行政院國家科學委員會補助專題研究計畫成果報告
※※※※※※※※※※※※※※※※※※※※※※※※※※※※※※※※※※※※※
※ 利用膀胱腫瘤特定促進質進行腺病毒媒介自殺基因方式治療膀胱腫瘤※
※
Treatment of Murine Bladder Cancer with Tumor-Specific Suicide Gene ※
※Expression Driven by Human Telomerase Reverse Transcriptase Promoter in ※
※a Syngeneic Mouse Tumor Model ※
※※※※※※※※※※※※※※※※※※※※※※※※※※※※※※※※※※※※※
計畫類別:
v
個別型計畫 □整合型計畫計畫編號:NSC
90-2314-B-006-162
執行期間: 90 年 8 月 1 日至 91 年 7 月 31 日 計畫主持人:
蔡宗欣
1教授共同主持人:
謝嘉興
2,蕭璦莉
3,吳朝良
4國立成功大學醫學院 泌尿學科 1
,臨床醫學研究所
2, 微免所
3,生化所
4台南市勝利路 138 號
電話:06-2766179 傳真:06-2383678 E-mail:
linym@mail.ncku.edu.tw
本成果報告包括以下應繳交之附件:
□赴國外出差或研習心得報告一份
□赴大陸地區出差或研習心得報告一份
□出席國際學術會議心得報告及發表之論文各一份
□國際合作研究計畫國外研究報告書一份
執行單位:
國立成功大學醫學院泌尿學科
中 華 民 國 九十一 年 十 月三十一 日
計劃名稱:利用膀胱腫瘤特定驅動子進行腺病毒媒介自殺基因方式治療小鼠膀胱腫瘤 中文摘要
目的:對於小鼠膀胱癌細胞,利用非複製 性腺病毒攜帶自殺基因,經由腫瘤細胞特定 驅動子調控,評估腺病毒對於小鼠膀胱癌細 胞的毒殺性及特異性。
方法:我們利用小鼠膀胱癌細胞(MBT-2),
小鼠胚胎纖維細胞及成熟纖維細胞進行實 驗。實驗細胞的 hTERT 驅動子的活性表現,
我們利用螢光載體偵測法定性。小鼠膀胱癌 細胞,小鼠胚胎纖維細胞及成熟纖維細胞 hTERT 驅動子表現量,我們利用 luciferase 相對活性表現定量。本實驗室製造一種複製 缺陷的腺病毒,此腺病毒經由 hTERT 特定驅 動子調控自殺基因(cytosine deaminase, CD),腺病毒對於小鼠膀胱癌細胞,小鼠胚 胎纖維細胞及成熟纖維細胞的毒殺作用,我 們利用 PreMix WST-1 test 測定。我們利用 小鼠皮下腫瘤模式(MBT-2)進行體內實驗。
結果:小鼠膀胱癌細胞及小鼠胚胎纖維細胞 的 hTERT 驅動子皆有螢光載體表現,然而成 熟纖維細胞則無螢光表現。hTERT 驅動子調 控 luciferase 相對活性表現量以小鼠膀胱癌 細胞最高,而成熟纖維細胞則只有少量。小 鼠膀胱癌細胞及小鼠胚胎纖維細胞經由此 複製缺陷腺病毒感染後,再加入無毒性的 5-fluorocytosine,經由 CD 酵素轉換唯有毒 性的 5-fluorouracil (5-FU),皆表現出細胞毒 殺效果,成熟纖維細胞則不受病毒感染及 5-fluorocytosine 影響細胞的存活。人類膀胱 癌細胞中 hTERT 的表現量,和細胞毒殺效果 亦呈現正比關係。於體內實驗,小鼠皮下腫 瘤模式接受複製缺陷腺病毒感染後,再加入 無毒性的 5-fluorocytosine,能減小腫瘤大小 及延長小鼠存活率。
結論:此複製缺陷腺病毒經由 hTERT 特定驅
動子調控自殺基因(cytosine deaminase, CD),於細胞實驗中確實能有效地殺死小鼠 膀胱癌細胞。小鼠皮下腫瘤模式中,能減小 腫瘤大小及延長小鼠存活。另外我們建立一 新的免疫完整的小鼠腫瘤模式,以了解小鼠 對於複製缺陷腺病毒,再加入無毒性的 5-fluorocytosin ,免疫系統真正反應及確實小
鼠存活。
Treatment of Murine Bladder Cancer with Tumor-Specific Suicide Gene Expression Driven by Human Telomerase Reverse Transcriptase Promoter in a Syngeneic Mouse Tumor Model Gia-Shing Shieh
1,2, Tzong-Shin Tzai
1, Ai-Li Shiau
2, Chao-Liang Wu
3,
National Cheng Kung University, Medical College, Department of Urology, Tainan, Taiwan.
ABSTRACT
INTRODUCTION AND OBJECTIVES:
Tissue-specific promoters represent one of the main approaches to induce therapeutic gene expression within the tumor cells and to prevent unwanted toxicity in the normal tissues.
Human telomerase reverse transcriptase (hTERT) is the key subunit of telomerase, which is highly active in immortalized cell lines and over 85% of human cancers but inactive in most somatic cells. Due to the high homology between human and mouse TERT core promoter regions, the transcriptional activity of hTERT promoter in syngeneic mouse bladder tumors and the potential toxicity of hTERT promoter-driven suicide gene in murine somatic cells are still unknown.
Therefore, we constructed a replication-incompetent adenoviral vector driving cytosine deaminase (CD) gene expression under the control of hTERT promoter and investigated its antitumor effect on mouse bladder cancer in vitro and in vivo.
We also determined the toxicity of this adenoviral vector on murine mature fibroblasts.
METHODS: The murine bladder carcinoma
cells (MBT-2), immortalized murine embryo
fibroblasts (NIH-3T3) and mature fibroblasts
derived from mouse skin were used in this
study. Transient transfection of the EGFP
reporter plasmid driven by hTERT promoter
(phTERT-EGFP) was performed by
lipofectamine gene transfer procedure to assess
the activity of hTERT promoter in murine cell lines. The levels of transcriptional activity of hTERT promoter in various cells were quantified using transient co-transfection of phTERT-Luciferase plasmid and a LacZ reporter plasmid by lipofectamine protocol. We constructed Ad-hTERT-CD adenovirus as the therapeutic vector and Ad-CMV-LacZ adenovirus as the control vector. In the presence of various concentrations of adenoviral vectors and 5-fluorocytosine (5-FC), the cell viability induced via the CD/5-FC system was analyzed with the WST-1 assay. To assess the bystander effect of CD/5-FC system, MBT-2 and NIH-3T3 cells were infected at different multiplicities of infection of Ad-hTERT-CD. The infected and non-infected cells were mixed in varying ratios, were incubated with 500 uM of 5-FC and examined for cell viability To determine the anti-tumor effect of Ad-hTERT-CD in vivo, we tested the targeted cancer gene therapy in syngeneic mouse tumor models.
RESULTS: The significant expression of EGFP reporter gene driven by the hTERT promoter was demonstrated in MBT-2 and NIH-3T3 cells, but not in murine mature fibroblasts. In quantification assay of transcriptional activity of hTERT promoter, the relative luciferase expression was highest in MBT-2 cells, moderate in NIH-3T3 cells, and lowest in mature fibroblasts. The hTERT promoter activity in MBT-2 cells was 10-fold higher compared with that in mature fibroblasts. In transfection study, the Ad-hTERT-CD adenoviral vector conferred 5-FC sensitivity to MBT-2 and NIH-3T3 cells, whereas mature fibroblasts remained
unaffected. The degree of cytotoxicity was correlated with the transcriptional activity of hTERT in murine cell lines. Analysis of the bystander effect on MBT-2 and NIH-3T3 cells showed that only a few infected cells were to kill the vast majority of infected as well as uninfected cells. The hTERT promoter-driven CD gene therapy followed by systemic administration of 5-FC suppressed MBT-2 tumor growth in syngeneic, immunocompetent mice. The long-term survival was prolonged in mice treated with Ad-hTERT-CD adenovirus followed by 5-FC treatment.
CONCLUSIONS: By restricting CD gene expression to MBT-2 cells, the hTERT promoter allowed the use of suicide genes for cancer therapy without detrimental effects on murine mature cells. In syngeneic mouse tumor models, the tumor-specific expression of suicide genes driven by hTERT promoter, therefore, may be a novel therapy for malignancy with telomerase activity
SOURCE OF FUNDING: The study was supported by NSC 90-2314-B-006-162
CONFLICT OF INTEREST AND
DISCLOSURE STATEMENT: The study is a basic research. All authors have participated in the study, which has not been supported by commercial companies.
TOPIC LIST: hTERT promoter-based suicide gene therapy in mouse bladder cancer
KEY WORDS: hTERT promoter, adenoviral
vector, cytosine deaminase
Introduction
Bladder cancer remains the most common neoplasm in the genitourinary malignancy.
About 75% to 80% of these neoplasms are superficial bladder cancers with a high rate of recurrence despite the transurethral resection of tumors and intravesical chemotherapy.
However, 15% of superficial tumors progress to muscle invasion and are required radical cystectomy. The prognosis for patients with advanced tumor is poor and the 5-year survival rate is about 50% even after multiple therapies.
Thus novel treatments are needed to improve the prognosis of bladder cancers.
The major challenge in cancer gene therapy is appropriate control of the expression of the therapeutic gene. In the cancer gene therapy, the extratumoral expression of transgene will produce its toxicity to normal cells and lead the destruction of normal tissues. Thus the control of gene expression via tissue- or cell-specific promoters can restrict the therapeutic gene to specific tissues or cell types. Several promoters have been identified to target transgene expression. However most of these promoters are weak and often useful only for the therapy of particular type of cancer cells.
Telomerase is a ribinucleoprotein enzyme that involves in the synthesis and maintenance of telomeric repeats in the ends of chromosomes. Telomerase is expressed in immortalized cell lines and in approximately 90% of human malignancies, whereas is silent
in most adult somatic tissues. Therefore telomerase may be a good candidate for cancer gene therapy. This enzyme complex composes of an essential RNA template (hTR), the telomerase-associated proteins and the telomerase reverse transcriptase (hTERT).
Recent studies suggest that hTERT is a key determinant of telomerase activity. The expression of hTERT is highly associated with telomerase activity. In urological malignancy, telomerase activity is present in more than 90% of bladder cancers, whereas a low incidence is also noted in normal tissues adjacent to bladder cancer. In recent reports, the expression of hTERT is detected in 100%
of bladder cancers but not in normal bladder tissues. In addition, the levels of hTERT expression are significantly associated with bladder cancer grade and stage. Therefore, hTERT subunit is expected to be a good role for targeted gene therapy of bladder cancers.
The delivery of a suicide gene into tumor
cells followed by the corresponding prodrug
treatment, such as the combination of
Escherichia coli cytosine deaminase (CD)
gene and 5-fluorocytosine (5-FC) therapy, is
one of the most promising approaches. CD, an
enzyme found only in many bacteria and fungi,
functions to deaminate nontoxic 5-FC to the
highly toxic 5-fluorouracil (5-FU). The CD
gene has been used in gene therapy to convert
intracellular 5-FC to 5-FU, and has been
reported to be effective in animal models of
human colon carcinoma. In addition, the
CD/5-FC system also induces the bystander
effect not only in vitro but also in vivo. The
existence of bystander effect is requisite for
successful gene therapy in cancers, because the transduced cells effectively kill surrounding non-transduced cells.
The mouse TERT (mTERT) promoter has been cloned and the homology between the hTERT and mTERT core promoter regions is about 50%. There is distinct in some of regulatory mechanisms between the hTERT and mTERT promoters. Thus, we hypothesized that the hTERT promoter may be used for tumor-specific expression of suicide genes into syngeneic mouse tumor models. In this present study, to test our hypothesis, we constructed the replication-incompetent adenoviral vectors driving cytosine deaminase gene expression under the control of hTERT promoter, and investigated its antitumoral effect on mouse bladder cancers in vitro and in vivo.
Results
Transgene expression induced by hTERT promoter in murine cell lines
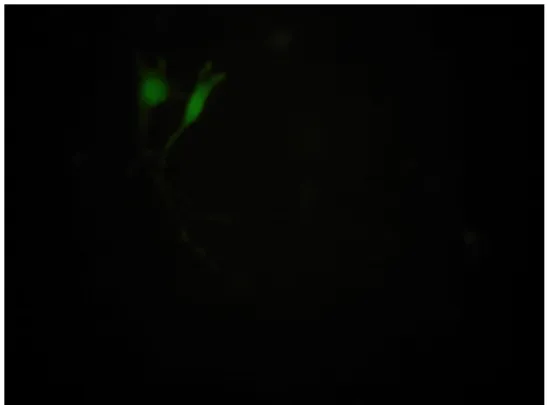
To assess the hTERT promoter activity in murine cell lines, a transient transfection of EGFP reporter plasmids was performed.
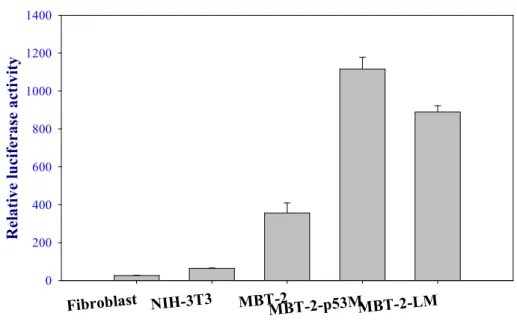
Expression of EGFP reporter gene was analyzed 48 hours after transfection. As shown in Fig. 1, significant expression of EGFP gene driven by the hTERT promoter was demonstrated in MBT-2 and NIH-3T3 cells, but this was not found in murine mature somatic fibroblasts. The duration of EGFP gene expression persisted for 9 days in MBT-2 cells and for 2 days in NIH-3T3 cells. To examine the transcriptional activity of the hTERT promoter in murine cell lines, a transient transfection of luciferase reporter plasmids was performed and co-transfection with β-galactosidase gene driven byβ-actin
promoter was used to monitor transfection efficiency. As shown in Fig. 2, the hTERT promoter construct showed the highest luciferase activity in MBT-2 cells. The relative luciferase activity was above 350. IN NIH-3T3 cell, a non-transformed murine embryonic fibroblast with immortalized capacity, the relative luciferase activity was moderate expression ranged between 63 and 66. In murine mature fibroblast, the relative luciferase activity, which ranged between 25 and 27, demonstrated the lowest expression.
These results indicated that the hTERT promoter could drive the mouse transcriptional mechinary and that hTERT promoter was significantly active in murine bladder tumor cells, but relatively quiescent in mature somatic cells
In vitro study of 5-FC toxicity of murine cells transduced with an adenoviral vectors carrying hTERT-mediated CD gene
To test the selectivity and toxicity of a CD
gene driven by the hTERT promoter in murine
cell lines, replication-incompetent adenoviral
vectors carrying hTERT-CD (Ad-hTERT-CD)
or CMV-LacZ plasmids (Ad-CMV-LacZ) were
constructed and amplified. We used the WST-1
assay to compare cell viability after treating
MBT-2 and NIH-3T3 cells with 2% media,
Ad-CMV-LacZ + 5-FC, 5-FC only,
Ad-hTERT-CD vectors only or Ad-hTERT-CD
+ 5-FC at different concentrations of
adenoviral vectors and 5-FC for 3 days. As
shown in Fig 3, no significant toxicity was
seen in MBT-2 and NIH-3T3 cells when 5-FC
only, Ad-CMV-LacZ + 5-FC or Ad-hTERT-CD
vectors only were used. In contrast, when
MBT-2 cells were infected with
Ad-hTERT-CD vectors (10 MOI), nearly 100% cell death was detected after exposure to different concentrations of 5-FC (50 uM to 5000 uM). At 1 or 0.1 MOI of Ad-hTERT-CD vectors, the severity of cytotoxicity to MBT-2 was dependent on the concentrations of 5-FC.
The Ad-hTERT-CD vectors with 5-FC exposure were less cytotoxic in NIH-3T3 than in MBT-2 cells (p < 0.01,Student t test). The cytotoxic results in NIH-3T3 cells were most probably attributable to the low level of transcriptional activity of the hTERT promoter.
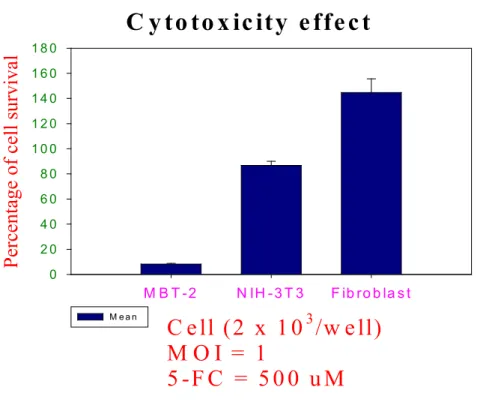
To test whether the Ad-hTERT-CD/5-FC system could be used to negate the toxic effect on murine mature cells while preserving its antitumoral activity, MBT-2 cells, NIH-3T3 cells and mature fibroblast were infected with Ad-hTERT-CD at 1 MOI. After adenoviral transfection, the cell lines were incubated in maintaining media supplemented with 500 uM of 5-FC for 3 days. As shown in Fig 4, no significant cytotoxicity was seen in mature fibroblast. In contrast, nearly 100% cell-killing effect on MBT-2 cells after exposure to the vectors and 5-FC, while only minimal cytotoxic effect on NIH-3T3 cells. These results demonstrated that the hTERT promoter could be used to drive suicide gene expression in murine bladder cancer cells and induce tumor cell death in vitro, while the cell toxicity was negated in normal cells.
Bystander effect in vitro
To test the bystander effect produced from infected cells on uninfected cells, MBT-2 and NIH-3T3 cells were infected at varying MOI (10 and 1 MOI) of Ad-hTERT-CD adenoviral vectors. Mixtures of infected and uninfected cells at varying ratio were generated and then
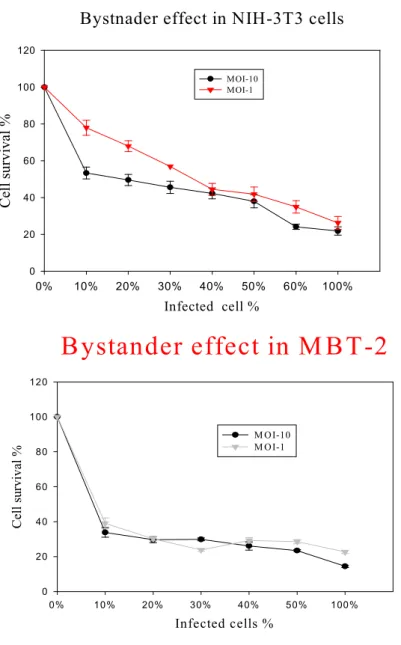
exposed to 500uM of 5-FC. The nontoxic prodrug 5-FC was converted to the toxic chemcal 5-FU by CD protein. The 5-FU could be released from infected cells and taken up by surrounding uninfected cell. Thus this was called the bystander effect, which killed the majority of uninfected cells and enhanced the antitumoral results of the CD protein. As shown in Fig 5, when as few as 10% of MBT-2 cells infected with Ad-hTERT-CD (10 and 1 MOI) vectors were mixed with 90% of uninfected cells, the majority of cells were killed when cells were exposed to 5-FC for 3 days. When the MBT-2 cells were infected with 10 or 1 MOI of Ad-hTERT-CD vectors, the bystander effects were similar. These results demonstrated that only few infected cells were required to kill the vast majority of infected as well as uninfected cells. The bystander effect was also found in NIH-3T3 cells which were hTERT-positive. Less cell death was seen in NIH-3T3 cells when the same experiments were carried out in MBT-2 cells. The difference in bystander effect between MBT-2 and NIH-3T3 cells might be attributable to the different transcriptional activity of hTERT promoter. The present data suggested that the bystander effect in MBT-2 cells infected with Ad-hTERT-CD vectors could create the high levels of cancer cell death at low infectivity status.
CD gene expression driven by the hTERT promoter suppressed tumor growth and prolonged survival in syngeneic murine model
To evaluate the possibility of using the CD
gene controlled by hTERT promoter for cancer
therapy in vivo, we established MBT-2 tumors
subcutaneously in immuno-competent C3H/HeN mice. The tumors were treated with intratumoral injection of Ad-hTERT-CD, Ad-CMV-LacZ vectors or PBS, and followed with intraperitoneal injection of 5-FC or PBS.
The treatment with three sequential intratumoral injection of Ad-hTERT-CD vectors followed by 5-FC resulted in significant inhibition of tumor growth. In contrast to Ad-hTERT-CD + 5-FC groups, MBT-2 tumor-bearing mice, receiving Ad-hTERT-CD + PBS, PBS only, PBS + 5-FC and Ad-CMV-LacZ + 5-FC treatment, did not show any delay in tumor progression (p <
0.001 for Ad-hTERT-CD + PBS, PBS only and PBS + 5-FC groups, and p < 0.005 for Ad-CMV-LacZ + 5-FC groups, Student t test).
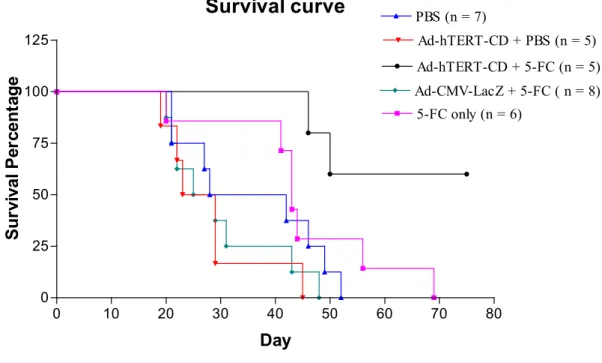
To test whether intratumoral delivery of Ad-hTERT-CD vectors followed by 5-FC treatment had effects to prolong the survival of tumor-bearing mice. In this study, there were significantly prolonged survival in Ad-hTERT-CD vectors and 5-FC-treated cohorts comparing with control groups (p <
0.01 for Ad-hTERT-CD + PBS and Ad-CMV-LacZ + 5-FC groups, and p < 0.02 for PBS only and PBS + 5-FC groups). In control groups, no mice survived past 68 days.
In contrast, two of 5 mice in Ad-hTERT-CD + 5-FC groups were dead before 68 days. Two of 5 mice in treated groups have been alive with complete remission of tumor burden for 150 days (data not shown). These results demonstrated that hTERT promoter effectively controlled CD gene expression to suppress tumor growth and prolong the survival of tumor-bearing mice in syngeneic, immuno-competent murine models.
Discussion
In cancer therapy by the transfer of therapeutic gene, it is essential to restrict the gene expression more selective for tumor cells and less toxic for normal tissues. One way to create this result would be to use the tumor-specific transcriptional promoter..
Several reports showed that the hTERT
promoter induced tumor-specific apoptotic
transgene expression in human
telomerase-posive cells and suppressed
xenograft tumor growth in nude mice. Yet
whether hTERT promoter can drive therapeutic
gene in syngeneic, immuno-competent murine
model was not described. The high homology
between hTERT and mTERT core promoter
regions had been reported. The E-box and two
SP1 binding sites in the core promoter regions
were conserved. We hypothesized that the
hTERT promoter could use the murine
transcriptional machinery. We showed here
that the hTERT promoter was functional in
murine cell lines. The hTERT promoter
activity was highest in murine bladder cancer
cells and nearly quiescent in mature murine
cells. The murine fibroblasts had detectable
telomerase activity by sensitive assays. Our
results showed that the cloned hTERT
promoter could control transgene expression
with the same specificity as the activity of
murine endogeneous telomerase promoter. In
in vitro assay, the cytotoxic effects of CD/5-FC
system driven by the hTERT promoter in
MBT-2, NIH-3T3 and mature fibroblast were
correlated with the activity levels of hTERT
promoter. It also prevented the toxicity of CD
gene for mature fibroblasts. These results
demonstrated that the varying activities of
hTERT promoter between tumor cells and mature fibroblasts could provide a “gap”, which produced and maintained the threshold level of CD gene expression effective to kill bladder cancer cells, and induced too low expression of the suicide gene to result in no therapeutic efficacy in mature fibroblasts. Thus, the present study demonstrated that the use of hTERT promoter was tumor-specific in murine bladder cancer models.
The replication-incompetent adenoviral vectors carrying the CD gene driven by hTERT promoter suppressed tumor growth and prolonged survival rate in syngeneic murine bladder cancer models. In general, it was expected that only a fraction of tumor cells were infected after locally intratumoral injection of replication-incompetent adenoviral vectors. In our in vitro study,
the bystander effect produced by Ad-hTERT-CD adenoviral vectors resulted in significant tumor cell death by few infected cells. Therefore our in vivo study indicated that the bystander effect of CD gene expression controlled by the hTERT promoter could increase significant tumor cell destruction and even complete remission of cancer masses.
Our in vitro study demonstrated that the cytotoxic and bystander effects induced by Ad-hTERT-CD vectors with 5-FC were found in NIH-3T3 cells, whose hTERT promoters were more active than in mature fibroblasts.
Therefore, the normal cells with high telomerase activity, such as stem cell, may be affected when hTERT promoter-driven suicide genes were expressed. Selection of a delivery vector with poor transfection to stem cells may limit the cytotoxic effects of hTERT promoter-mediated transgene expression. In previous reports, only a limited percentage of stem cells were infected with very high doses of adenoviral vectors and increased cell-vector contact. Adenoviral vectors poorly infected stem cells. So, if the systemic delivery of Ad-hTERT-CD adenoviral vectors with 5-FC, the toxicity of hTERT promoter-mediated suicide gene expression in stem cells would be
minimal. Furthermore, a more selective delivery vector with limited tropism to stem cells will be investigated to prevent any detrimental effect of hTERT promoter-mediated transgene expression.
Tumor regrowth in some of the treated mice decreased the survival prognosis in this study.
It was difficult to measure precisely the adenoviral infection percentage in tumors. In addition, our in vitro data demonstrated that few tumor cells survived after infection of Ad-hTERT-CD vectors and exposure to high doses of 5-FC. Therefore inefficient vector delivery to tumor cells or drug resistance to 5-FU in cancer cells were likely to be the cause of incomplete eradication of tumors in tumor-regrowing mice. Future in vivo studies will design to improve the survival with different adenoviral infection protocol or more potent cytotoxic genes.
In summary, we reported that the hTERT
promoter restricted the suicide gene expression
in bladder cancer cell lines without cytotoxic
effect in mature cells. In addition, the
Ad-hTERT-CD adenoviral vectors suppressed
tumor growth in syngeneic,
immuno-competent mice. These
immuno-competent mice were especially
useful tools in understanding the immune responses and actual treatment results of adenoviral vectors carrying therapeutic gene driven by hTERT promoter in vivo. The telomerase activity expressed in vast tumor cells indicated that this approach in immuno-competent mice may be an useful in virtually all types of cancers.
Materials and methods
Construction of recombinant adenoviral vectors carrying CD genes driven by hTERT promoter
To construct the replication-incompetent adenoviral vectors carrying hTERT promoter-mediated cytosine deaminase gene, the hTERT promoter-luciferase (PGl3-hTERT-Luciferase, a gift from Dr. M Takakura) constructs were used. The replication-incompetent adenovirus was created with AdEasy system. The fragment containing hTERT promoter was digested with
HindIII and KpnI from
PGL3-hTERT-luciferase plasmid and cloned into th HindIII and KpnI sites of pShuttle plasmids (a gift from Dr. T.C. He ) to generate pShuttle-hTERT plasmid. The pCD2 plasmid was digested with EcoRI and BamHI to isolate CD gene, which was filled in with klenow enzyme. The klenow fragments were cloned into HindIII-digested pShuttle-hTERT plasmids to generate pShuttle-hTERT-CD plasmids. The PmeI-digested pShuttle-hTERT-CD plasmid was mixed with AdEasy-1 plasmid (a gift from Dr. T.C. He ) to generate recombinant adenoviral plasmid by homologous recombination in E coli BJ5183 cells. The proper homologous recombinant plasmid was digested with PacI and transfected
into 293 cells to generate replication-incompetent adenovirus, Ad-hTERT-CD, which was purified by CsCl density gradient centrifugation. Viral titers were determined by plaque assay.
Analysis of in vitro hTERT promoter activity in murine cell lines
Normal murine fibroblast cells were isolated from the skin of C3H/HeN mice using conventional techniques. MBT-2 (murine bladder transitional cell carcinoma) and NIH-3T3 (non-transformed, immortalized mouse embryo fibroblast) were maintained in our laboratory. All Cell lines were cultured in Dulbecco’s medified Eagle’s medium (DMED, Life Technologies, Inc.) supplemented with 10% fetal bovine serum (FBS, Life Technologies, Inc.), 2 mM glutamine, and 50ug/ml gentamycin. Cells at densities of 1 x 105/ well were plated in 24-well plates 1 day before transient transfection. The transient transfection of hTERT-EGFP plasmid was performed by LipofectAMINE-mediated gene transfer protocol (GIBCO). Forty-eight hours after transfection, the cellular expression of EGFP gene driven by hTERT promoter was visualized by fluorescence microscopy.
Transient luciferase assay in murine cell lines
The transcriptional activity of the hTERT promoter in MBT-2, NIH-3T3 and mature fibroblast was determined by the luciferase reporter plasmids. Cells were plated at densities of 1 x 105/ well were plated in 24-well plates 1 day before transfection.
Twenty-four hours later, co-transfection of 2
ug of hTERT-luciferase plasmid and 1 ug of
β-galactosidase plasmid into each well was
performed by lipofectAMINE method.
Forty-eight hours later, cells were washed twice with PBS and lysed in lysis buffer (Applied Biosystems). The Dual-Light System (Applied Biosystems), a chemiluminescent reporter gene assay system for the combined detection of luciferase and β -galactosidase, and luminometer were used to evaluate the luciferase activity. The relative luciferase activity were normalized for transfection efficiency by the corresponding β -galactosidase values.
In vitro cell viability assay
Cells were plated on 96-well plates at densities of 2 x 103 per well 1 day prior to virus infection. Cells were then infected with adenoviral vectors (Ad-hTERT-CD) at varying MOI (10, 1 and 0.1 MOI). Cells were incubated at 37 ℃ for 3 hours, followed by removal of media with adenoviral vectors and addition of 100 ul of maintaining media which containing varying concentration of 5-FC (50, 500, 5000 uM). 2% maintaining media was used as mock control. 500 uM of 5-FC and 10 MOI of Ad-CMV-LacZ were used as control.
The cell viability was determined by WST-1 assay (TaKaRa Biomedicals) according to the manufacturer’s protocol. In each treatment group, quadruplicate wells were measured for cell viability.
In vitro bystander effect
To evaluate the bystander effect from infected cells on uninfected cells, MBT-2 and NIH-3T3 cell lines were infected at 10 or 1MOI of Ad-hTERT-CD vectors and incubated at 37 ℃ for 3 hours. Then the media containing adenoviruses were removed and 10% media were added. Cells were incubated
at 37 ℃ for 9 hours and then trypsized as infected cohorts. The infected and uninfected cells were mixed in varying ratios to generate 0, 10, 20, 30, 40, 50, 60 and 100% infected cells. Cells were plated on 96-well plates at 2 x 103 per well, and incubated for 24 hours .Then cells was incubated subsequently with 500 uM of 5-FC for 3 days. The cell viability was determined with WST-1 assay.
Effect of the CD gene expression under hTERT promoter in vivo
For the subcutaneous tumor models, MBT-2 cells (2 x 106) in PBS were inoculated subcutaneously into the dorsal flank of 6- to 8-week-old C3H/HeN mice to establish tumors.
Two weeks later, the tumor sizes were measured, and the tumor volumes were calculated using the formula a x b2 x 0.45, where a and b represented the larger and smaller diameters, respectively. On day 1 of treatment, mice bearing subcutaneous tumors of 100- 400 mm3 were pooled and randomly assigned to treatment groups. Tumor sizes were measured three times a week.
Ad-hTERT-CD or Ad-CMV-LacZ vectors were
administered by intratumoral injection at a
dose of 1 x 109 pfu in 100 ul PBS daily for 3
consecutive days. Different tracks and
injection sites of the tumor were chosen for
each treatment. Control was injected with 100
ul PBS as the same procedures. After
intratumoral injections, 500 ul of 5-FC (500
mg/kg) or PBS was injected into the peritoneal
cavity once a day for 14 days. Survival of
animals was observed everyday.
Fig 1 Analysis of in vitro hTERT promoter activity in murine cell lines. EGFP gene expression was visualized with fluorescent microscopy. The name of each cell lines and day are indicated at the bottom of the panels
MBT-2 , Day 4 NIH-3T3, Day 4
MBT-2, Day 10
Fig 2 Transcriptional activity of hTERT promoter in murine cell lines.
Transient transfection assay
Fibroblast NIH-3T3 MBT-2MBT-2-p53MMBT-2-LM
Relative luciferase activity
0 200 400 600 800 1000 1200 1400
Fig 3 In vitro cell viability assay in murine cell lines.
Fig 4 In vitro bystander effect in murine cell lines
C y to to x ic ity e ffe c t
C e ll (2 x 1 0
3/w e ll) M O I = 1
5 -F C = 5 0 0 u M
M B T -2 N IH -3 T 3 F ib ro b la s t
Percentage of cell surviva l
0 2 0 4 0 6 0 8 0 1 0 0 1 2 0 1 4 0 1 6 0 1 8 0
M e a n
Fig 5 suppression of syngeneic tumor growth by hTERT promoter-mediated CD gene expression.
Bystnader effect in NIH-3T3 cells
Infected cell %
0% 10% 20% 30% 40% 50% 60% 100%
Cell survival %
0 20 40 60 80 100 120
MOI-10 MOI-1
Bystander effect in M BT-2
Infected cells %
0% 10% 20% 30% 40% 50% 100%
Cell survival %
0 20 40 60 80 100 120
M OI-10 M OI-1
T u m o r V o l u m e
D a y
0 5 1 0 1 5 2 0 2 5 3 0 3 5
tumor volume
0 2 0 0 0 4 0 0 0 6 0 0 0 8 0 0 0 1 0 0 0 0 1 2 0 0 0
A d - h T E R T - C D + 5 - F C P B S + 5 - F C
A d - h T E R T - C D + P B S P B S + P B S
A d - C M V - L a c Z + 5 - F C
Fig 6 Survival of mice following adenoviral adeministration and 5-FC treatment.
Survival curve
0 10 20 30 40 50 60 70 80
0 25 50 75 100 125
5-FC only (n = 6) PBS (n = 7)
Ad-hTERT-CD + PBS (n = 5) Ad-CMV-LacZ + 5-FC ( n = 8)
Ad-hTERT-CD + 5-FC (n = 5)
Day
Survival Percentage
References:
(1) Sagar,R et al: Senescence as a mode of tumor suppression. Environ.
Health
Perspect. 93, 59-62 (1991)
(2) Harley, C.B. et al: Telomerase, cell immortality, and cancer. Cold Spring Harb. Symp. Quant. Biol. 59, 307-315 (1994)
(3) Harley, C.B. et al: Telomeres shorten during aging of human fibroblasts.
Nature 346, 458-460 (1990)
(4) Hastie, N.D. et al: Telomere reduction in human colorectal carcinoma and with aging. 866-868 (1990)
(5) Counter, C.M. et al: Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 11, 1921-1929 (1992).
(6) Feng, J. et al: The RNA component of human telomerase. Science 269,
1236-1241 (1995)
(7) Nakamura, T.M. et al: Telomerase catalytic subunit lomologs from fission yeast and human. Science 277, 955-959 (1997).
(8) Meyerson,M. et al: hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell 90, 785-794 (1997)
(9) Nakayama, J. et al: Telomerase activation by hTNT in human normal fibroblasts and hepatocellular carcinomas. Nature Genet. 18, 65-68 (1998)
(10) Ramakrishnan, S. et al: Expression profile of putative catalytic subunit of the telomerase gene. Cancer Res.
58, 622-625 (1998).
(11) Hahn, W.C. et al: Creation of human tumor cells with defined genetic elements. Nature
400,464-468 (1999).
(12) Shay, J.W. et al: A survey of telomerase activity in human cancer.
Eur. J. Cancer 33, 787 (1997)
(13) Rahat, M.A. et al: Telomerase activity in patients with transitional cell carcinoma. Cancer 85, 919-924 (1999)
(14) Suzuki, T. et al: Expression of the catalytic subunit associated with telomerase gene in human urinary bladder cancer. J. Urol. 162, 2217-2220 (1999).
(15) Mullen, C.A. et al: Tumors expressing the cytosine deaminase suicide gene can be eliminated in
vivo with 5-fluocytosine and induce protective immunity to wild-type tumor. Cancer Res 54, 1503-1506 (1994).
(16) Huber, B.E. et al: Transfer of the bacterial gene for cytosine deaminase to mammalian cells confers lethal sensitivity to 5-fluorocytosine: a negative selestion system. Proc. Natl. Acad.
Sci. 91, 8302-8306 (1994).
(17)Shiau, A.L. et al: Provision of positive and negative selections in retroviral vectors containing the cytosine deaminase gene. Gene therapy 5, 1571-1574 (1998)