It is known that electrochemical reaction and mechanical abrasion are both involved in the chemical mechanical polishing (CMP) processes. The associated mechanism is rather complicated. Accord- ing to the model proposed by Kaufman et al.,
1the removal of tungsten by CMP is the result of repeated processes of film formation (passiva- tion), film abrasion, and repassivation in the slurry. This mechanism has also been applied to explain the processes for metal removal and planarization for Cu
2and Al
3,4CMP. Obviously, as indicated by this mechanism, passivation of metal in slurry plays very important role in CMP. Hence, oxidants such as H
2O
2,
2,5,6KMnO
4,
5KIO
3,
5and K
3Fe(CN)
61were frequently added to slurries in order to enhance passivation and to increase the metal removal rate during CMP.
Although passivation behavior is essential to the film formation, film abrasion, and film reformation mechanism, Stein et al.
7and Kneer et al.
5indicated that the passive film might not exist on the metal surface during CMP. The results of their investigations re- vealed that the role of passive film on the metal removal was still not clear. Since passivation behavior strongly depends on the electro- chemical potential across the metal/electrolyte interface, the effect of passive film on the polishing process may be evaluated under potentiostatic conditions. In this study, the electrochemical behavior of Al in phosphoric acid base slurry under CMP was investigated.
Furthermore, the respective roles of electrochemical reaction and mechanical abrasion on the CMP removal rates of Al under con- trolled potential condition were also explored.
Experimental
Specimen and slurry preparations.—A commercial pure Al sheet with a chemical composition of Al-0.1Si-0.2Fe-0.01Ti was used.
The specimens both for polarization measurement and CMP had the same dimension of 1 3 1 3 0.2 cm. Each specimen was mounted with epoxy resin with one surface exposed to air while a Cu wire was connected to the other side for electric conduction. The specimen was then mounted to a specimen holder of the polisher as described in the following section. In each case, the surface to be exposed to the slurry was ground with SiC paper to a finish of no. 800.
Phosphoric acid base slurry containing alumina powders was pre- pared. The composition of the slurry was 5 vol % H
3PO
41 0.5 M citric acid 1 5 wt % Al
2O
3powders. The average particle size of Al
2O
3powders was about 0.05 mm. Potassium hydroxide was used to adjust the slurry to pH 4.
Polishing apparatus.—A novel polisher simulating the polishing mechanism of CMP was designed and used in this study. The system
consists of an 8 in. platen and a 3 in. carrier. The platen was bonded with slurry holder to avoid splash of slurry during polishing. The platen speed was set with a rotating speed at 100 rpm. The down force between the specimen carrier and the platen could be varied from 3 to 9 psi. A Rodel Politex polish pad was affixed to the platen for polishing. During the polishing process, auxiliary specimens also made of Al specimens were added to the carrier for weight balance.
The main features of the polisher layout and electrochemical setup are drawn schematically in Fig. 1. All electrochemical tests were performed in this polisher.
Effect of Applied Potential on the Chemical Mechanical Polishing of Aluminum in Phosphoric Acid Base Slurry
Hong-Shi Kuo* and Wen-Ta Tsai**
Department of Materials Science and Engineering, National Cheng Kung University, Tainan, Taiwan
The electrochemical behavior of Al in phosphoric acid base slurry was investigated under chemical mechanical polishing (CMP) condition. The effect of applied potential on the CMP metal removal rate was also evaluated. The results from potentiodynamic polarization curve measurements indicated that a contact pressure in the range of 3 to 9 psi greatly modified the passivation behav- ior of Al in 5 vol % H
3PO
41 0.5 M citric acid 1 5 wt % Al2O
3slurry by a decrease in corrosion potential and an increase in pas- sive current density. The experimental results also showed that CMP removal rate strongly depended on the contact pressure and potential applied. The potential dependence behavior of the removal rate could be divided into three regions depending on the direc- tion of polarization and the magnitude of potential applied. The results of electrochemical impedance spectroscopy and Auger electron spectroscopy examinations showed that passive film formation on Al in the testing slurry was affected by the applied potential, which in term caused a change in the relative contribution of the corrosion and mechanical abrasion to the total removal rate of Al.
© 2000 The Electrochemical Society. S0013-4651(99)07-090-1. All rights reserved.
Manuscript submitted July 20, 1999; revised manuscript received February 15, 2000.
** Electrochemical Society Student Member.
** Electrochemical Society Active Member.
Figure 1. Schematic diagram showing the apparatus used for electrochemi-
cal and CMP tests.
Electrochemical tests and removal rate measurements.—The po- tentiostatic polarization tests were conducted in the conditions while the specimens were under CMP. The instruments employed were an EG&G model 362 electrochemical system coupled with a Yokogawa LR4110 recorder to monitor the potential and current during exper- iment. A saturated calomel electrode (SCE) was used as the refer- ence electrode, and the carrier made of AISI 304 stainless steel served as the counter electrode. Electrochemical impedance spec- troscopy (EIS) measurements were performed with a Gamry EIS 900 system. Impedance measurements were performed in the fre- quency range of 100 K to 0.01 Hz at controlled potentials. The am- plitude of potential modulation was 10 mV.
The weight loss of the specimen after CMP was also measured.
The removal rate (average value of more than two tests) of each specimen was then calculated.
Auger electron spectroscopy (AES).—The chemical analysis of the passive film after CMP at various applied potentials for 30 min was performed by AES. A Fison Microlab 310D Auger electron spec- troscope was used. Surface composition depth profile of each element analyzed was accomplished using Ar ion sputtering. The sputtering rate was determined to be 0.45 Å s
21on Ta
2O
5.
Results
Electrochemical behavior and removal rate.—The effect of con- tact pressure on the potentiodynamic polarization curves of Al in 5 vol % phosphoric acid 1 0.5 M citric acid solution of pH 4 with 5 wt % Al
2O
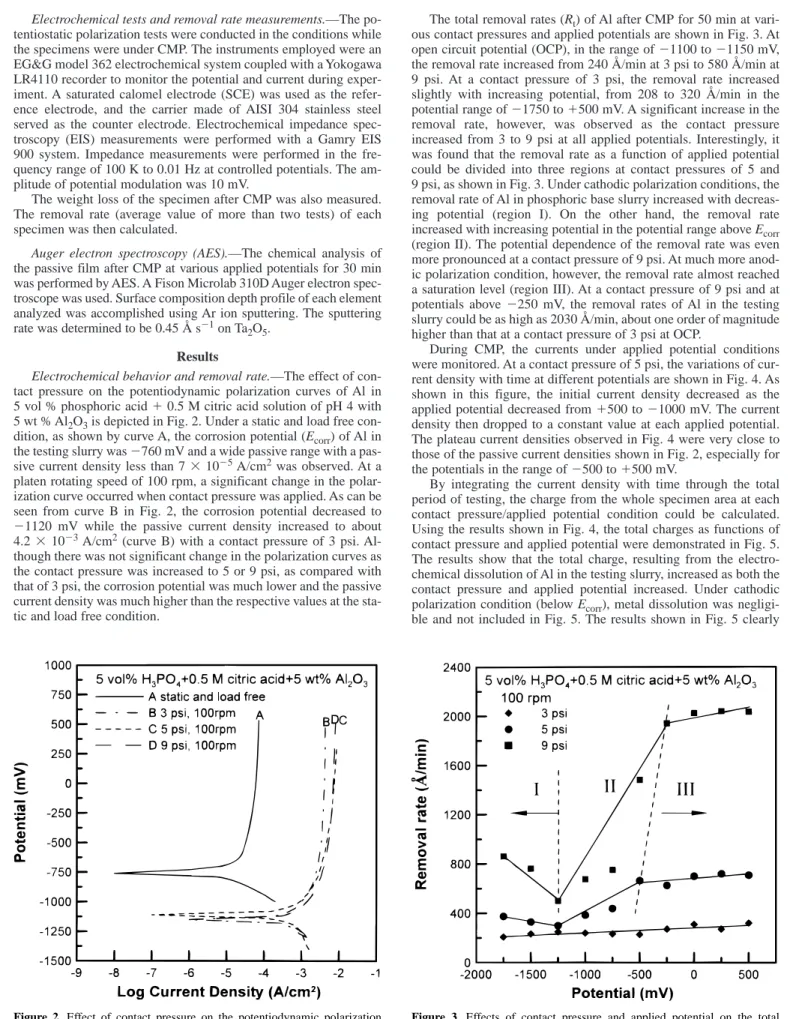
3is depicted in Fig. 2. Under a static and load free con- dition, as shown by curve A, the corrosion potential (E
corr) of Al in the testing slurry was 2760 mV and a wide passive range with a pas- sive current density less than 7 3 10
25A/cm
2was observed. At a platen rotating speed of 100 rpm, a significant change in the polar- ization curve occurred when contact pressure was applied. As can be seen from curve B in Fig. 2, the corrosion potential decreased to 21120 mV while the passive current density increased to about 4.2 3 10
23A/cm
2(curve B) with a contact pressure of 3 psi. Al- though there was not significant change in the polarization curves as the contact pressure was increased to 5 or 9 psi, as compared with that of 3 psi, the corrosion potential was much lower and the passive current density was much higher than the respective values at the sta- tic and load free condition.
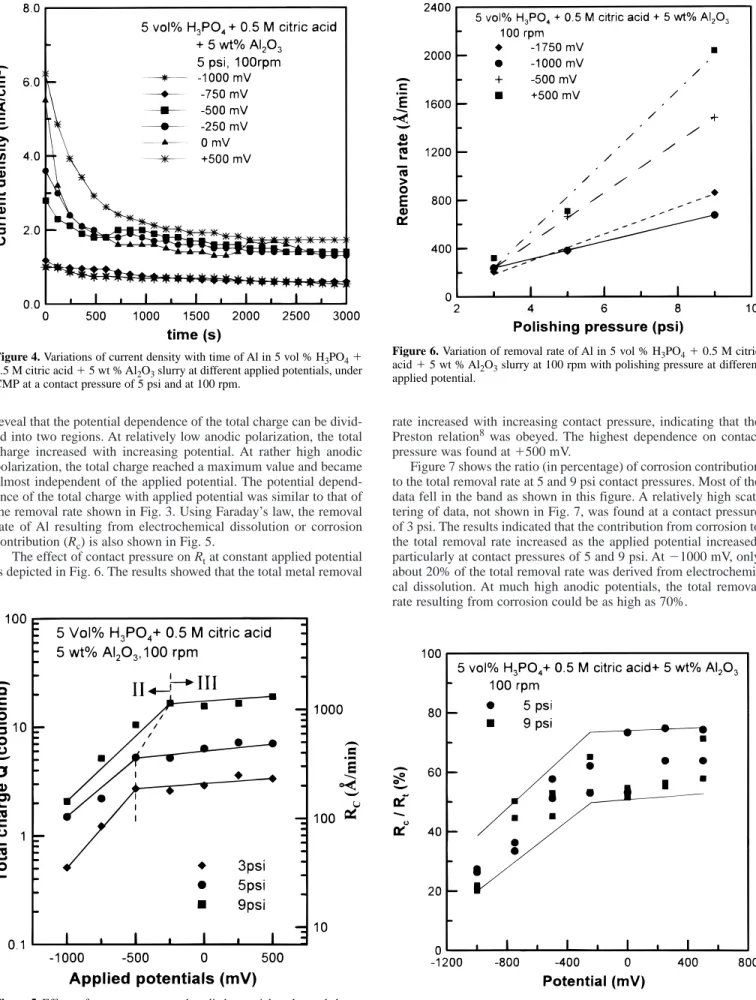
The total removal rates (R
t) of Al after CMP for 50 min at vari- ous contact pressures and applied potentials are shown in Fig. 3. At open circuit potential (OCP), in the range of 21100 to 21150 mV, the removal rate increased from 240 Å/min at 3 psi to 580 Å/min at 9 psi. At a contact pressure of 3 psi, the removal rate increased slightly with increasing potential, from 208 to 320 Å/min in the potential range of 21750 to 1500 mV. A significant increase in the removal rate, however, was observed as the contact pressure increased from 3 to 9 psi at all applied potentials. Interestingly, it was found that the removal rate as a function of applied potential could be divided into three regions at contact pressures of 5 and 9 psi, as shown in Fig. 3. Under cathodic polarization conditions, the removal rate of Al in phosphoric base slurry increased with decreas- ing potential (region I). On the other hand, the removal rate increased with increasing potential in the potential range above E
corr(region II). The potential dependence of the removal rate was even more pronounced at a contact pressure of 9 psi. At much more anod- ic polarization condition, however, the removal rate almost reached a saturation level (region III). At a contact pressure of 9 psi and at potentials above 2250 mV, the removal rates of Al in the testing slurry could be as high as 2030 Å/min, about one order of magnitude higher than that at a contact pressure of 3 psi at OCP.
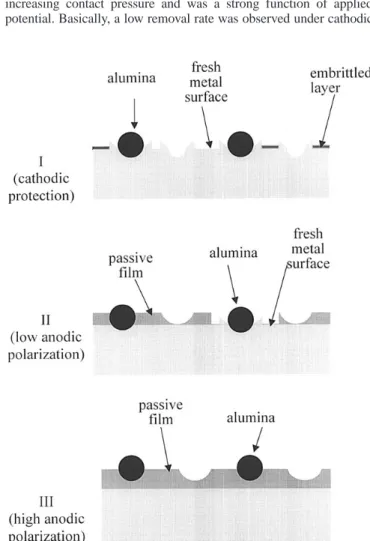
During CMP, the currents under applied potential conditions were monitored. At a contact pressure of 5 psi, the variations of cur- rent density with time at different potentials are shown in Fig. 4. As shown in this figure, the initial current density decreased as the applied potential decreased from 1500 to 21000 mV. The current density then dropped to a constant value at each applied potential.
The plateau current densities observed in Fig. 4 were very close to those of the passive current densities shown in Fig. 2, especially for the potentials in the range of 2500 to 1500 mV.
By integrating the current density with time through the total period of testing, the charge from the whole specimen area at each contact pressure/applied potential condition could be calculated.
Using the results shown in Fig. 4, the total charges as functions of contact pressure and applied potential were demonstrated in Fig. 5.
The results show that the total charge, resulting from the electro- chemical dissolution of Al in the testing slurry, increased as both the contact pressure and applied potential increased. Under cathodic polarization condition (below E
corr), metal dissolution was negligi- ble and not included in Fig. 5. The results shown in Fig. 5 clearly
Figure 2. Effect of contact pressure on the potentiodynamic polarization behavior of Al in 5 vol % H
3PO
41 0.5 M citric acid 1 5 wt % Al
2O
3slurry at 100 rpm.
Figure 3. Effects of contact pressure and applied potential on the total
removal rate of Al in 5 vol % H
3PO
41 0.5 M citric acid 1 5 wt % Al
2O
3slurry at 100 rpm.
reveal that the potential dependence of the total charge can be divid- ed into two regions. At relatively low anodic polarization, the total charge increased with increasing potential. At rather high anodic polarization, the total charge reached a maximum value and became almost independent of the applied potential. The potential depend- ence of the total charge with applied potential was similar to that of the removal rate shown in Fig. 3. Using Faraday’s law, the removal rate of Al resulting from electrochemical dissolution or corrosion contribution (R
c) is also shown in Fig. 5.
The effect of contact pressure on R
tat constant applied potential is depicted in Fig. 6. The results showed that the total metal removal
rate increased with increasing contact pressure, indicating that the Preston relation
8was obeyed. The highest dependence on contact pressure was found at 1500 mV.
Figure 7 shows the ratio (in percentage) of corrosion contribution to the total removal rate at 5 and 9 psi contact pressures. Most of the data fell in the band as shown in this figure. A relatively high scat- tering of data, not shown in Fig. 7, was found at a contact pressure of 3 psi. The results indicated that the contribution from corrosion to the total removal rate increased as the applied potential increased, particularly at contact pressures of 5 and 9 psi. At 21000 mV, only about 20% of the total removal rate was derived from electrochemi- cal dissolution. At much high anodic potentials, the total removal rate resulting from corrosion could be as high as 70%.
Figure 4. Variations of current density with time of Al in 5 vol % H
3PO
41 0.5 M citric acid 1 5 wt % Al
2O
3slurry at different applied potentials, under CMP at a contact pressure of 5 psi and at 100 rpm.
Figure 5. Effects of contact pressure and applied potential on the total charge and removal rate resulting from electrochemical reaction of Al in 5 vol % H
3PO
41 0.5 M citric acid 1 5 wt % Al
2O
3slurry at 100 rpm.
Figure 6. Variation of removal rate of Al in 5 vol % H
3PO
41 0.5 M citric acid 1 5 wt % Al
2O
3slurry at 100 rpm with polishing pressure at different applied potential.
Figure 7. Contribution of corrosion to the total removal rate of Al in 5 vol %
H
3PO
41 0.5 M citric acid 1 5 wt % Al
2O
3slurry at 100 rpm and at various
contact pressures.
EIS measurements.—EIS measurements were conducted during CMP after the start-up of CMP for different time intervals. Figure 8a shows the Nyquist plots for Al in the testing slurry at 21000 mV after the start-up of CMP at 100 rpm and 5 psi for 10, 1800, and 3600 s. Two semicircular loops, indicating the presence of two time
constants were found for each condition. The close similarity among these curves indicated that the surface property did not vary with time at 21000 mV. At 1500 mV, the Nyquist plots for Al after CMP for different times were shown in Fig. 8b. Two semicircles were still observed. But the semicircle of low frequency part enlarged as the time interval increased, indicating the time dependent electrochemi- cal behavior of the surface. The increase in the impedance was be- lieved due to the thickening of the passive film even during CMP.
The results shown in Fig. 8a and b reveal that the electrochemi- cal reaction at the Al/slurry interface could be simulated by an equiv- alent circuit as depicted in Fig. 8c. The equivalent circuit consists of the elements corresponding to solution resistance R
s, double-layer capacitance C
dl, charge-transfer resistance R
ct, film capacitance C
f, and film resistance R
f. The polarization resistance R
pis the sum of R
ctand R
f. The simulated circuit fits the data points very well as shown in Fig. 8a and b. The equivalent circuit has also been applied to the Al and Al alloys/NH
4Cl systems,
9-11and Al/phosphoric acid base slurry system.
12Based on the EIS data obtained, the values of the elements depicted in Fig. 8c are summarized in Tables I and II.
AES analysis.—The concentration (in terms of Auger electron intensity) profiles along the thickness direction (estimated from the sputtering time) for Al, O, and P elements after CMP at various con- ditions were determined. Figure 9a shows the results for the speci- men after polishing in deionized (DI) water with 5 wt % Al
2O
3for 30 min. The concentration profiles for the specimens polished in 5 vol % phosphoric acid 1 0.5 M citric acid with 5 wt % Al
2O
3slur- ry at 21000 and 1500 mV are shown in Fig. 9b and c, respectively.
The enrichment of O near the specimen surface indicated that an oxide film was formed on the surface. The thickness (in terms of sputtering time) of the oxide film might be estimated from the con- centration profile at the point where the intensity of Al was equal to that of O. Accordingly and as expected, the oxide film formed at 1500 mV was much thicker than that at low potentials. The results also showed that the passivation rate in DI water was quite low. The detection of P suggested that aluminum phosphate might exist on the surface, which has also been reported elsewhere.
13,14It was noted that phosphate was identified at 21000 mV but not at 1500 mV (Fig. 9b and c). At more negative potentials, the oxide film formed was thinner than that formed under high anodic polarization condi- tion. As a result, the ratio of the amount of phosphate to the amount of oxide was higher as compared with that obtained at high anodic potentials. Therefore, P was only detected at 21000 mV rather than at 1500 mV.
Discussion
Based on the mechanism proposed by Kaufman et al.,
1CMP involves the repeated processes of passive film formation, removal and repassivation of passive film. According to this model, the removal rate depends on the formation and abrasion of the passive film. In other words, the presence of surface film is necessary and essential in the process of CMP. As can be seen in Fig. 2, Al could be easily passivated in the phosphoric base slurry used in this inves- tigation in static and load free condition. At a platen rotation speed of 100 rpm and at a different applied contact pressure, a shift of the corrosion potential toward the negative direction was found. This in- dicated that mechanical abrasion could assist in maintaining the specimen surface in fresh condition, which consequently caused the corrosion potential to move toward negative direction. It is known that Al could be easily repassivated with a high repassivation rate,
15-19a passive film might be formed again at a very fast rate after the film was destroyed. The existence of a passive region under each rotating and abrasive condition as shown in Fig. 2 was attributed to the high repassivation rate of Al in the testing slurry. Under rotating and abrasive conditions, however, the passive film formed was sub- jected to a successive mechanical force induced either from the pol- ishing pad or the flowing abrasive alumina particles. As a result, the repassivated film might not provide a complete coverage of the metal surface. Furthermore, under a chronic abrasive condition, the surface Figure 8. Nyquist plots for Al, determined during CMP, in 5 vol % H
3PO
41
0.5 M citric acid 1 5 wt % Al
2O
3slurry after the start-up of CMP for 10,
1800, and 3600 s at 5 psi and at 100 rpm: (a) 21000 mV
SCE, (b)
1500 mV
SCE, and (c) the corresponding equivalent circuit.
film formed might not be compacted and protective but rather porous in nature. Therefore, a high passive current density was observed as compared with that in a static and load free condition.
During CMP at 100 rpm and 5 psi contact pressure, the repassi- vation reaction continued as indicated by the drop of current density
as shown in Fig. 4. The fact that two semicircles present in the Nyquist plot determined during Al CMP in phosphoric acid base slurry also indicated the existence of a passive film on the specimen surface (Fig. 8a and b). These experimental results gave a clear indi- cation that the repassivation rate of Al was certainly extremely rapid.
Table I. Electrochemical parameters estimated from EIS measurements of Al CMP in 5 vol % H
3PO
41 0.5 M citric acid 1 5 wt % Al
2O
3slurry at 100 rpm and 21000 mV
SCE.
Time after starting CMP
Rs Cf Rf Cdl Rct Rp(s) ( V cm
2) ( mF) ( V cm
2) (mF) ( V cm
2) ( V cm
2)
0 010
70.04 5.203 231 10.830 127 358
1800 56.54 7.265 231 8.276 104 335
3600 58.52 6.925 229 7.021 102 331
Table II. Electrochemical parameters estimated from EIS measurements of Al CMP in 5 vol % H
3PO
41 0.5 M citric acid 1 5 wt % Al
2O
3slurry at 100 rpm and 1500 mV
SCE.
Time after starting CMP
Rs Cf Rf Cdl Rct Rp(s) ( V cm
2) ( mF) ( V cm
2) (mF) ( V cm
2) ( V cm
2)
0 010
70.52 2.660 235 1.724 1827 2062
1800 63.20 3.316 359 2.076 2328 2687
3600 59.76 3.428 356 1.908 5461 5817
Figure 9. AES for Al after CMP at 5 psi and 100 rpm for 30 min in (a, top left) distilled water 1 5 wt % Al
2O
3slurry, (b, above) 5 vol % H
3PO
41 0.5 M citric acid 1 5 wt % Al
2O
3slurry, 21000 mV
SCE, and (c, left) in 5 vol
% H
3PO
41 0.5 M citric acid 1 5 wt % Al
2O
3slurry, 1500 mV
SCE.
The removal rate of metal in CMP might sometimes be described to follow the Preston equation,
8namely
R 5 K
P?P?V
where R is the removal rate, K
Pis a constant, P is the pressure ap- plied, and V is the relative velocity between the surface and the abra- sive materials such as pad or solid particles. The experimental results shown in Fig. 6 revealed that the Preston relation was obeyed for Al in 5 vol % phosphoric acid 1 0.5 M citric acid of pH 4 with 5 wt % Al
2O
3abrasive particles. The slopes in Fig. 6 increased as the ap- plied potential increased, indicating that K
Pwas a function of applied potential.
Concerning that the removal rate was dependent on the amount of passive film removed as proposed by Kaufman et al.,
1a higher passive film formation rate would favor a higher metal removal rate.
At high anodic polarization (i.e. 1500 mV), a much thicker of pas- sive film on the Al surface would be formed per unit of time as com- pared with that at low potentials. Therefore, more amount of passive film could be removed at a high anodic polarization condition, which subsequently resulted in a higher total removal rate of Al as demon- strated in Fig. 6. At low anodic potential or under cathodic polariza- tion, mechanical abrasion of metal besides passive film would occur.
A lower removal rate was thus observed.
The dissolution or oxidation of Al in aqueous environment would lead to oxide or hydroxide precipitation due to their low solubility of product. Therefore, the average dissolution rate might be considered as the passivation rate. At low anodic potential, the dissolution rate would be small on the film free surface. As the potential increased, the dissolution rate increased on the bare surface with most of the Al
13ion produced incorporated into the passive film with a small amount in ionic form dissolved in the electrolyte. But since the film could be destroyed under stress, the surface might be only partially passivated. These combined effects led to the increasing contribution of metal dissolution to the total removal rate as the potential was raised as depicted in Fig. 7.
Since full passivation might not be accomplished at low anodic potential, metal in addition to passive film could be abraded away during CMP. The absence of a blanket passive film on metal surface has been reported by Stein et al.,
7Kneer et al.,
5and Tsai et al.
20The experimental results obtained in this study were in agreement with those appearing in the literature, suggesting that the passive film abrasion proposed by Kaufman et al.,
1was not the sole fact deter- mining the total removal rate. As the potential increased, the contri- bution of film to the total metal loss also became important due to the increase of passive film formed. Thus, the percentage of metal corrosion to the total metal removal rate increased with increasing potential as shown in Fig. 7. At higher anodic potentials, perhaps the passive film formed was so thick that it could not be totally removed.
Furthermore, at 100 rpm and at a contact pressure applied (3-9 psi), the passive film removal rate might reach a saturation level and almost equal the repassivation rate. Thus, the percentage of corro- sion contribution to the total removal rate also reached a high con- stant value (approximately 70%). A synergistic effect between elec- trochemical and mechanical reactions always exists in wear corro- sion or erosion corrosion processes.
21-25This might also account for the high total removal rate observed at high anodic potential as revealed in Fig. 7.
It was found that the potential dependence of the removal rate could be divided into three regions (Fig. 3). Similar observations have been found in the wear corrosion of stainless steel in chloride solution
21and in the erosion corrosion of Al in silica aqueous slur- ries.
22It has been pointed out that the wear rate of stainless steel in chloride solution determined at cathodic potential was caused by mechanical action because the steel was cathodically protected.
21The wear rate was independent of potential under cathodic polariza- tion. In this investigation, however, the removal rate of Al increased as the cathodic polarization increased (more negative potential) with a contact pressure of 5 and 9 psi. The increased removal rate might be associated with the increasing tendency to H embrittlement re-
sulting from H pick-up under cathodic polarization in acidic slurry.
But since metal dissolution was negligible at cathodic potential, a low metal removal rate mainly caused by mechanical wear of metal was observed (region in Fig. 3).
At low anodic polarization, the surface was partially passivated under rotating and abrasive condition as described above. Mechani- cal wear of both metal and oxide would occur. The metal dissolution also proceeded in the film free or film damaged area. Since the dis- solution rate increased with increasing anodic polarization, the total metal removal rate increased as depicted as region II in Fig. 3. In region III, a balance between passive film abrasion rate and the repassivation rate was achieved, hence, the total removal rate became independent of potential.
A schematic diagram illustrating the effect of potential on Al removal in CMP is given in Fig. 10. In region I (at cathodic poten- tials), the amount of metal removed is dominated by mechanical wear. At low anodic potential region, metal removal caused by the abrasion of either metal or passive film on the partially passivated surface is responsible for metal removal. At high anodic potentials (region III), the repassivation rate is high enough and the passive film is rather thick. The total removal rate is determined by how fast the passive film is abraded away.
Conclusions
Potentiodynamic polarization results indicate that Al could be pas- sivated in 5 vol % H
3PO
41 0.5 M citric acid 1 5 wt % Al
2O
3slurry.
However, contact pressure in the range of 3 to 9 psi would cause a decrease of corrosion potential of about 360 mV and an increase of passive current density of about two order of magnitude for Al in the slurry under rotating condition. The removal rate of Al increased with increasing contact pressure and was a strong function of applied potential. Basically, a low removal rate was observed under cathodic
Figure 10. Schematic diagram illustrating the effect of potential and the
interaction between the electrochemical and mechanical reactions on CMP.
polarization, which was dominated by mechanical wear of metal. At low anodic potentials, the removal rate increased with increasing po- tential. The contribution of film formation and abrasion to the total re- moval rate became increasingly important as the applied potential increased. At much higher anodic polarization, a balance between the repassivation rate and the film abrasion rate gave rise to a high removal rate which became independent of potential.
Acknowledgment
The authors are grateful for the support of this research by National Science Council of the Republic of China under contract no. NSC 87-2216-E-006-019. The authors would also like to thank R. C. Lee for AES analysis.
National Cheng Kung University assisted in meeting the publication costs of this article.
References
1. F. B. Kaufman, D. B. Thompson, R. E. Broadie, M. A. Jaso, W. L. Guthrie, D. J.
Pearson, and M. B. Small, J. Electrochem. Soc., 138, 3460 (1991).
2. D. Zeidler, Z. Stavreva, M. Plötner, and K. Drescher, Microelectron. Eng., 33, 259 (1997).
3. M. A. Fury, D. L. Scherber, and M. A. Stell, Mater. Res. Soc. Bull., 61 (Nov 1995).
4. C. C. Yu, T. T. Doan, and A. E. Laulusa, U.S. Pat. 5,209,816 (1993).
5. E. A. Kneer, C. Raghunath, V. Mathew, S. Raghavon, and J. S. Jeon, J. Elec-
trochem. Soc., 144, 3041 (1997).
6. W. T. Tseng, J. Wu, Y. S. Chang, Mater. Res. Soc. Symp. Proc., 477, 125 (1997).
7. D. J. Stein, D. Hetherrington, T. Guilinger, and J. L. Cerchi, J. Electrochem. Soc., 145, 3190 (1998).
8. L. M. Cook, J. Non-Cryst. Solids, 120, 152 (1990).
9. S. E. Hernandez, A. J. Griffin, Jr. , F. R. Brotzen, and C. F. Dunn, J. Electrochem.
Soc., 142, 1215 (1995).
10. A. J. Griffin, Jr., F. R. Brotzen, and C. F. Dunn, J. Electrochem. Soc., 141, 3473 (1994).
11. A. J. Griffin, Jr., F. R. Brotzen, and C. F. Dunn, J. Electrochem. Soc., 139, 699 (1992).
12. H. S. Kuo and W. T. Tsai, J. Electrochem. Soc., 147, 149 (2000).
13. A. A. Mazhar, F. E. Heakal, and K. M. Award, Thin Solid Films, 192, 193 (1990).
14. G. E. Thompson, R. C. Furneaux, G. C. Wood, and R. Hutehings, J. Electrochem.
Soc., 125, 1480 (1978).
15. F. P. Ford, G. T. Burstein, and T. P. Hoar, J. Electrochem. Soc., 127, 1325 (1980).
16. T. Hagyard and W. B. Earl, J. Electrochem. Soc., 114, 694 (1967).
17. T. Hagyard and W. B. Earl, J. Electrochem. Soc., 115, 623 (1968).
18. G. S. Frankel, C. V. Jahnes, V. Brusic, and A. J. Davenport, J. Electrochem. Soc., 142, 2290 (1995).
19. G. S. Frankel, B. M. Rush, C. V. Jahnes, and C. E. Farrell, J. Electrochem. Soc., 138, 643 (1991).
20. W. T. Tsai and C. M. Huang, Thin Solid Films, Accepted for publication (1999).
21. H. Abd-El-Kader and S. M. El-Raghy, Corros. Sci., 26, 647 (1986).
22. Y. Li, G. T. Burstein, and I. M. Hutchings, Wear, 181-183, 70 (1995).
23. S. Zhou, M. M. Stack, and R. C. Newman, Corrosion, 52, p.934 (1996).
24. M. H. Hong and S. II. Pyun, Wear, 147, 59 (1991).
25. A. B. Ijzermans, Wear, 14, 397 (1969).