Research Express@NCKU Volume 13 Issue 5 - April 9, 2010 [ http://research.ncku.edu.tw/re/articles/e/20100409/4.html ]
Novel cathode structure for organic light-emitting
diodes
Tzung-Fang Guo
1*, Fuh-Shun Yang
1, J. C. A. Hunag
2, Tsung-Hsun Lee
2, and Ten-Chin Wen
3 1Institute of Electro-Optical Science and Engineering, Advanced Optoelectronic TechnologyCenter, National Cheng Kung University
2Department of Physical Engineering, National Cheng Kung University 3Department of Chemical Engineering, National Cheng Kung University
guotf@mail.ncku.edu.tw
Advanced Functional Materials 18, 3036-3042 OCT 9(2008)
1.
I
ntroductionOur recent works have shown that an improved “high-yellow” phenyl-substituted poly-(paraphenylenevinylene) copolymer (HY-PPV) based polymer light emitting diode can be constructed by using an insulated polymer between the metal cathode and light emissive layer. [Ref.1] The turn-on voltage for the insulated polymer incorporated device is markedly reduced. Furthermore, the EL intensity is about a factor of two orders of magnitude higher than that of a similar device without an insulated polymer. The thickness of the insulated
film is just a few (3~5) nanometers. The interfacial layer becomes a buffer medium that enables efficient injection of electron through the aluminum cathode into the organic/polymer light-emissive layer.
The polymer/metal junction in polymer light-emitting diodes (PLEDs) with and without depositing an ultra-thin organic oxide interlayer is studied by X-ray photoelectron spectroscopy. Experimental results indicate that the deposition of an Al electrode causes the oxidation at the surface of the light-emissive polymer layer. Introducing an organic-oxide cathode buffer layer suppresses the oxidation and the diffusion of the Al atoms into the functional polymer layer. The introduction of a carbide-like (negative carbon) thin layer, which accompanies interfacial interactions, is critical to the injection of electrons through the Al cathode. [Ref.2] The balanced charge injection is responsible for the substantially improved device performance. This process is specific to the organic oxide/Al interface, as revealed by a comparison with similar device configurations that have Ag as the electrode, in which no significant interaction in the interface is observed.
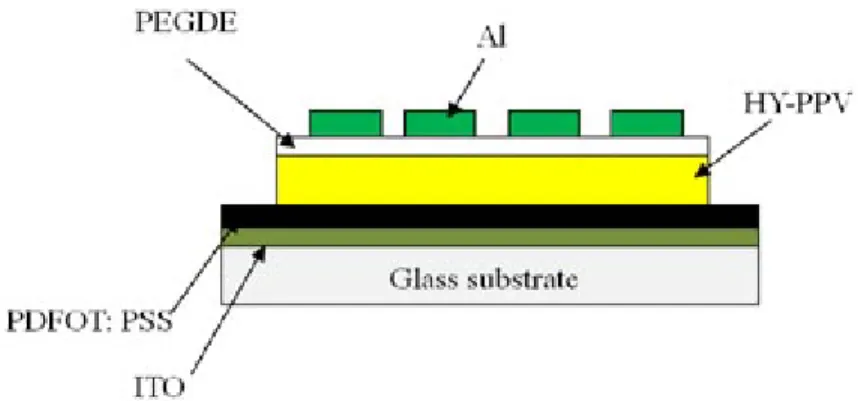
FIG. 1. Configuration of a polymer light-emitting diode (PLED) that incorporates a novel organic oxide/Al cathode.
2. Experimental results and discussion
The configuration of the PLED that incorporates the organic/Al cathode structure is presented in Figure 1. From the bottom of the stack: a layer of indium-tin-oxide (ITO) acts as the anode.
Poly(3,4- ethylenedioxythiophene):polystyr-enesulfonate (PEDOT-PSS) as the hole transport layer by spin casting onto an ITO/glass substrate. A “high-yellow” layer of phenyl-substituted
deposition of 45 Å thick poly(ethylene glycol) dimethyl ether (PEGDE) film. Al electrodes were
vacuum-deposited to form LEDs with groups of pixels of 6 mm2 area.
2.1 Electrophosphorescent device performance
Figure 2(a) presents the current-brightness-voltage (I-L-V) curves for devices of the HY-PPV based PLEDs PDGDE (45 Å)/Al cathode shows a significant improvement compared Al cathode. The emission maxima (560 nm) of the two devices are the same. The EL intensity of the HY-PPV/PEGDE45/Al800 device exceeds 85,000 cd/
m2 when biased at ~10.0 V. The maximum luminous efficiency is approximately 14.53 cd/A at 6.80V, as shown in
Figure 2(b). However, the EL intensity of the HY-PPV/Al800 device biased at ~10.0 V is only 641.80 cd/m2 and
the maximum luminous efficiency is ~0.16 cd/A at 8.61V, also shown in Figure 2(b). Moreover, the light turn-on voltage of the HY-PPV/PEGDE45/Al800 device was reduced to ~2.50 V from the corresponding value of 3.5 V for the HY-PPV/Al800 device. This result also infers that the voltage for the effective injection of minority carriers was brought forward to the lower electrical bias, in which the shift results from the interfacial dipoles or the decline in the metal work function when the PEGDE buffer layer is introduced at the HY-PPV/Al junction.
FIG. 2(a). I-L-V curves of (○) HY-PPV/Al800 and (□) HY-PPV/PEGDE45/Al800 devices; (b). The luminous efficiency versus current density of (○) HY-PPV/Al800 and (□) HY-PPV/PEGDE45/Al800 devices.
2.2 EA spectroscopy
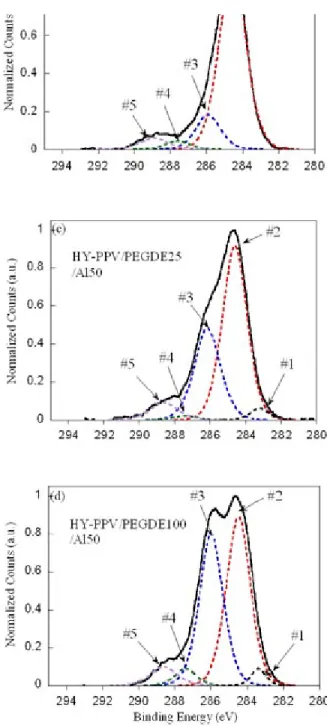
Figures 3(a)-(d) present the de-convolutions of the C 1s core level signals based on the minimum Gaussian peaks to yield the best fit after the background substration. Fig. 3(a) shown the pristine HY-PPV, peaks at 284.5 eV and 286.0 eV and are associated with hydrocarbon atoms (C-C and C-H) and carbon atoms attached to the oxygen (C-O) of HY-PPV molecules. The 287.9 eV peak is attributed to C=O bond and the 288.5 eV belongs to the carbon atoms in a highly oxidative environment. The intensity of peaks #4 and #5 in Fig. 3(a) is low, suggesting some
unintentional oxidation or contamination of the polymer surface. In Figs. 3(c) and (d), the #1 (283.1 eV) peak is observed for the HY-PPV films on which
FIG. 3. The deconvolutions of the normalized and corrected C 1s core level signals from (a) HY-PPV, (b) HY-PPV/Al50, (c) HY-PPV/PEGDE25/Al50, and (d) HY-PPV/PEGDE100/Al50 surfaces.
had been deposited PEGDE and Al were deposited. The binding energies of carbon atoms in this range typically correspond to the carbide-like bonds. The origin or formation of the carbide-like carbons is probably correlated with the interaction between the PEGDE buffer film and Al, as will be discussed below. In Fig. 3(b), the evaporation of a thin Al layer on an HY-PPV surface changes the relative ratio of the intensities of the deconvoluted peaks and increases the intensity of the high-energy tail in the C 1s core level spectrum above that in Fig. 3(a). The intensity of the #3 peak in Fig. 3(b) is approximately halved and is simultaneously accompanied by a relative increase in the #2 peak (C-C and C-H bond). This result supports the assertion that some C-O bonds at the HY-PPV surface will break following the deposition of the Al layer. Furthermore, the marked increase in the intensity of the #5 peak above that in Figs. 3(b) to 3(a),
associated with electron-deficient carbons, indicates the oxidation of the conjugated polymer. Very likely, many benzeneC-O-Calkyl groups are transformed to the
ester moieties by the addition of the second (carbonyl) oxygen to the Calkyl-atom that is directly attached to the oxo-bridge in the side chain of HY-PPV. As a result, the high electron affinity of oxygen atoms causes a large chemical shift of ~4.0 eV in the C 1s core level spectrum, which is reasonably consistent with the literature. The carbonyl-containing moieties and/or various oxidizing defects in poly(phenylene vinylene) (PPV)-based polymers are known to be the quenching sites for electroluminescence. The oxidized interface is also expected to raise the series resistance of the devices, causing the low EL efficiency of PLEDs.
When a thin layer of Al is deposited on the surface of HY-PPV covered with an ultrathin 25Å or 100Å PEGDE layer for XPS measurement, the ~50Å Al metal layer interferes with the penetration of the incident X-rays. The excited photoelectrons that are carried with the C 1s core level signals mainly escape from the PEGDE-rich region located directly beneath the Al layer. The PEGDE film has a higher C-O bond ratio than the HY-PPV film. As a result, in Fig. 3(c), the relative intensity of the #3 peak is higher than that of Fig. 3(a), partially because of the change in the XPS probing depth. The intensity of the #3 peak in Fig. 3(d) is even larger than that in Fig. 3(c) because of the better coverage of the thicker (100Å) PEGDE layer on the HY-PPV surface, as also suggested by a decline in the full-width-half-maximum (FWHM) of the #3 peak in Fig. 3(d). The #5 peak which corresponds to highly oxidized ester-like carbon species presented in Fig. 3(b) is still observed in Fig. 3(c) and 3(d), suggesting oxidation at the surface of the PEGDE-rich region induced by the deposited Al layer. Noticeably, the #1 peak at 283.1 eV is observed in both Figs. 3(c) and 3(d), revealing that the formation of a carbide-like thin layer at the
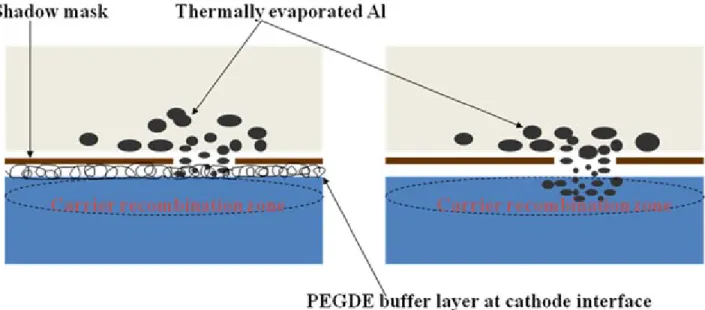
interface is accompanied by an interaction between PEGDE and Al. The reaction of the PEGDE layer with the thermally evaporated Al probably suppresses the diffusion of Al into the HY-PPV layer, potentially inhibiting further oxidization or generation of metal-induced EL quenching sites in the HY-PPV layer near the recombination zone. Figure 4 schematically presents the diffusion of Al atoms into the HY-PPV layer during vacuum thermal deposition, in which the inhibition of diffusion of Al atoms by the PEGDE buffer occurs at the modified polymer/ meal junction.
FIG. 4. A schematic plot presents; (a) the diffusion of Al atoms into the HY-PPV layer during vacuum thermal deposition; (b) the inhibition of diffusion of Al atoms by the PEGDE buffer at the polymer/meal junction. The "cleaner" excitons recombination zone is expected in (b).
3. Conclusions
Incorporating a buffer layer (PDGDE) into a yellow-emitting LED provided significant improvements in brightness and efficiency which can be attributed to the more facile injection of electrons into the emitting layer. The polymer/metal junctions of HY-PPV-based PLEDs with and without introducing the PEGDE buffer layer are investigated by the high-resolution XPS. The formation of an ultra-thin Al-C interlayer, which accompanies the interaction of thermally evaporated Al with the PEGDE buffer layer, is presumed to be based on the C 1s and Al 2p core level spectra. The specific organic oxide/Al complex at the cathode interface effectively facilitates the injection of electrons through the Al electrode at a low bias voltage and also suppresses the formation of metal-induced EL quenching sites in the HY-PPV layer.
Acknowledgments
The authors would like to thank the National Science Council (NSC) of Taiwan (NSC96-2113-M-006-009-MY3), the Asian Office of Aerospace Research and Development (AOARD-08-4076), and NCKU Landmark project for financially supporting this research.
References
1. T. F. Guo,. F. S. Yang, Z. J. Tsai, T. C. Wen, S. N. Hsieh, and Y. S. Fu, Appl. Phys. Lett. 87, 013504
(2005).