DOI 10.1378/chest.07-1769 2008;133;190-196 Chest
Shih-Ann Chen
Nan-Hung Pan, Hsuan-Ming Tsao, Nen-Chung Chang, Yi-Jen Chen and
Fibrillation
Implications for the Genesis of Atrial
:
* Aging Dilates Atrium and Pulmonary Veins
http://chestjournal.chestpubs.org/content/133/1/190.full.html services can be found online on the World Wide Web at:
The online version of this article, along with updated information and
ISSN:0012-3692
)
http://chestjournal.chestpubs.org/site/misc/reprints.xhtml(
written permission of the copyright holder.
this article or PDF may be reproduced or distributed without the prior Dundee Road, Northbrook, IL 60062. All rights reserved. No part of Copyright2008by the American College of Chest Physicians, 3300 Physicians. It has been published monthly since 1935.
is the official journal of the American College of Chest
Chest
Aging Dilates Atrium and Pulmonary Veins*
Implications for the Genesis of Atrial Fibrillation
Nan-Hung Pan, MD; Hsuan-Ming Tsao, MD; Nen-Chung Chang, MD, PhD;
Yi-Jen Chen, MD, PhD; and Shih-Ann Chen, MD
Backgrounds: Aging plays a critical role in the pathophysiology of atrial fibrillation (AF). The left atrium (LA) and pulmonary veins (PVs) are essential components for the genesis and maintenance of AF. The purpose of this study was to investigate the effects of aging on the AF substrate and the initiator (PVs).
Methods: A total of 180 patients undergoing multidetector CT were enrolled and classified into six groups according to the decade of their age. LA, LA appendage (LAA), and orifice of the four PVs were measured.
Results: The LA anterior-posterior diameter and wall thickness became increased with aging after the age of 50 years (p < 0.001). Similarly, the LAA and four PV trunks also became dilated after the patients were > 50 years old (p < 0.001). The anterior wall was consistently thicker than the posterior wall in each group. Aging also increased both anterior and posterior wall thickness after the patients became > 50 years old. However, LA diameter, PV diameter, and LA wall thickness in the patients aged 70 to 79 years and > 80 years did not significantly differ. Age correlated well with the four PVs, LA diameter, and wall thickness with linear regression.
Conclusions: Age significantly determines LA and PV structures. These findings show the important contributing effects involved in aging-induced AF in the general population.
(CHEST 2008; 133:190–196)
Key words: atrial fibrillation; multidetector CT; pulmonary vein
Abbreviations: AF⫽ atrial fibrillation; BMI ⫽ body mass index; CAD ⫽ coronary artery disease; LA ⫽ left atrium/atrial;
LAA⫽ left atrial appendage; LIPV ⫽ left inferior pulmonary vein; LSPV ⫽ left superior pulmonary vein; LV ⫽ left ventricle/ventricular; MDCT⫽ multidetector CT; PV ⫽ pulmonary vein; RIPV ⫽ right inferior pulmonary vein;
RSPV⫽ right superior pulmonary vein
A trial fibrillation (AF) is the most common cardiac arrhythmia observed in clinical practice and in- duces cardiac dysfunction and strokes.1,2 The preva- lence of AF has been reported to be 1% in humans
⬎ 60 years old and up to ⬎ 5% in humans ⬎ 70 years old.
3The estimated risk of AF developing during one’s life is approximately 2% in humans ⬎ 30 years old according to the Framingham study.
4These findings suggest that aging plays an important role in AF genesis. However, the mechanisms of aging-induced AF have not been fully elucidated. Aging can induce myocyte loss or increase fibrosis and reactive cellular hypertrophy, which will produce ventricular hypertro- phy and stiffness.
5In addition, aging induces mitochon- drial damage associated with cell dysfunction in cardi- omyocytes.
6,7An animal study
8showed that aging may alter cardiac electrophysiology to cause AF. However, information about the effects of aging on human cardiac structures in the general population has been
limited. Knowledge about cardiac structures in very old patients (⬎ 80 years old) is also not available.
Pulmonary veins (PVs) are the most important source of ectopic beats with the initiation of paroxysmal AF or foci of ectopic atrial tachycardia and focal AF.
9Previous studies
10 –11have shown that morphology changes of PVs have significant effects on PV arrhyth- mogenesis. Enlarged PVs may cause a higher PV arrhythmogenesis to induce AF. However, it is not clear whether aging alters the PV structure resulting in an increase in the PV arrhythmogenesis. Multidetector CT (MDCT) provides better and reliable imaging of smaller cardiac structures.
11–14A previous study
15has also shown that MDCT provides accurate and detailed imaging of the left atrium (LA) and PVs. Therefore, by using 64-row scan MDCT, the purpose of this study was to investigate the effects of aging on the atrium (AF substrate) and PVs (AF initiators).
Original Research
CARDIOVASCULAR DISEASE
Methods and Materials
Patient Selection
This study received institutional review board approval and enrolled 180 consecutive individuals (126 men and 54 women; mean age, of 56⫾ 12 years [⫾ SD]) undergoing 64-row scan MDCT for evaluation of the coronary artery. One hundred thirty-nine patients (77%) from the community, 29 patients (16%) from outpatient clinics, and 12 patients (7%) from hospitals were included. During the study, all subjects were in sinus rhythm and did not have any coronary artery disease (CAD) [⬎ 50% stenosis], with a zero coronary calcium score diagnosed by MDCT. The subjects were classified into six age groups according to their decade of life. The lowest age group was⬍ 40 years, and the highest age group was ⬎ 80 years. Each participant underwent a medical history, laboratory assessment, and measurement of weight, height, body mass index (BMI), and BP. Metabolic syndrome was defined according to the 1999 World Health Organization definition as
the presence of hyperglycemia (an impaired fasting glucose, impaired glucose tolerance, type 2 diabetes, or insulin resistance) and at least two of the following: dyslipidemia (triglycerides⬎ 150 mg/dL and/or high- density lipoprotein cholesterol⬍ 35 mg/dL in men and ⬍ 39 mg/dL in women), elevated BP⬎ 140/90 mm Hg, obesity (BMI ⬎ 30 kg/m2or waist/hip ratios⬎ 0.9 in men and ⬎ 0.85 in women), or microalbu- miuria (⬎ 20 g/min).
CT
The patients underwent a 64-row scan (Light Speed VCT; GE Healthcare; Milwaukee, WI) using an ECG-synchronized tube-modu- lation system. Patients with a heart rate⬎ 70 beats/min were adminis- tered a single oral dose of propranolol (10 to 40 mg) at least 40 min before the examination. Images were reconstructed retrospectively in the diastolic phase (at 60% of the start of the RR interval). Nonionic contrast medium was administered in a test dose of 250 mL.
Measurement of the LA, Left Ventricular Dimensions, and PVs PV diameters were measured using the maximal transverse diameter of the four PV trunk orifices in a virtual endoscopic view. LA diameters were measured with the maximal anterior-posterior distance in the oblique-sagittal view. The orifice of the LA appendage (LAA) was defined as the deflection between the LAA and LA free wall. The largest diameter was measured in the oblique- sagittal view. Anterior and posterior wall thickness were measured by the axial view. Left ventricle (LV) dimensions were measured as the maximal distance from the septum to the lateral free wall at the level of the papillary muscle in the end-diastolic phase in the four-chamber axial view. LV ejec- tion fraction was calculated by integrated computer software in a workstation (AW 4.3; GE Healthcare), which traced automatically in the end-diastolic volume and end-systolic volume phases. Two independent observers were asked to analyze the image measurements in a blinded fashion. The
*From the Division of Cardiovascular Medicine (Drs. Pan and Chang), Taipei Medical University and Hospital, Taipei; I-Lan Hospital (Dr. Tsao), Taiwan; Graduate Institute of Clinical Medicine and Topnotch Stroke Research Center (Dr. Y-J Chen), Taipei Medical University; and Division of Cardiology and Cardiovascular Research Center (Dr. S-A Chen), Veterans Gen- eral Hospital-Taipei, Taipei, Taiwan.
This work was supported by the Topnotch Stroke Research Center Grant, Ministry of Education and grants NSC 95-2314- B-016-015, NSC 95-2314-B-038-026.
The authors have no conflicts of interest to disclose.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestjournal.
org/misc/reprints.shtml).
Correspondence to: Yi-Jen Chen, MD, PhD, Division of Cardio- vascular Medicine, Taipei Medical University-Wan Fang Hospi- tal, 111, Hsin-Lung Rd, Section 3, Taipei, Taiwan; e-mail:
a9900112@ms15.hinet.net DOI: 10.1378/chest.07-1769
Table 1—Patient Characteristics*
Characteristics
Age Range, yr
⬍ 40 (n ⫽ 9) 40–49 (n⫽ 53) 50–59 (n⫽ 57) 60–69 (n⫽ 41) 70–79 (n⫽ 13) ⱖ 80 (n ⫽ 7) p Value
Age, yr 33⫾ 6 46⫾ 2 54⫾ 3 65⫾ 3 73⫾ 4 86⫾ 2 ⬍ 0.001
Male gender 6 (67) 35 (66) 42 (74) 28 (68) 10 (77) 5 (71) 0.947
Body weight, kg 63⫾ 10 65⫾ 10 65⫾ 10 66⫾ 11 68⫾ 66⫾ 11 0.894
Height, m 1.6⫾ 0.14 1.6⫾ 0.15 1.6⫾ 0.18 1.6⫾ 0.12 1.6⫾ 0.19 1.6⫾ 0.14 0.988
BMI, kg/m2 24.7⫾ 3.2 25.2⫾ 3.7 24.9⫾ 2.8 25.5⫾ 3.6 23.7⫾ 2.6 24.6⫾ 4.6 0.628
Serum creatinine, mg/dL 1.3⫾ 0.3 1.2⫾ 0.2 1.2⫾ 0.3 1.1⫾ 0.3 1.3⫾ 0.3 1.3⫾ 0.2 0.218
Ejection fraction, % 72⫾ 3† 63⫾ 8 62⫾ 7 63⫾ 7 57⫾ 5 56⫾ 3 ⬍ 0.001
Systolic BP, mm Hg 127⫾ 7 130⫾ 11 129⫾ 11 131⫾ 9 134⫾ 4 138⫾ 9 0.156
Diastolic BP, mm Hg 73⫾ 4 76⫾ 7 77⫾ 7 77⫾ 8 79⫾ 6 80⫾ 4 0.185
Hypertension 1 (11) 4 (8) 5 (9) 4 (10) 1 (7) 1 (14) 0.505
Diabetes 0 4 (8) 5 (9) 3 (7) 2 (15) 1 (14) 0.835
Dyslipidemia 0 10 (19) 22 (39) 13 (32) 5 (38) 4 (57) 0.036
Smoking 0 0 5 (9) 12 (29) 5 (38) 0 ⬍ 0.001
Metabolic syndrome 0 5 (9) 6 (11) 3 (7) 3 (23) 2 (29) 0.292
CAD family history 2 (22) 6 (11) 8 (14) 5 (12) 5 (38) 1 (14) 0.233
Aspirin use 0 2 (4) 4 (7) 4 (10) 3 (23) 3 (33) 0.110
ACEI or ARB use 0 4 (8) 4 (7) 4 (10) 4 (31) 1 (11) 0.141
CCB use 1 (14) 2 (4) 1(2) 2 (5) 1 (8) 1 (11) 0.150
-Blocker use 1 (14) 2 (4) 2 (4) 3 (7) 0 1 (11) 0.170
*Data are presented as mean⫾ SD or No. (%). ACEI ⫽ angiotensin-converting enzyme inhibitor; ARB ⫽ angiotensin II receptor blocker;
CCB⫽ calcium channel blocker.
†p⬍ 0.05 vs other age groups.
interobserver reproducibility and intraobserver reproducibility were 91% and 97%, respectively.
Statistical Analysis
Continuous variables are expressed as mean⫾ SD. Comparisons among the six age groups were analyzed by a one-way analysis of variance with a post hoc Student-Newman-Keuls method. Nominal variables were compared by a2analysis with a Yates correction or Fisher exact test. Multivariate regression analysis was used to assess the independence of the variables. A paired Student t test was used to
compare the LA anterior and posterior wall thickness. Linear regression was used to evaluate the correlation between the age and the structure of the LA and PVs. A p value ⬍ 0.05 was considered statistically significant.
Results Patient Characteristics
Table 1 shows the patient characteristics from the six age groups. The incidence of dyslipidemia increased with
Figure 1. Oblique-sagittal (upper panels) and oblique-coronal (lower panels) views during MDCT in patients aged⬍ 40 years (left panels, A), 50 to 59 years (center panels, B), and 70 to 79 years (right panels, C). The largest LA anterior-posterior distance was measured in the oblique-sagittal view. The oblique-coronal view exhibited the largest distance from the LSPV to the RSPV. Left panels, A:
LA⫽ 30 mm. Center panels, B: LA ⫽ 35 mm. Right panels, C: LA ⫽ 41 mm. Ao ⫽ aorta.
Table 2—Comparison Between Aging and the Anatomies of the LA and PVs and LV Dimension*
Variables
Age, yr
⬍ 40 (n ⫽ 9) 40–49 (n ⫽ 53) 50–59 (n ⫽ 57) 60–69 (n ⫽ 41) 70–79 (n ⫽ 13) ⱖ 80 (n ⫽ 7) p Value LA diameter, mm 30⫾ 6.2 30.1⫾ 7.9 33.9⫾ 8.9† 38.3⫾ 10†‡§ 43.2 ⫾ 5.4†‡§ 48.2 ⫾ 5.9†‡§ ⬍ 0.001 LA anterior wall thickness, mm 2.0⫾ 0.9 2.1⫾ 0.5 2.5⫾ 0.7† 3.2⫾ 0.2†‡§ 3.6⫾ 0.4†‡§ 3.7⫾ 0.9†‡§储 ⬍ 0.001 LA posterior wall thickness, mm 0.7⫾ 0.2 1.1⫾ 0.3 1.5⫾ 0.3†‡ 1.8⫾ 0.2†‡§ 1.9⫾ 0.2†‡§ 2.4⫾ 0.4†‡§储 ⬍ 0.001 Anterior and posterior wall
thickness difference, mm
1.2⫾ 0.4 1.1⫾ 0.5 1.0⫾ 0.7 1.9⫾ 1.1†‡§ 1.4⫾ 0.5†‡§ 1.3⫾ 1.2†‡§储 ⬍ 0.001 LAA orifice, mm 15.3⫾ 0.6 16.2⫾ 0.9 17.4⫾ 1.8†‡ 22.3⫾ 1.4†‡§ 24.6 ⫾ 0.8†‡§ 24.8 ⫾ 0.9†‡§储 ⬍ 0.001 LSPV, mm 12.0⫾ 0.6 12.7⫾ 0.9 15.0⫾ 1.4†‡ 18.6⫾ 1.6†‡§ 19.6 ⫾ 1.8†‡§ 20.5 ⫾ 1.1†‡§储 ⬍ 0.001 LIPV, mm 12.8⫾ 0.5 13.9⫾ 0.6 15.9⫾ 1.4†‡ 17.7⫾ 0.6†‡§ 19.9 ⫾ 0.8†‡§ 19.0 ⫾ 0.5†‡§储 ⬍ 0.001 RSPV, mm 12.6⫾ 0.7 13.5⫾ 1.3 16.3⫾ 1.4†‡ 18.5⫾ 1.2†‡§ 19.1 ⫾ 1.2†‡§ 20.2 ⫾ 0.9†‡§储 ⬍ 0.001 RIPV, mm 12.5⫾ 0.8 13.2⫾ 1.2 16.4⫾ 1.5†‡ 18.5⫾ 1.4†‡§ 19.6 ⫾ 0.9†‡§ 20.7 ⫾ 0.6†‡§储 ⬍ 0.001 LV dimension, mm 40⫾ 4.2 41⫾ 4.8 44⫾ 5.3 45⫾ 4.2†‡ 51⫾ 5.7†‡§储 52⫾ 2.7†‡§储 ⬍ 0.001
*Data are presented as mean⫾ SD.
†p⬍ 0.05 vs ⬍ 40 years.
‡p⬍ 0.05 vs 40 to 49 years.
§p⬍ 0.05 vs 50 to 59 years.
储p ⬍ 0.05 vs 60 to 69 years.
aging (Table 1). Body weight, height, and BMI in each age group did not significantly differ. Patients aged 50 to 59 years and 60 to 69 years had higher incidences of smoking as compared to those aged ⬍ 40 years or 40 to 49 years.
Patients aged ⬍ 40 years had a better ejection fraction than the other groups. However, drug therapies, family history of CAD, systolic BP, diastolic BP, presence of hypertension, diabetes, and metabolic syndrome were not
statistically different among the six age groups (Table 1), although on multivariate analysis, age group still remains an independent factor for the incidence of dyslipidemia and smoking.
Structural Changes Among Different Age Individuals
Table 2 shows the PV and LA structural parame- ters in the six age groups. Aging had significant effects on LA diameter. LA diameter increased after the patients became ⬎ 50 years old (p ⬍ 0.001).
However, LA diameter in patients aged 70 to 79 years and ⬎ 80 years was similar. Compared to those aged ⬍ 40 years, patient aged 50 to 59 years, 60 to 69 years, 70 to 79 years, and ⬎ 80 years had a larger LA diameter by 13%, 28%, 44%, and 61%, respectively.
Figure 1 shows an example of the different LA sizes among the six age groups. In addition, aging also increased both anterior and posterior wall thickness after the patients became ⬎ 50 years old. However, anterior and posterior wall thickness in the patients aged 70 to 79 years and ⬎ 80 years did not signifi- cantly differ. The anterior wall was consistently thicker than the posterior wall in each age group (p ⬍ 0.05). Figure 2 shows an example demonstrat- ing that aging increased the LA anterior wall and posterior wall thickness. Compared to those aged
⬍ 40 years, patients aged 50 to 59 years, 60 to 69 years, 70 to 79 years, and ⬎ 80 years had larger LA anterior and posterior wall thickness by 25%, 60%, 80%, and 85% for the anterior wall, and by 114%, 157%, 171%, and 242% for the posterior wall, respectively. Furthermore, compared to those aged
⬍ 40 years, anterior and posterior wall thickness differ- ences increased in patents aged 60 to 69 years, 70 to 79 years, and ⬎ 80 years (Table 2).
Figure 2. Axial views show the LA anterior wall thickness (1) and posterior wall thickness (2) in patients aged⬍ 40 years (top, A) and 70 to 79 years (bottom, B). Aging increases both anterior and posterior wall thickness. The anterior wall was significantly more thickened than the posterior wall.
Figure 3. Intra-atrial oblique-sagittal views during MDCT from patients aged⬍ 40 years (left, A), 50 to 59 years (center, B), and 70 to 79 years (right, C). The largest diameters of the LSPV and LIPV were measured using the virtual intra-atrial view. LAA orifice diameter was measured using the oblique- sagittal view. Left, A: LSPV⫽ 12 mm, LIPV ⫽ 13 mm, and LAA ⫽ 15 mm. Center, B: LSPV ⫽ 15 mm, LIPV⫽ 16 mm, and LAA ⫽ 17 mm. Right, C: LSPV ⫽ 19 mm, LIPV ⫽ 19 mm, and LAA ⫽ 24 mm.
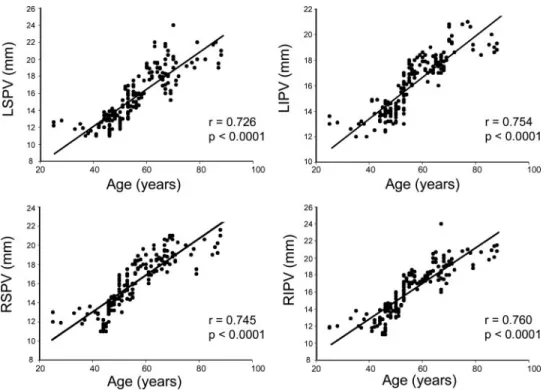
Figure 3 shows examples of LAA diameter from the different age groups. Aging increased LAA diameter after the patients were ⬎ 50 years old. However, LAA diame- ters in the patients aged 70 to 79 years and ⬎ 80 years were similar. Compared to those aged ⬍ 40 years, pa- tients aged 50 to 59 years, 60 to 69 years, 70 to 79 years, and ⬎ 80 years had larger LAA diameters by 14%, 46%, 61%, and 62%, respectively. Moreover, aging correlated well with LA diameter, LAA diameter, and anterior and posterior wall thickness using linear regression (Fig 4).
Comparisons of the four PV trunk diameters among the six age groups showed that the four PV diameters in- creased after the patients became ⬎ 50 years old (Table 2;
Fig 3). Compared to the right superior pulmonary vein (RSPV), left superior pulmonary vein (LSPV), right inferior pulmonary vein (RIPV), and left inferior pul- monary vein (LIPV) in patients aged ⬍ 40 years, PVs (RSPV, LSPV, RIPV, and LIPV) in those aged 51 to 60 years were larger by 29%, 25%, 31%, and 24%; in those aged 60 to 69 years were larger by 47%, 55%, 48%, and 38%; in those aged 70 to 79 years were larger by 52%, 63%, 57%, and 55%; and in those aged ⬎ 80 years were larger by 60%, 71%, 66%, and 58%, respectively. Aging correlated well with all four PV diameters using linear regression (Fig 5). Moreover, aging also had significant effects on LV dimensions (Table 2). Through multivar-
iate analysis, age group was an independent factor for LV dimensions, LA wall thickness, and the diameters of LA, LAA, and the four PVs.
Discussion
Aging has significant cardiovascular effects and in- creases the occurrence of AF. However, an extensive understanding of the aging effects on the AF substrate and initiators has not been elucidated. Huonker et al
16reported age-related cardiac structural changes in the thickening of the myocardium and arrythmias. In this study, we found that aging significantly dilated the atrium and PVs, which may cause aging-related AF. In addition, this study showed that aging increased LA and PV size after the patients become ⬎ 50 years old. Patients aged 60 to 69 years had a greater extent of structure changes of the atrial diameter and thickness.
17All of those results may explain the dramatic increase in the AF in patients aged 60 to 70 years, which then slowly increases after 70 years.
18,19Moreover, the good liner correlation between aging and the LA or PV structure highly suggests the critical risk effects of aging on AF.
The genesis of AF arises from the changes in the AF substrate (atrium) and initiators (PVs). Atrial enlargement
Figure 4. Correlation between changes in age and LA chamber size, LAA orifice diameter, and LA anterior and posterior wall thickness.
may facilitate the maintenance of AF due to the wave- length theory. Dilated PVs may enhance PV arrhythmo- genesis and induce more AF.
20Therefore, in addition to structure changes, aging may increase AF through mecha- noelectrical feedback in the PVs and atrium. Gardin et al
21reported that aging-related increases in LV mass will add load to the heart and further enlarge LA chamber size and pressure. That effect may lead to fibrosis and electrical remodeling in the atrium and provide a substrate for the development of AF. Moreover, aging could directly im- pair the ventricular relaxation and increase atrial size.
22,23Heart failure is an important risk factor for AF.
24–26It is known that heart failure is very common in elderly population. Risk factors for heart failure are also increased with aging. Similarly, LV ejection fraction was better in patients ⬍ 40 years old. Therefore, structure changes occurring during aging may partially arise from subclinical heart failure, although our patients did not have any evidence of heart failure. In addition, the incidence of dyslipidemia also increased during aging in study patients.
All of these aging effects can increase the risk for AF.
27In this study, the incidence of hypertension did not signifi- cantly differ among the six age groups, although aging still has a trend to increase BP. It is known that aging increases the hypertension population. Therefore, our patients may not be completely correlated with the general population.
The similar hypertension incidence in our patients may reduce the potential hypertension effects and demon- strate more uncontaminated aging effects on the atrium and PVs. Metabolic syndrome is known to induce inflam-
mation and thereby may increase AF risk.
28However, the similar incidence of metabolic syndrome among the dif- ferent age groups suggests that metabolic syndrome may not play a significant role in this study.
Aging may accelerate the wall thickness and stiffness by a process of fibrosis and depletion of the elastin and collagen.
29In this study, for the first time we found that aging increased wall thickness using the MDCT. There was general agreement that MDCT is superior to transthoracic echocardiography or transoesophageal echocardiography for LA or PV measurements. LA wall thickness was difficult to detect by transthoracic echocardiography or transoesophageal echocardiog- raphy. Moreover, we demonstrated a consistently thinner wall for the LA posterior wall than for the LA anterior wall using MDCT. These findings may result in a higher arrhythmogenesis in the LA pos- terior wall than in the anterior wall
30,31because the thinner wall should have a higher wall stress. Our previous animal study
8also found that a thinner LA posterior wall may have a higher arrhythmogenesis for inducing AF.
Data from this study should be interpreted with caution due to the limitations of this study. First, we could not completely exclude occult CAD in the study patients because MDCT could not evaluate small vessels (diame- ters ⬍ 1 to 2 mm) accurately or because the patients may have insignificant CAD. Second, the structures measured are three dimensional and not uniformly shaped in all individuals, which may limit the comparative utility of the
Figure 5. Correlation between changes in age and diameters of the LSPV, LIPV, RSPV, and RIPV.
linear measurements. Third, we did not evaluate LV diastolic function in this study. Aging has been shown to impair LV diastolic function. This effect may result in the changes in LA and PV structures.
In conclusion, aging has significant effects on LA and PV structure. The anatomic dilation and mechanoelectri- cal feedback caused by the aging effects may facilitate the occurrence of aging-related AF.
References
1 Krahn AD, Manfreda J, Tate RB, et al. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow-up Study. Am J Med 1995; 98:476 – 484 2 Psaty BM, Manolio TA, Kuller LH, et al. Incidence of and risk
factors for atrial fibrillation in older adults. Circulation 1997; 96:
2455–2461
3 Furberg CD, Psaty BM, Manolio TA, et al. Prevalence of atrial fibrillation in elderly subjects (the Cardiovascular Health Study).
Am J Cardiol 1994; 74:236–241
4 Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an indepen- dent risk factor for stoke: the Framingham Study. Stroke 1991;
22:983–988
5 Chen CH, Nakayama M, Nevo E, et al. Coupled systolic-ventricular and vascular stiffening with age: implications for pressure regulation and cardiac reserve in the elderly. J Am Coll Cardiol 1998; 32:1221–
1227
6 Terman A, Brunk UT. The aging myocardium: roles of mitochon- drial damage and lysosomal degradation. Heart Lung Circ 2005;
14:107–114
7 Brunk UT, Terman A. Lipofuscin: mechanisms of age-related accumulation and influence on cell functions. Free Radic Biol Med 2002; 33:611–619
8 Wongcharoen W, Chen YC, Chen YJ, et al. Effects of aging and ouabain on left atrial arrhythmogenicity. J Cardiovasc Electrophysiol 2007; 18:526–531
9 Chen SA, Hsieh MH, Tai CT, et al. Initiation of atrial fibrillation by ectopic beats originating from the pulmonary veins: electrophysio- logical characteristics, pharmacological responses, and effects of radiofrequency ablation. Circulation 1999; 100:1879–1886 10 Tsao HM, Wu MH, Huang BH, et al. Morphologic remodeling of
pulmonary veins and left atrium after catheter ablation of atrial fibrillation: insight from long-term follow-up of three-dimensional magnetic resonance imaging. J Cardiovasc Electrophysiol 2005;
16:7–12
11 Nieman K, Cademartiri F, Lemos PA, et al. Reliable noninvasive coronary angiography with fast submillimeter multislice spiral com- puted tomography. Circulation 2002; 106:2051–2054
12 Mollet NR, Cademartiri F, Nieman K, et al. Multislice spiral computed tomography coronary angiography in patients with stable angina pectoris. J Am Coll Cardiol 2004; 43:2265–2270
13 Mollet NR, Cademartiri F, Nieman K, et al. Noninvasive assess- ment of coronary plaque burden using multislice computed tomog- raphy. Am J Cardiol 2005; 95:1165–1169
14 Ropers D, Baum U, Pohle K, et al. Detection of coronary artery stenoses with thin-slice multi-detector row spiral computed tomog- raphy and multiplanar reconstruction. Circulation 2003; 107:664–
666
15 Schwartzman D, Lacomis J, Wigginton WG. Characterization of left atrium and distal pulmonary vein morphology using multidimen- sional computed tomography. J Am Coll Cardiol 2003; 41:1349–
1357
16 Huonker M, Schmidt-Trucksass A, Heiss HW, et al. Effects of physical training and age-induced structural and functional changes in cardiovascular system and skeletal muscles. Z Gerontol Geriatr 2002; 35:151–156
17 Gardin JM, Henry WL, Savage DD, et al. Echocardiographic measurements in normal subjects: evaluation of an adult population without clinically apparent heart disease. J Clin Ultrasound 1979;
7:439–447
18 Feinberg WM, Blackshear JL, Laupacis A, et al. Prevalence, age distribution, and gender of patients with atrial fibrillation: analysis and implications. Arch Intern Med 1995; 155:469–473
19 Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study.
Circulation 2004; 110:1042–1046
20 Wongcharoen W, Chen YC, Chen YJ, et al. Aging increases pulmonary veins arrhythmogenesis and susceptibility to calcium regulation agents. Heart Rhythm 2007; 4:1338–1349
21 Gardin JM, Arnold A, Gottdiener JS, et al. Left ventricular mass in the elderly: the Cardiovascular Health Study. Hypertension 1997;
29:1095–1103
22 Hundley WG, Kitzman DW, Morgan TM, et al. Cardiac cycle- dependent changes in aortic area and distensibility are reduced in older patients with isolated diastolic heart failure and correlate with exercise intolerance. J Am Coll Cardiol 2001; 38:796–802 23 Vaziri SM, Larson MG, Lauer MS, et al. Influence of blood
pressure on left atrial size: the Framingham Heart Study. Hyper- tension 1995; 25:1155–1160
24 Chae CU, Pfeffer MA, Glynn RJ, et al. Increased pulse pressure and risk of heart failure in the elderly. JAMA 1999; 281:634–639 25 Vasan RS, Larson MG, Benjamin EJ, et al. Congestive
heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population-based cohort. J Am Coll Cardiol 1999; 33:1948 – 1955
26 Kitzman DW. Diastolic dysfunction in the elderly: genesis and diagnostic and therapeutic implications. Cardiol Clin 2000; 18:597–
617
27 Tsang TS, Gersh BJ, Appleton CP, et al. Left ventricular diastolic dysfunction as a predictor of the first diagnosed nonvalvular atrial fibrillation in 840 elderly men and women. J Am Coll Cardiol 2002;
40:1636–1644
28 Festa A, D’Agostino R Jr, Howard G, et al. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS). Circulation 2000; 102:
42–47
29 Najjar SS, Scuteri A, Lakatta EG. Arterial aging: is it an immutable cardiovascular risk factor? Hypertension 2005; 46:454–462 30 Huang JL, Tai CT, Lin YJ, et al. The mechanisms of an increased
dominant frequency in the left atrial posterior wall during atrial fibrillation in acute atrial dilatation. J Cardiovasc Electrophysiol 2006; 17:178–188
31 Kalifa J, Tanaka K, Zaitsev AV, et al. Mechanisms of wave fraction- ation at boundaries of high-frequency excitation in the posterior left atrium of the isolated sheep heart during atrial fibrillation. Circula- tion 2006; 113:626–633