Block Copolyetheresters. Part 3: Preparation of
Block Copolyetheresters by a Terephthalic Acid
Process in the Presence of Salts
SHINN-JEN CHANG, FENG-CHIH CHANG andHONG-BING TSAI* Institute of Applied Chemistry National Chiao
Tung University
Hsinchu, Taiwan, Republic of China*
Union Chemical LaboratoriesIndustrial
Technology Research lnstituteHsinchu, Taiwan, Republic of China
The block copolyetheresters with hard segments of poly(buty1ene terephthalate) and soft segments of poly(tetramethy1ene ether) were prepared by a terephthalic acid (TPA) process in the presence of some salts. The preparations of a block copolyetherester under various conditions were first studied in a 1 L stainless steel reactor to find the best method. Then, the preparations of four block copolyetheresters were run in a pilot plant comprising a 200 L polyesterifcation reactor and a 200 L polymerization reactor under the suitable condition. The presence of some salts reduced the formation of tetrahydrofuran (THF), and also reduced the total reaction time in the pilot plant. The thermal properties and various mechanical properties of the block copolyetheresters prepared by the pilot plant were investigated to evaluate the feasibility of this method.
INTRODUCTION lar to the case of preparation of poldbutylene tereph-
he concept of block copolymer can be used to
T
design thermoplastic elastomers. Typical exam- ples are styrene-diene-styrene triblock copolymers (1-41, block copolyurethanes ( 1 , 2,51,
block copolyetheramides (1, 2, 61, and block copolyether- esters (1, 2, 7-10). In our laboratories, the designs of various block copolymers a s thermoplastic elas- tomers have become our major aims, and some block copolymers, such as block copolyetheramides ( 1 1). block copolyesters (121, and thermotropic block copolyetheresters (13) have been developed. It is a n alternative approach intended to improve the prepa- ration of the well-known commercial block copolymers.The block copolyetheresters with hard segments of poldbutylene terephthalate) and soft segments of poly(tetramethy1ene ether) have been commercialized as thermoplastic elastomers for a period of time (1, 2,
10). They are produced by melt polycondensation of dimethyl terephthalate (DMT), 1.4-butanediol, and a poly(tetramethy1ene ether)glycol (PTMEG), namely a DMT process. However, the preparation of these block copolyetheresters by melt polycondensation of terephthalic acid (TPA), 1,4-butanediol, and a
PT-
MEG, namely a TPA process, has been given little attention by the industry. The situation may be simi-
thalate) (PBT) by a TPA process in which the forma- tion of tetrahydrofuran (THF) by cyclization is a se- vere problem. We have used some salts to reduce the formation of THF in the preparation of PBT by a TPA process, and some good results were found (14). In this study, we use some salts to improve the prepara- tion of the block copolyetheresters by a TPA process, and a better condition was set u p in a 200 L pilot plant to evaluate this modified TPA process. The ther- mal and mechanical properties of these block copolyetheresters were also investigated.
EXPERIMENTAL
Poly(tetramethy1ene ether) glycol (PTMEG 1000) with a molecular weight of 1000 (Terathane 1000) and 1,4-butanediol were obtained from DuPont, terephthalic acid was supplied by China America Petrochemical Co. Ltd. (Taiwan), and isophthalic acid was supplied by Sisas (Italy). Irganox 1010 (an an- tioxidant), Tinuvin 770 (a U V stabilizer), and Tinuvin 370 (a U V stabilizer) were obtained from Ciba-Geigy. Tetrabutyl orthotitanate (TBT) and other chemicals were all Merck reagent grade. All the chemicals were used as received.
The block copolyetherester was prepared by melt polycondensation of terephthalic acid, 1,4-butanediol,
Block Copolyetheresters. Part 3
-
and ITMEG1000, similar to a process for PBT de-scribed previously (14). The reactions of 166
g
(1 .Omoll of terephthalic acid, 162
g
(1.8 mol) of 1,4- butanediol, 123g
(0.123 moll of PTMEGlOOO in the presence of catalysts and various salts in a 1 L stain- less steel reactor were carried out to evaluate the effect of the salts. The content of THF of the distillate (containing water and THF) a t the esterification stage and the conversion of esterification were determined as described previously (14). After the evaluation in the 1 L reactor, a better condition was chosen to scale up the process in a stainless steel pilot plant, which is composed of a 200 L esterification reactor and a 200 L polymerization reactor.The products of the block copolyetheresters pro- duced by the pilot plant were evaluated. The intrinsic viscosity of the products in phenol/syn-tetrachloro- ethane (60/40 wt/wt) was determined by a n Ubbelohde viscometer at 30°C. The thermal proper- ties from 50 to 250°C were measured by a DuPont DSC 910 a t a heating rate of 10"C/min under nitro- gen. Their 'H NMR spectra were determined by a Bruker AM-400 NMR.
The products were injection molded into test speci- mens by a n injection molding machine (Fu Chen Shine KT- 150, Taiwan) under suitable conditions de- pending on the composition. The test specimens were used for the determination of tensile properties, flex- ural modulus, and hardness. The tensile properties were determined according to ASTM D-638, the Shore
D hardness was measured according ASTM D-2240, and the flexural modulus was determined according to ASTM D-790.
RESULTS
AND DISCUSSIONIn the 1 L reactor, the reactions in the presence of various amounts of salts were carried out to evaluate the effect of the salts. Considering the reduction of the content of THF in the distillate, and the time required to reach a conversion of esterification at about 90%, we found that in the presence of some salts the reactions gave a lower content of THF and a shorter time of esterifkation. In the presence of 0.332
g
(0.2 wt% based on terephthalic acid) of TBT a s the catalyst system, 0.019 g (0.0115 wt% based on terephthalic acid) of sodium phosphate, and 0.0083g
(0.005 wt% based on terephthalic acid) of potassium
c E 2 -60
p
a v 0 - 4 0p
z
.- L L .- 20-.
L - 5 - & c-
-
$
x
- > - 0terephthalate as the salt system, the reaction gave a better result. Thus, this condition was chosen to scale up the TPA process.
Four block copolyetheresters were prepared in the pilot plant under a condition based on the above evaluation. The charged compositions are shown in
Table I. Three additional stabilizers were also used to
improve the aging resistance.
To check the significance of the effect of the salts, a blank reaction with the same charged composition a s that of TPEE3 but without salt was also carried out in the pilot plant. Figure I shows the process of the blank reaction and that of TPEE3. In the 200 L esteri- fication reactor, the temperature was held a t 220°C and the reactants were charged. The reaction temper- ature first decreased a s a result of the charge of the
-
25c ? F a E t- 2oc U-
c 5 > E F v u 0 I- \ \ - \ \ \ \- 1 \ \ I - - + I ' I \ I 1 I 1 II
2 4 6 0 Time ( h )Fig. I . Process curves of the preparation of "PEE3 in the presence of the salt system (solid lines) and the blank reac
tion (dash lines).
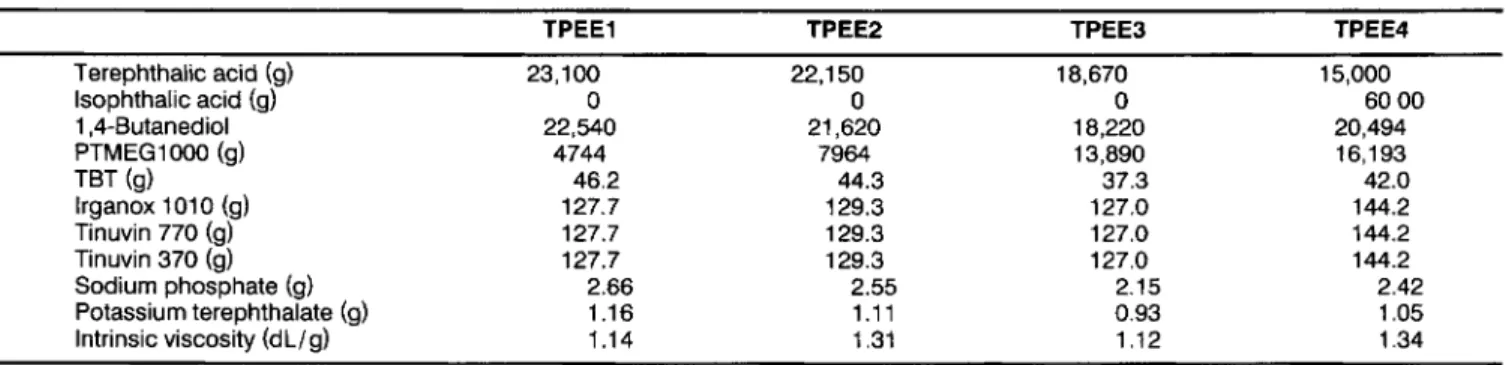
Table 1. The Charged Composition and Intrinsic Viscosity of the Block Copolyetheresters.
TPEEl TPEEP TPEEJ TPEE4
Terephthalic acid (9) 23,100 22.1 50 18,670 15,000 lsophthalic acid (9) 0 0 0 60 00 1,4-Butanediol 22,540 21,620 18,220 20,494 PTMEG1000 (9) 4744 7964 13,890 16,193 TBT (9) 46.2 44.3 37.3 42.0 lrganox 101 0 (9) 127.7 129.3 127.0 144.2 Tinuvin 770 (g) 127.7 129.3 127.0 144.2 Tinuvin 370 (g) 127.7 129.3 127.0 144.2 Sodium phosphate (9) 2.66 2.55 2.15 2.42 Potassium terephthalate (9) 1.16 1.11 0.93 1.05 Intrinsic viscosity (dL/g) 1.14 1.31 1.12 1.34
reactants and then increased gradually to 220°C. As the esterification reaction proceeded, the by-products including water and THF were distilled out. The blank reaction required 3 h to complete the esterification stage. However, the esterification stage for the reac- tion of TPEE3 required only 2 h. For the blank reac- tion, the THF content of the distillate was 25 wt% and the conversion of esterification was 9 1 %. For the reaction of TPEE3, the THR content and the conver- sion of esterification were 15 wt% and 95% respectively.
After the esterification stage was finished, the reac- tion mixture was transferred to the 200 L polymeriza- tion reactor, of which the temperature was held at 260°C and the stirring speed was held a t 60 rpm for a period of time and then decreased gradually to 20 rpm
(FQ.
1 ) . The reaction temperature increased gradually to 260°C. Low vacuum (10 torr) was first applied to evacuate the excess 1,4-butanediol. After most excess 1,4-butanediol was distilled out, high vacuum was applied. The degree of vacuum increased as the reaction time increased. Then, the torque of the stirrer increased slowly, indicating the increase of the molecular weight. At the time near the completion of the polymerization stage, the torque increased sig- nificantly. Finally, when the torque reached a given value (5.5 mV), the reaction was stopped by discharg- ing the product. The reaction time of the polymeriza- tion stage was about 6 h for the blank reaction and 3.5 h for the reaction of TPEE3. The intrinsic viscosity of product of the blank reaction and that of TPEE3 were found to be 1.18 and 1.12 dL/g respectively.For comparison, some data are listed in Table 2.
The advantage of the preparation method in the pres- ence of the salt system is obvious. First, the reduc- tion of the formation of THF was significant in the presence of the salt system. Second, in the presence of the salt system, the reaction time required to com- plete the whole process was within 6 h and consider- ably shorter than that in the absence of any salt
Table 2. Comparison of the Effect of the Salt System on the Preparation of TPEE3 in the Pilot Plant.
Salt System No Yes
THF content in the distillate (??) 25 15 Conversion of esterification (%) 91 95 Time of esterification stage (h) 3 2 Time of polymerization stage (h) 6 3.5
(about 9 h). When the time for the whole process for a pilot plant or manufacturing plant is within 6 h, it
has an economical advantage in that the same work- ers can run the plant within the normal working time.
The reason for the reduction of the whole process time in the presence of the salt system was twofold. First, the esterification rate was enhanced in the presence of some salts (14) and the reaction time of the esterification stage could be reduced. Second, the conversion of esterification reached a higher value in the presence of the salt system, and this could re- duce the reaction time of the polymerization stage. In the presence of the salt system, only about 2 h were needed to finish the esterification stage, and the con- version of esterification could be a s high as 95%. However, in the absence of the salt system, about 3 h were needed to finish the esterification stage, and the conversion of esterification was about 90%. It would be not economical to reach a conversion of over 95% since it might require about 5 h based on our experi- ences. When the conversion of esterification was higher, it was easier to finish the polymerization stage. Thus, the time required to finish the polymerization stage for the reaction in the presence of the salt system (about 3.5 h) was shorter than that of the blank reaction (about 6 h).
The intrinsic viscosity of the block copolyether- esters was controlled to be close to 1.2 dL/g by s t o p ping the reaction when the torque of the stirrer reached about 5.5 mV. The intrinsic viscosity values of the block copolyetheresters were all within 1.1 to
1.4 dL/g, as shown in Table 1 .
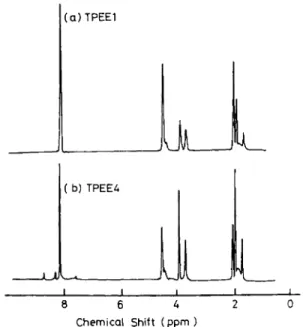
The compositions of these four block copolyether- esters were analyzed by 'H NMR Typical 'H NMR spectra (TPEE1 and TPEE4) are shown in
FQ.
2. The assignments of the resonance peaks are shown inTable 3. Based on the charged compositions, the ra-
tios of H 1 : H2 : H 3 : H4 for the four TPEEs are calcu- lated and listed in Table 4. The ratios of integrated intensity of H 1 : H2 : H3 : H4 of these four TPEEs mea- sured by 'H NMR are also listed in Table 4. Each determined ratio was close to the calculated value. In the preparation of TPEE4, isophthalic acid (IPA) was used additionally. The analysis by 'H NMR showed that the molar ratio of the terephthalate unit to the isophthalate unit for TPEE4 was 1.0 : 0.4, which was the same as the charged molar ratio.
The DSC heating curves from 50 to 250°C of the Table 3. The Assignments of Resonance Peaks.
Protons Chemical Shift (ppm)
H I of terephthalate unit H I of isophthalate unit H2 H3 H4 (- CO -Ar - CO
-
0-
CH 2CH,CH,CH2 - 0 -) 8.1 ~3.7~8.3, 7.6 4.5 3.9, 3.7 2.05, 1.95, 1.7 1 2 4 4 2 ~-CO-Ar-CO-O-CH2CH,CH2CH2-~O-CH2CH,CHz~,z-O-CHzCH2CHzCH~-O-) 1 2 4 4 3 3 4 4 3 3 4 4 2 192 ~Block Copolyetheresters. Part 3 four block copolyetheresters are shown in Flg. 3. An
endotherm corresponding to the melting of the hard polyester segments was observed for the block copolyetheresters. Their melting point (T,) and heat of fusion are listed in Table 5. Their thermal proper- ties were significantly affected by .the composition. There was no isophthalate unit in TPEE 1, TPEE2, or TPEE3. For these three block copolyetheresters, as the content of the polyether segment increased, TPEE1
<
TPEE2<
TPEE3, the sequence length of the polfibutylene terephthalate) hard segment decreased (10). and thus the melting point ( T , ) decreased (Table5). The heat of fusion also decreased as the content of the polyether segment increased as a result of the decreasing order of both the content and the se-
quence length of the polfibutylene terephthalate) segment. TPEE4 showed a considerably lower T,. The charged molar ratio of diacids (TPA and IPA) to
PT-
I I 1 I I
8 6 4 2 0
Chemical Shift ( ppm )
Fig. 2. ' H NMR spectra of TPEEl and TPEE4.
Table 4. The Ratios of H1 : H2: H3 : H4 Calculated, Based on Charged Compositions and Determined by 'H NMR.
Calculated Ratio Determined Ratio
TPEE1 1 .O : 1.04 : 0.48 : 1.54 TPEE2 1.0:1.0:0.78:1.78 1.0:1.05:0.71 :1.79 TPEE3 1 .O : 1.03 : 1.55 : 2.69 TPEE4 1 .O : 1.05 : 1.76 : 2.80 1 .O: 1 .O : 0.44 : 1.44 1 .O : 1 .O : 1.60 : 2.60 1 .O : 1 .O : 1.66 : 2.66 TPEEl 1 I I I 0 50 100 150 200 Temperature ('C)
Flg. 3. DSC heating curves of the block copolyetheresters.
0
MEG was 1.0: 0.128 for TPEE4, and that of TPA to PTMEG was 1.0: 0.123 for TPEE3. Thus the content of the polyether segment of TPEE4 was close to that of TPEE3. However, TPEE4 showed a considerably lower T, (153°C) than that of TPEE3 (202°C). It was due to the copolymerization effect in the polyester hard segment. For TPEE4, the presence of the isoph- thalate unit rendered a random structure in the polyester segment and affected the crystallization. Thus, TPEE4 showed a considerably lower
T,
and also a lower heat of fusion when compared to TPEE3. The tensile stress-strain curves and the corre- sponding tensile properties of the injection molded specimens are shown in Fig. 4 and Table 5 respec- tively. The measured flexural modulus and Shore D hardness are also listed in Table 5. As the content of polyether segment increased, the tensile yield strength, tensile modulus, flexural modulus, and Shore D hardness decreased as expected. This trend could correlate with the decreasing crystallinity as indicated by the heat of fusion (Table 5).Table 5. The Thermal Properties and Mechanical Properties of the Block Copolyetheresters.
Properties TPEEl TPEE2 TPEE3 TPEE4
T, PC) 21 9 21 1 202 153
Heat of fusion (J/g) 41.4 30.1 21.3 12.5
Yield strength (MPa) 44 27 17 13
Tensile modulus (MPa) 1250 440 200 84
Elongation (%) > 500 > 500 > 500 > 500
Flexural modulus (MPa) 1040 420 200 62
Hardness (Shore D) 80 73 63 50
50 40 h
2
30 I-
m E! 20 10 0 0 100 200 300 400 500 Strain(%)Frg. 4. Tensile stress-strain curves of the block copolyether- esters.
CONCLUSION
The block copolyetheresters were prepared suc- cessfully in a pilot plant by a TPA process in the presence of a salt system. The presence of this salt system reduced the formation of THF, and also re- duced the reaction time of the whole process. The compositions of the block copolyetheresters deter-
mined by ' H NMR agreed well with the charged com- positions. The thermal properties and mechanical properties were measured and found to be dependent on the composition.
REFERENCES
1. N. R. Legge, G. Holder, and H. E. Schroeder, Eds., Ther- moplastic Elastomers: A Comprehensive Reuieru. Hanser Publishers, New York (1987).
2. A. K. Bhowmick and H. L. Stephens, Eds., Handbook of Elastomers: New Developments and Technology, Marcel Dekker, New York (1988).
3. L. M. Porter, U.S. Pat. 3,149,182 (1964).
4. R. F. Zelinski, U.S. Pat. 3,28 1,383 ( 1966).
5. G. Oertel, Polyurethane Handbook, Hanser Publishers,
6. P. Foy, C. Jungblut, and G. Deleens, U.S. Pat. 4,332,920 7. J. C. Shivers and W. Chester, U.S. Pat. 3,023,192 (1962). 8. W. K. Witsiepe, U.S. Pat. 3,651,014(1972).
9. G. K. Hoeschele, U.S. Pat. 3,801,547 (1974).
10. H. Schroeder and R. J. Cella, in Encyclopedia ofPolymer Science a n d Engineering, 2nd Ed., Vol. 12, pp. 75-1 17,
H. F. Mark, N. M. Bikales, C. G . Overberger, G. Menges, and J. I. Kroschwits, Eds., Wiley, New York (1988). 11. L. 2. Chung, D. L. Kuo, A. T. Hu, and H. B. Tsai, J . Polym.
Sci. PartA: Polym. Chem, 30, 951 (1992).
12. H. B. Tsai, H. C. Li, S. J. Chang, and F. S. Yu, Polym BulL, 27, 141 (1991).
13. H. B. Tsai, C. Lee, and N. S. Chang, Polym J., 24, 157
( 1992).
14. S. J. Chang and H. B. Tsai, J . AppL Polym Sci., 45, 371 ( 1992).
Received Nov. 15, 1992 New York (1985).
( 1982).