthesis of
D-Asp would greatly advance the current
understanding of the role and regulation of
D-Asp in
the mammalian body.
ACKNOWLEDGMENTSThe authors express their sincere appreciation to Prof. M. Wa-tanabe (Univ. Hokkaido, School of Medicine) for his generous gifts of anti-rat Glu transporter antisera. This work was supported in part by a Grant-in Aid for Scientific Research 11672163 (to H.H.) from the Ministry of Education, Science, Sports and Culture of Japan.
REFERENCES
1. Takigawa, Y., Homma, H., Lee, J.-A., Fukushima, T., Santa, T., Iwatsubo, T., and Imai, K. (1998) Biochem. Biophys. Res. Com-mun. 248, 641– 647.
2. Ishio, S., Yamada, H., Hayashi, M., Yatsushiro, S., Noumi, T., Yamaguchi, A., and Moriyama, Y. (1998) Neurosci. Lett. 249, 143–146.
3. Govitrapong, P., and Ebadi, M. (1988) Neurochem. Int. 13, 223– 230.
4. Sato, K., Kiyama, H., Shimada, S., and Tohyama, M. (1993) Neuroendocrinology 58, 77–79.
5. Yamada, H., Yatsushiro, S., Ishio, S., Hayashi, M., Nishi, T., Yamamoto, A., Futai, M., Yamaguchi, A., and Moriyama, Y. (1998) J. Neurosci. 18, 2056 –2062.
6. Nagata, Y., Homma, H., Lee, J.-A., and Imai, K. (1999) FEBS Lett. 444, 160 –164.
7. Nagata, Y., Homma, H., Matsumoto, M., and Imai, K. (1999) FEBS Lett. 454, 317–320.
8. Long, Z., Homma, H., Lee, J.-A., Fukushima, T., Santa, T., Iwa-tsubo, T., Yamada, R., and Imai, K. (1998) FEBS Lett. 434, 231–235.
9. Schell, M. J., Cooper, O. B., and Snyder, S. H. (1997) Proc. Natl. Acad. Sci. USA 94, 2013–2018.
FIG. 4. Localization of Glu transporter in the rat adrenal and pituitary glands. (A) Immunolocalization of GLAST in the adrenal cortex of a 3-week-old rat. GLAST reactivity is predominantly present in the ZF and ZR (ZR is not shown in this figure), and weak in the ZG. Bar, 120 m. This GLAST localization pattern is almost identical to those of both endogenous and exogenousD-Asp. (B) Double staining of GLAST and PRL in the anterior lobe of the pituitary gland of an 8-week-old rat. The lobe was probed with anti-PRL antiserum (red) and anti-GLAST antiserum (green). PRL-positive cells are only rarely superimposed (yellow) on GLAST-positive cells, suggesting that PRL-containing cells do not contain the Glu transporter. Bar, 60m.
10. Wolosker, H., Sheth, K. N., Takahashi, M., Mothet, J.-P., Brady J., R. O., Ferris, C. D., and Snyder, S. H. (1999) Proc. Natl. Acad. Sci. USA 96, 721–725.
11. Wolosker, H., Blackshaw, S., and Snyder, S. H. (1999) Proc. Natl. Acad. Sci. USA 96, 13409 –13414.
12. D’Aniello, A., Di Cosmo, A., Di Cristo, C., Annunziato, L., Petru-celli, L., and Fisher, G. (1996) Life Sci. 59, 97–104.
13. Imai, K., Fukushima, T., Santa, T., Homma, H., Suguhara, J., Kodama, H., and Yoshikawa, M. (1997) Proc. Jn Acad. 73B, 48 –52.
14. Hashimoto, A., Kumashiro, S., Nishikawa, T., Oka, T., Taka-hashi, K., Mito, T., Takashima, S., Doi, N., Mizutani, Y., Yamazaki, T., Kaneko, T., and Ootomo, E. (1993) J. Neurochem.
61, 348 –351.
15. Dunlop, D., S., Neidle, A., McHale, D., Dunlop D., M., and Lajtha, A. (1986) Biochem. Biophys. Res. Commun. 141, 27–32. 16. Neidle, A., and Dunlop, D. S. (1990) Life Sci. 46, 1517–1522. 17. Hashimoto, A., Oka, T., and Nishikawa, T. (1995) Eur. J.
Neu-rosci. 7, 1657–1663.
18. Lee, J., Homma, H., Sakai, K., Fukushima, T., Santa, T., Tashiro, K., Iwatsubo, T., Yoshikawa, M., and Imai, K. (1997) Biochem. Biophys. Res. Commun. 231, 505–508.
19. Sakai, K., Homma, H., Lee, J.-A., Fukushima, T., Santa, T., Tashiro, K., Iwatsubo, T., and Imai, K. (1997) Biochem. Biophys. Res. Commun. 235, 433– 436.
20. Sakai, K., Homma, H., Lee, J.-A., Fukushima, T., Santa, T., Tashiro, K., Iwatsubo, T., and Imai, K. (1998) Arch. Biochem. Biophys. 351, 96 –105.
21. Sakai, K., Homma, H., Lee, J.-A., Fukushima, T., Santa, T., Tashiro, K., Iwatsubo, T., and Imai, K. (1998) Brain Res. 808, 65–71.
22. Lee, J., Homma, H., Tashiro, K., Iwatsubo, T., and Imai, K. (1999) Brain Res. 838, 193–199.
23. D’Aniello, A., D’Onofrio, G., Pischetola, M., D’Aniello, G., Vetere, A., Petrucelli, L., and Fisher, G. H. (1993) J. Biol. Chem. 268, 26941–26949.
24. Nagasaki, H. (1994) Int. J. Biochem. 26, 415– 423.
25. Kera, Y., Aoyama, H., Matsumura, H., Hasegawa, A., Nagasaki, H., and Yamada, R.-H. (1995) Biochim. Biophys. Acta 1243, 282–286.
26. Balcar, V. J., and Johnston, G. A. R. (1972) J. Neurochem. 19, 2657–2666.
27. Christensen, H., Greene, A. A., Kakuda, D. K., and MacLeod, C. L. (1994) J. Exp. Biol. 196, 297–305.
28. Cooper, B., Chebib, M., Shen, J., King, N. J. C., Darvey, I. G., Kuchel, P. W., Rothstein, J. D., and Balcar, V. J. (1998) Arch. Biochem. Biophys. 353, 356 –364.
29. Hamase, K., Homma, H., Takigawa, Y., Fukushima, T., Santa, T., and Imai, K. (1997) Biochim. Biophys. Acta 1334, 214 –222. 30. Pines, G., Danbolt, N. C., Bjørås, M., Zhang, Y., Bendahan, A.,
Eide, L., Koepsell, H., Storm-Mathisen, J., Seeberg, E., and Kanner, B. I. (1992) Nature 360, 464 – 467.
31. Storck, T., Schulte, S., Hofmann, K., and Stoffel, W. (1992) Proc. Natl. Acad. Sci. USA 89, 10955–10959.
32. Kanai, Y., and Hediger, M. (1992) Nature 360, 467– 471. 33. Fairman, W. A., Vandenberg, R. J., Arriza, J. L., Kavanaugh,
M. P., and Amara, S. G. (1995) Nature 375, 599 – 603.
34. Yamada, H., Yatsushiro, S., Yamamoto, A., Hayashi, M., Nishi, T., Futai, M., Yamaguchi, A., and Moriyama, Y. (1997) J. Neu-rochem. 69, 1491–1498.
35. Furuta, A., Rothstein, J. D., and Martin, L. J. (1997) J. Neurosci.
17, 8363– 8375.
36. D’Aniello, A., Di Fiore, M. M., D’Aniello, G., Colin, F. E., Lewis, G., and Setchell, B. P. (1998) FEBS Lett. 436, 23–27.
37. Arriza, J. L., Eliasof, S., Kavanaugh, M. P., and Amara, S. G. (1997) Proc. Natl. Acad. Sci. USA 94, 4155– 4160.
38. Long, Z., Lee, J.-A., Okamoto, T., Nimura, N., Imai, K., and Homma, H. (2000) Biochem. Biophys. Res. Commun., 276, 1143– 1147, doi:10.1006/bbrc.2000.3573.
39. Imai, K., Fukushima, T., Santa, T., Homma, H., Hamase, K., Sakai, K., and Kato, M. (1996) Biomed. Chromatogr. 10, 303– 312.