Ms No.: QJM-2014-608, QJM
The Risk of Chronic Kidney Disease in Tuberculosis:
A Population-based Cohort Study
Running head: Risk of CKD in TB
— Original Article —
Te-Chun Shen 1, 2, Kuo-Yang Huang 2, Chih-Hao Chao 3, Yu-Chiao Wang 4, Chih-Hsin Muo 4, Chang-Ching Wei 5, Chih-Yen Tu 2, Te-Chun Hsia 2, Chuen-Ming Shih 2, Wu-Huei Hsu 2, Fung-Chang Sung 1, Chia-Hung Kao 1, 6,
1 Graduate Institute of Clinical Medicine Science, College of Medicine, China
Medical University, Taichung, Taiwan;
2 Division of Pulmonary and Critical Care Medicine, Department of Internal
Medicine, China Medical University Hospital, China Medical University, Taichung,
Taiwan;
3 Division of Pulmonary Medicine, Department of Internal Medicine, Chang Bing
Show Chwan Memorial Hospital, Changhua, Taiwan;
4 Management Office for Health Data, China Medical University Hospital, China
Medical University, Taichung, Taiwan;
5 Division of Nephrology, Department of Pediatrics, China Medical University
Hospital, China Medical University, Taichung, Taiwan;
6 Department of Nuclear Medicine and PET Center, China Medical University Hospital, China Medical University, Taichung, Taiwan
Corresponding author:
Chia-Hung Kao, MD Professor
1 Graduate Institute of Clinical Medicine Science, College of Medicine, China
Medical University, Taichung, Taiwan
2 Department of Nuclear Medicine and PET Center, China Medical University
Hospital, China Medical University, Taichung, Taiwan
Postal address: No. 91 Hsueh-Shih Road, Taichung 404, Taiwan E-mail address: d10040@mail.cmuh.org.tw
Telephone: +886-4-22053366 Ext 7412
Keywords: tuberculosis (TB); chronic kidney disease (CKD); epidemiology.
Competing interests: there are no competing interests.
Funding information:
This work was supported by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW103-TDU-B-212-113002). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study.
Abstract
Background:
The relationship between tuberculosis (TB) and subsequent chronic kidney disease (CKD) remains unclear. Therefore, we examined the risk of CKD among TB patients
in a nationwide study.
Methods:
We conducted a retrospective cohort study using data from the National Health Insurance (NHI) system of Taiwan. The cohort included 8,735 patients who were newly diagnosed with TB. Patients were recruited between 1998 and 2002, and the date of diagnosis was defined as the index date. Each patient was randomly matched with four people from the general population without TB, according to age, gender, and the index year. The occurrence of CKD was followed up until the end of 2011.
The relative risks of CKD were estimated using the Cox proportional hazard model
after adjusting for age, gender, index year, and comorbidities.
Results:
The overall incidence of CKD was 1.27-fold greater in the TB cohort than in the non-TB cohort. The adjusted hazard ratio (HR) of CKD associated with TB was higher in women (1.72; 95% CI, 1.33–2.22), those aged less than 50 years (1.67; 95%
CI, 1.15–2.41), and those without comorbidities (1.39; 95% CI, 1.06–1.83). In addition, patients with more comorbidities among hypertension, diabetes, and
hyperlipidemia have a greater risk of developing CKD in both cohorts, and the
adjusted HRs were higher in the TB cohort than in the non-TB cohort.
Conclusion:
TB patients had a significantly higher risk of developing CKD than the general population. The detailed mechanisms need further investigation.
Introduction
Tuberculosis (TB) is an infectious disease caused by Mycobacterium tuberculosis, which can affect multiple organs. It remains the most prevalent infectious disease and major leading cause of death worldwide. Every year about 9 million people develop this disease, and 1.4 million die of it, over 95% of them are from developing countries (1). TB is endemic in Taiwan; more than 10,000 new TB cases are diagnosed every year. The annual incidence is approximately 62–75 per 100,000 people (2). TB is associated with several factors, including age (3), sex (4), malnutrition (5), diabetes mellitus (6), organ transplantation (7–9), malignancy (10), human immunodeficiency
virus (HIV) infection (11), and chronic renal disease (12).
Chronic kidney disease (CKD) is a progressive loss in renal function over a period of months or years. The symptoms of worsening kidney function are non-specific, and might include feeling generally unwell and experiencing a reduced appetite. It is differentiated from acute kidney injury in that the reduction in kidney function must be present for over 3 months. Taiwan is reported to have the highest incidence and prevalence of CKD worldwide; the overall prevalence of end stage renal disease (ESRD) is 6.9% for CKD stages 3–5 and 11.9% for CKD stages 1–5 (13). The four most common causes of CKD in Taiwan are diabetes mellitus, chronic glomerulonephritis, hypertension, and chronic interstitial nephritis (13).
CKD patients have higher risks of developing TB than the general population, a result of CKD itself or treatment-related immunosuppression. Li et al. conducted a 9- year longitudinal cohort study and found that the risk of TB is 4.5 times greater in ESRD patients than the general population (14). However, TB is considered to be a chronic infectious disease and the causality between TB and CKD remained unknown. There is also some evidence that TB may result in CKD and ESRD in several case reports (15–17). Furthermore, several chemotherapeutic agents that are used for TB treatment, including rifampin, isoniazid, and ethambutol, have
nephrotoxicity (18).
Therefore, we attempted to determine if there is an increased risk of CKD in patients with TB by conducting a nationwide, population-based retrospective cohort study in Taiwan with data obtained from the National Health Insurance Research Database (NHIRD).
Materials and Methods
Data Source
The Taiwan Department of Health launched the social insurance system, the National Health Insurance (NHI) program, which requires only minimal copayment fees and covers more than 99% of Taiwanese (23.74 million). We used the Longitudinal Health Insurance Database (LHID), established by the National Health Research Institutes (NHRI), Ministry of Health and Welfare, Taiwan, for this study.
The LHID database includes claims data from 1996 to 2011 for one million people covered in the Taiwan NHI program. The LHID database has information on basic patient demographics and medical care received, including outpatient and inpatient claims. The International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) diagnostic codes are accurate and a valid tool for identifying patients with specific diseases. Personal identification information was encrypted before the release of the research database to protect patient privacy and data security. This study was censored by the institutional review board of China
Medical University (CMU-REC-101-012).
Study design and Study participants
This study is a retrospective, population-based cohort study. We identified 8,735 subjects with TB (ICD-9-CM code 010–018) from 1998 to 2002 as the TB cohort, and the date of diagnosis as the index date. Four control subjects were randomly selected
for each TB case, frequency-matched for age, sex, and index year using a non-TB cohort (n = 34,940). The risk of developing CKD (ICD-9-CM 585) was calculated in both cohorts. Subjects in both cohorts with a history of any kidney disease (ICD-9- CM 580–589) before the index date were excluded from this study. The urbanization level of where the study subjects resided were also included in this study. There are seven levels of urbanization in Taiwan, divided on the basis of the Taiwan National Health Research Institutes report, with the most urbanized area as level 1. Because the number of subjects is too small in levels 6 and 7, we combined urbanization levels 5 to 7 into level 5. All of the study subjects were followed from the index date until the diagnosis of CKD, withdrawal from the LHID database, or December 31, 2011.
Subjects with history of hypertension (ICD-9-CM 401–405), diabetes (ICD-9-CM 250), and/or hyperlipidemia (ICD-9-CM 272) were identified before the index date to
deal with the potential confounding risk factor of CKD.
Statistical analysis
To compare the differences of demographic status and proportion of comorbidity between the TB cohort and non-TB cohort, Student's t-test for continuous variables and Chi-square test for categorical variables was used. The incidence rate of CKD was calculated by using the number of CKD incidents divided by the number of people (per 1,000 person-years) at risk in both cohorts. We used the Cox proportional
hazards model to evaluate the crude hazard ratios (HR) and the 95% confidence interval (CI) of CKD in the TB and non-TB cohorts for various variables. After controlling for potential confounding risk factors including sex, age, urbanization, and comorbidities, we calculated the adjusted HR and 95% CI of CKD in both cohorts by the multivariable Cox proportional hazards model. We also used the Cox model to estimate the HR of CKD associated with comorbidity, compared to the non-TB
cohort.
All statistical analyses used SAS 9.3 statistical package (SAS Institute Inc., NC, USA), with p value < 0.05 in two-tailed tests considered significant. We also utilized Statistical Product and Service Solutions, IBM, Chicago (SPSS statistics (version 18.0)), to conduct a Kaplan-Meier analysis to measure the cumulative CKD incidence for TB and comparison cohorts, and used the log-rank test to estimate the differences between the two cumulative incidence curves.
Results
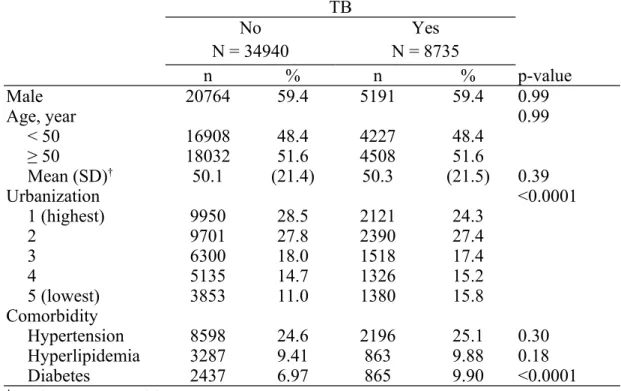
We analyzed 8,735 patients in the TB cohort and 34,940 participants in the non-TB cohort (Table 1). After frequency matching, the TB and non-TB cohorts had similar distributions of sex and age; a majority of the subjects enrolled in this study were male (59.4%) and over 50 years old of age (51.6%). We calculated the mean age ± standard deviation (SD) by Student's t test; 50.3 ± 21.5 years old for the TB cohort and 50.1 ± 21.4 years old for the non-TB cohort. In both cohorts, over 50% of subjects lived in an urbanization level of 1 and 2 (51.7% for the TB cohort and 56.3%
for the non-TB cohort). Patients with TB showed higher proportions of comorbidity than the non-TB cohort, including hypertension (25.1% vs. 24.6%), hyperlipidemia (9.88% vs. 9.41%), and diabetes (9.9% vs. 6.97%), but only diabetes achieved
significance.
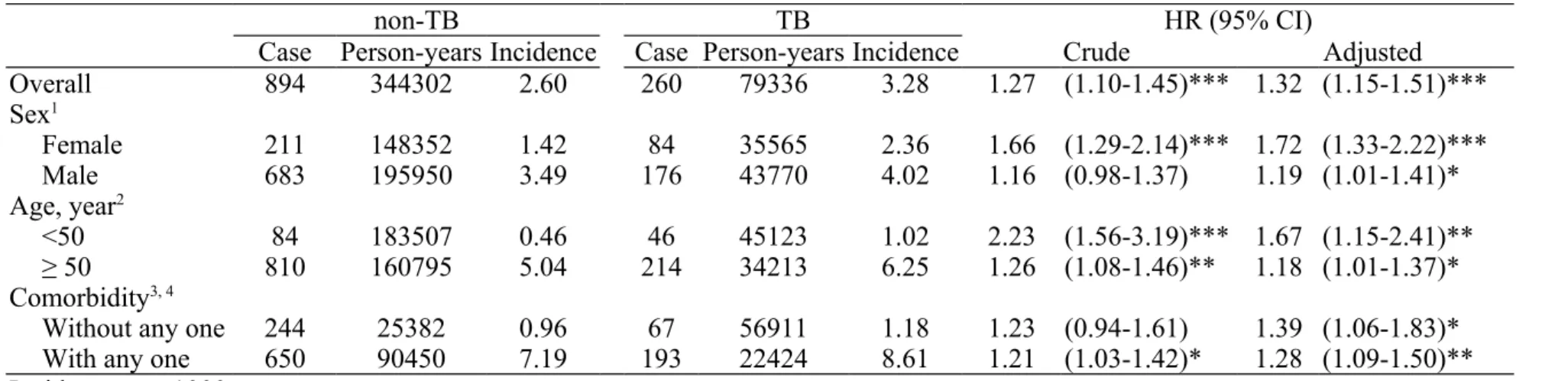
Table 2 shows the overall incidence of CKD, which was 27% higher in the TB cohort than in the non-TB cohort (3.28 vs. 2.60 per 1000 person-years). The crude HR of CKD was 1.27 (95% CI: 1.10–1.45), and adjusted HR of CKD was 1.32 (95% CI:
1.15–1.51) in the TB cohort, compared to subjects without TB. After comparing the adjusted HR of CKD in the TB cohort with the non-TB cohort by stratified analyses of sex, the adjusted HR was 1.72 (95% CI: 1.33–2.22) in females and 1.19 (95% CI:
1.01–1.41) in males. The incidence of CKD was higher in TB patients with age over
50 years, and the adjusted HR was 1.18 (95% CI: 1.01–1.37). The incidence of CKD was higher in TB patients with any comorbidity, and the adjusted HR was 1.28 (95%
CI: 1.09–1.50).
We also evaluated the contribution comorbidities to the risk of CKD. The incidence of CKD was higher as the number of comorbidities increased in both cohorts (Table 3). In the non-TB cohort, the adjusted HR was increased with greater number of comorbidities, with an adjusted HR of 3.05 (95% CI: 2.57–3.62) with a single comorbidity, 4.37 (95% CI: 3.59–5.32) with two types of comorbidity, and 7.97 (95%
CI: 6.19–10.3) with three types of comorbidity, compared to without any comorbidity.
Similarly, the distinct dose effect between the number of comorbidities and CKD risk was obvious in the TB cohort. Compared to the TB cohort without any comorbidity, the adjusted HR was 3.28 (95% CI: 2.57–4.20) with a single comorbidity, 7.83 (95%
CI: 6.03–10.2) with two types of comorbidity, and 10.1 (95% CI: 6.78–15.1) with three types of comorbidity. Figure 1 shows that the cumulative incidence of CKD by the end of the follow-up period was higher in the TB cohort than in the non-TB cohort (log-rank test, p = 0.002).
Discussion
To the best of our knowledge, this is the first nationwide, population-based study evaluating the relationship between TB and the subsequent risk of CKD. We identified a significant risk of CKD among TB patients compared to the general population. Further analyses showed that the adjusted HR was higher in females than males, in young than elderly, and in ones without comorbidity than those with any comorbidity. In addition, we found participants with greater comorbidities had a higher incidence of developing CKD in both cohorts, and the adjusted HRs were higher in the TB cohort than in the non-TB cohort. We suggest that TB may play an
independent role in the development of CKD.
There is little information on the contribution that TB makes to the burden of renal disease. Data obtained from the European Dialysis and Transplant Association registry revealed 0.65% of patients had renal failure caused by TB in 1991 (19).
Another data on primary renal diagnosis due to TB were 0.7% in Europe, 0.16% in Australasia, and 0.004% in the United States (20). However, we should make it clear that, in our study, the CKD incidence in the TB cohort (3.48 per 1000 person-years) did not mean the causal effect was only due to TB. Furthermore, all stages of CKD in our study were enrolled, not only ESRD. This is because we considered at most a 13- year follow-up period, so the effect of TB may influence renal function but may not
have dramatic effects within this time period.
It is believed that 8% to 15% of patients with pulmonary TB develop infection in the genitourinary system via hematogenous spray (21). Genitourinary TB was the second most common extrapulmonary TB and was a commonly missed genitourinary infection (22). Genitourinary TB should be considered in any case of urinary symptoms, such as pyeuria or hematuria, which does not respond to common antibacterial drugs. sterile pyuria, voiding symptoms, resistant urinary tract infection, obstructive nephropathy, interstitial nephritis, or rarely, amyloidosis or glomerulonephritis (23). The kidney is the most common genitourinary organ to be affected (24). The clinical manifestations of renal TB are nonspecific. In most cases, the disease results in calcification, atrophy, or necrosis of the renal parenchyma (25).
Features of renal TB in imaging studies are multiple and complex. Tubercular granulomas in the renal pyramids coalesce to form ulcers. The lesions enlarge, and a tubercular abscess may form in the parenchyma. Later in the course of the disease, a perinephric abscess forms, and the kidney is replaced by caseous material (“putty kidney”), which may become calcified (“cement kidney”) and nonfunctional (26).
Genitourinary TB is often secondary to pulmonary TB; however, the bacilli can reach the genitourinary system even after the primary focus has healed or is quiescent (16).
The pathophysiology through which how TB damages the kidney and affects renal
function is still not fully understood. First, a tuberculous kidney becomes calcified and gross anatomic distortion occurs. Not surprising, the glomerular filtration rate (GFR) falls and usually, will not recover (27). Second, TB can induce chronic granulomatous tubulointerstitial nephritis, even if imaging shows equal-sized smooth kidneys (16, 28). Third, TB associates with various forms of glomerulonephritis. In some situations, complications occur due to TB-related amyloidosis (29). Shribman et al. have reported a case with military TB presented with immune deposits and focal proliferative glomerulonephritis (30). Furthermore, any ureteral obstruction with
resultant hydronephrosis can lead to irreversible changes in renal function (17).
Another concern we should always keep in mind is related to drug complications.
Kidney injury is a rare and severe complication during anti-TB treatment that can cause permanent kidney damage (31). Rifampin is the most common anti-TB drug responsible for kidney injury, although isoniazid and ethambutol have also been reported (32–35). Chang et al. reported 7.1% (99/1394) patients on anti-TB treatment were identified to have acute kidney injury (AKI), 30% (30/99) of patients had comorbid CKD or ESRD, and 27% (26/99) of patients did not recover from AKI in 6 months (18). However, this study may not provide an enough follow-up time to observe the subsequent effects of anti-TB medications.
In addition, it is common knowledge that TB patients are typically characterized
by low educational attainment, low financial status, blue-collar occupations, rural dwelling, low body mass index, malnutrition, immunocompromised status, and potential corticosteroid use. These attributes may be shared risk factors in both diseases, and potential confounding factors that cannot be totally corrected in this
study.
With regard to the validity of diagnosis, TB could be diagnosed by its typical presentation and most by bacteriological or pathological evidence. Taiwan lists TB as a type 3 notifiable disease; every newly diagnosed case has to be reported within a week to the Taiwan Centers for Disease Control (CDC). The government takes part in the treatment of every TB patient. Therefore, the data regarding TB diagnoses were
reliable (36–38).
A major strength of our study is that it was performed using population-based data that are highly representative of the general population. However, certain limitations should be considered. First, the detailed stages of CKD in this study were unavailable.
Second, NHIRD does not contain detailed information regarding smoking habits, diet preference, occupational exposure, drug history, and family history of systemic diseases, all of which may be risk factors for TB and CKD. In addition, evidence derived from a retrospective cohort study is generally lower in statistical quality than that from a randomized trial because of potential biases associated with adjustments
for confounding variables. Despite our meticulous study design and adjustment for confounding factors, biases resulting from unknown confounders may have affected our results. Last, all data in the NHIRD are anonymous. Therefore, relevant clinical variables such as serum laboratory analyses, pulmonary function tests, imaging results, and pathology findings of the subjects were unavailable in our study.
Conclusion
TB patients had a significantly higher risk of developing CKD than the general population. The detailed mechanisms need further investigation.
References
1. Lawn SD, Zumla AI. Tuberculosis. Lancet 2011; 378: 57–72.
2. Lo HY, Chou P, Yang SL, Lee CY, Kuo HS. Trends in tuberculosis in
Taiwan, 2002-2008. J Formos Med Assoc 2011; 110: 501–10.
3. Donald PR, Marais BJ, Barry CE 3rd. Age and the epidemiology and
pathogenesis of tuberculosis. Lancet 2010; 375: 1852–4.
4. Allotey P, Gyapong M. Gender in tuberculosis research. Int J Tuberc Lung Dis
2008; 12: 831–6.
5. Cegielski JP, McMurray DN. The relationship between malnutrition and tuberculosis: evidence from studies in humans and experimental animals. Int J
Tuberc Lung Dis 2004; 8: 286–98.
6. Dooley KE, Chaisson RE. Tuberculosis and diabetes mellitus: convergence of
two epidemics. Lancet Infect Dis 2009; 9: 737–46.
7. Munoz P, Palomo J, Munoz R, Rodriguez-Creixems M, Pelaez T, Bouza E.
Tuberculosis in heart transplant recipients. Clin Infect Dis 1995; 21: 398–402.
8. Meyers BR, Halpern M, Sheiner P, Mendelson MH, Neibart E, Miller C.
Tuberculosis in liver transplant patients. Transplantation 1994; 58: 301–6.
9. Akan H, Arslan O, Akan OA. Tuberculosis in stem cell transplant patients. J Hosp Infect 2006; 62: 421–6.
10. Kamboj M, Sepkowitz KA. The risk of tuberculosis in patients with cancer.
Clin Infect Dis 2006; 42: 1592–5.
11. Antonucci G, Girardi E, Raviglione MC, Ippolito G. Risk factors for tuberculosis in HIV-infected persons. A prospective cohort study. The Gruppo
Italiano di Studio Tubercolosi e AIDS (GISTA). JAMA 1995; 274: 143–8.
12. Hussein MM, Mooij JM, Roujouleh H. Tuberculosis and chronic renal disease.
Semin Dial 2003; 16: 38–44.
13. Hwang SJ, Tsai JC, Chen HC. Epidemiology, impact and preventive care of chronic kidney disease in Taiwan. Nephrology (Carlton) 2010; 15 Suppl 2: 3–
9.
14. Li SY, Chen TJ, Chung KW, Tsai LW, Yang WC, Chen JY, et al.
Mycobacterium tuberculosis infection of end-stage renal disease patients in Taiwan: a nationwide longitudinal study. Clin Microbiol Infect 2011; 17:
1646–52.
15. Daher Ede F, Silva Junior GB, Damasceno RT, Santos GM, Corsino GA, Silva SL, et al. End-stage renal disease due to delayed diagnosis of renal
tuberculosis: a fatal case report. Braz J Infect Dis 2007; 11: 169–71.
16. Zumrutdal A, Yildiz I, Ozelsancak R, Canpolat T. Chronic renal failure:
unexpected late sequela of pulmonary tuberculosis after 30 years. Mikrobiyol Bul 2011; 45: 366–70.
17. de Oliveira JL, da Silva Junior GB, Daher Ede F. Tuberculosis-associated
chronic kidney disease. Am J Trop Med Hyg 2011; 84: 843–4.
18. Chang CH, Chen YF, Wu VC, Shu CC, Lee CH, Wang JY, et al. Acute kidney injury due to anti-tuberculosis drugs: a five-year experience in an aging
population. BMC Infect Dis 2014; 14: 23.
19. Eastwood JB, Zaidi M, Maxwell JD, Wing AJ, Pazianas M. Tuberculosis as
primary renal diagnosis in end-stage uraemia. J Nephrol 1994; 7: 290–3.
20. Maisonneuve P, Agodoa L, Gellert R, Stewart JH, Buccianti G, Lowenfels AB, et al. Distribution of primary renal diseases leading to end-stage renal failure in the United States, Europe, and Australia/New Zealand: Results from
an international comparative study. Am J Kidney Dis 2000; 35: 157–65.
21. Wise GJ , Merella VK. Genitourinary manifestations of tuberculosis. Urol
Clin North Am 2003; 30: 111–21.
22. Golden MP, Vikram HR. Extrapulmonary tuberculosis: an overview. Am Fam
Physician 2005; 72: 1761–8.
23.Waikhom R, Sarkar D, Bennikal M, Pandey R. Rapidly progressive glomerulonephritis in tuberculosis. Saudi J Kidney Dis Transpl 2014 ; 25:
872–5.
24.Dharmapalan A, Vijaykumar R, Bhoopal S. Renal tuberculosis presenting as thigh abscess. Indian J Surg 2013; 75 (Suppl 1): 446–8.
25.Kumar S, Shankaregowda SA, Choudhary GR, Singla K. Rare presentation of
genitourinary tuberculosis masquerading as renal cell carcinoma: a
histopathological surprise. J Clin Imaging Sci 2014; 4: 26.
26.Lima NA, Vasconcelos CC, Filgueira PH, Kretzmann M, Sindeaux TA, Feitosa Neto B, Silva Junior GB, Daher EF. Review of genitourinary tuberculosis with focus on end-stage renal disease. Rev Inst Med Trop Sao
Paulo 2012; 54: 57–60.
27. Eastwood JB, Corbishley CM, Grange JM. Tuberculosis and the kidney. J Am
Soc Nephrol 2001; 12: 1307–14.
28. Mallinson WJW, Fuller RW, Levison DA, Baker LRI, Cattell WR. Diffuse interstitial renal tuberculosis—An unusual cause of renal failure. QJMed 1981;
50: 137–48.
29. Chugh KS. Pattern of renal amyloidosis in Indian patients. Postgrad Med J
1981; 57: 31–5.
30. Shribman JH, Eastwood JB, Uff JS. Immune-complex nephritis complicating
miliary tuberculosis. Br Med J 1983; 287: 1593–4.
31. De Vriese AS, Robbrecht DL, Vanholder RC, Vogelaers DP, Lameire NH.
Rifampicin-associated acute renal failure: pathophysiologic, immunologic, and clinical features. Am J Kidney Dis: Off J Nat Kidney Found 1998; 31: 108–
15.
32. Qunibi WY, Godwin J, Eknoyan G. Toxic nephropathy during continuous
rifampin therapy. Southern Med J 1980; 73: 791–2.
33. Winter RJ, Banks RA, Collins CM, Hoffbrand BI. Rifampicin induced light
chain proteinuria and renal failure. Thorax 1984; 39: 952–3.
34. Trainin EB, Turin RD, Gomez-Leon G. Acute renal insufficiency
complicating isoniazid therapy. Int J Pediatric Nephrol 1981; 2: 53–4.
35. Kwon SH, Kim JH, Yang JO, Lee EY, Hong SY. Ethambutol-induced acute renal failure. Nephrol Dial Transpl: Off Publ European Dial Transpl Assoc
European Renal Assoc 2004; 19: 1335–6.
36. Lin YC, Liang SJ, Liu YH, Hsu WH, Shih CM, Sung FC, et al. Tuberculosis as a risk factor for systemic lupus erythematosus: results of a nationwide study
in Taiwan. Rheumatol Int 2012; 32: 1669–73.
37. Chung WS, Lin CL, Hung CT, Chu YH, Sung FC, Kao CH, et al.
Tuberculosis increases the subsequent risk of acute coronary syndrome: a nationwide population-based cohort study. Int J Tuberc Lung Dis 2014; 18:
79–83.
38. Chung WS, Lin CL, Chen YF, Hsu WH, Kao CH. Pulmonary tuberculosis increases the risk of pulmonary thromboembolism: a nationwide population- based cohort study. Thromb Haemost 2014; 112(5).
Table 1. Demographics between patients with and without tuberculosis TB
No N = 34940
Yes N = 8735
n % n % p-value
Male 20764 59.4 5191 59.4 0.99
Age, year 0.99
< 50 16908 48.4 4227 48.4
≥ 50 18032 51.6 4508 51.6
Mean (SD)† 50.1 (21.4) 50.3 (21.5) 0.39
Urbanization <0.0001
1 (highest) 9950 28.5 2121 24.3
2 9701 27.8 2390 27.4
3 6300 18.0 1518 17.4
4 5135 14.7 1326 15.2
5 (lowest) 3853 11.0 1380 15.8
Comorbidity
Hypertension 8598 24.6 2196 25.1 0.30
Hyperlipidemia 3287 9.41 863 9.88 0.18
Diabetes 2437 6.97 865 9.90 <0.0001
†Student's t test;Chi-square test
Table 2. Incidence and hazard ratio for CKD
non-TB TB HR (95% CI)
Case Person-years Incidence Case Person-years Incidence Crude Adjusted
Overall 894 344302 2.60 260 79336 3.28 1.27 (1.10-1.45)*** 1.32 (1.15-1.51)***
Sex1
Female 211 148352 1.42 84 35565 2.36 1.66 (1.29-2.14)*** 1.72 (1.33-2.22)***
Male 683 195950 3.49 176 43770 4.02 1.16 (0.98-1.37) 1.19 (1.01-1.41)*
Age, year2
<50 84 183507 0.46 46 45123 1.02 2.23 (1.56-3.19)*** 1.67 (1.15-2.41)**
≥ 50 810 160795 5.04 214 34213 6.25 1.26 (1.08-1.46)** 1.18 (1.01-1.37)*
Comorbidity3, 4
Without any one 244 25382 0.96 67 56911 1.18 1.23 (0.94-1.61) 1.39 (1.06-1.83)*
With any one 650 90450 7.19 193 22424 8.61 1.21 (1.03-1.42)* 1.28 (1.09-1.50)**
Incidence, per 1000 person-years
Adjusted model, adjusted for age, sex, urbanization and diabetes
1 Adjusted model, adjusted for age, urbanization and diabetes
2 Adjusted model, adjusted for sex, urbanization and diabetes
3 Adjusted model, adjusted for age, sex and urbanization
4 Comorbidity included gypertension, hyperlipidemia, and diabetes
Table 3. Incidence and hazard ratio for CKD and CKD-associated risk factor
non-TB TB
Case Person-years Incidence HR (95% CI) Case Person-years Incidence HR (95% CI) Comorbidity
0 244 253852 0.96 1.00 67 56911 1.18 1.35 (1.03-1.77)*
1 372 61444 6.05 3.05 (2.57-3.62)*** 90 15316 5.88 3.28 (2.57-4.20)***
2 192 23097 8.31 4.37 (3.59-5.32)*** 76 5602 13.57 7.83 (6.03-10.2)***
3 86 5910 14.55 7.97 (6.19-10.3)*** 27 1506 17.92 10.1 (6.78-15.1)***
Incidence, per 1000 person-years
Adjusted model, adjusted for age, sex and urbanization
*p<0.05, *** p<0.001
Figure 1. Kaplan-Meier analysis for CKD between TB and non-TB cohort
27