Principles of Principles of
BIOCHEMISTRY BIOCHEMISTRY
Third Edition Third Edition
HORTON MORAN OCHS RAWN SCRIMGEOUR
Chapter 8 - Carbohydrates
• Carbohydrates (“hydrate of carbon”) have empirical formulas of (CH2O)n , where n ≥ 3
• Monosaccharides one monomeric unit
• Oligosaccharides ~2-20 monosaccharides
• Polysaccharides > 20 monosaccharides
• Glycoconjugates linked to proteins or lipids
8.1 Most Monosaccharides are Chiral Compounds
• Aldoses - polyhydroxy aldehydes
• Ketoses - polyhydroxy ketones
• Most oxidized carbon: aldoses C-1, ketoses usually C-2
• Trioses (3 carbon sugars) are the smallest monsaccharides
Aldoses and ketoses
• Aldehyde C-1 is drawn at the top of a Fischer projection
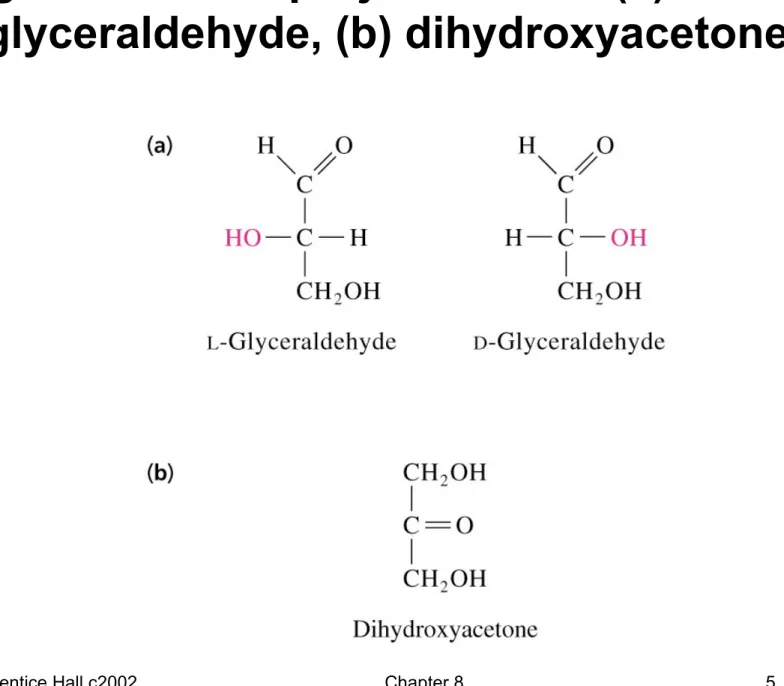
• Glyceraldehyde (aldotriose) is chiral (C-2 carbon has 4 different groups attached to it)
• Dihydroxyacetone (ketotriose) does not have an asymmetric or chiral carbon and is not a chiral compound
Fig 8.1 Fischer projections of: (a) L- and D- glyceraldehyde, (b) dihydroxyacetone
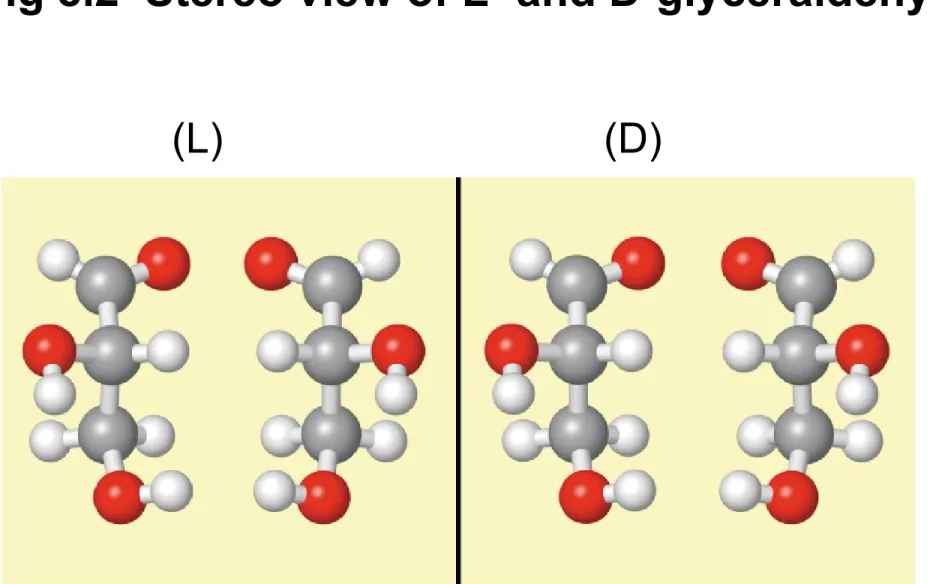
Fig 8.2 Stereo view of L- and D-glyceraldehyde
(L) (D)
Fig 8.3 Fisher projections of 3 to 6 carbon D-aldoses
• D-sugars have the same configuration as D-glyceraldehyde in their chiral carbon
most distant from the carbonyl carbon
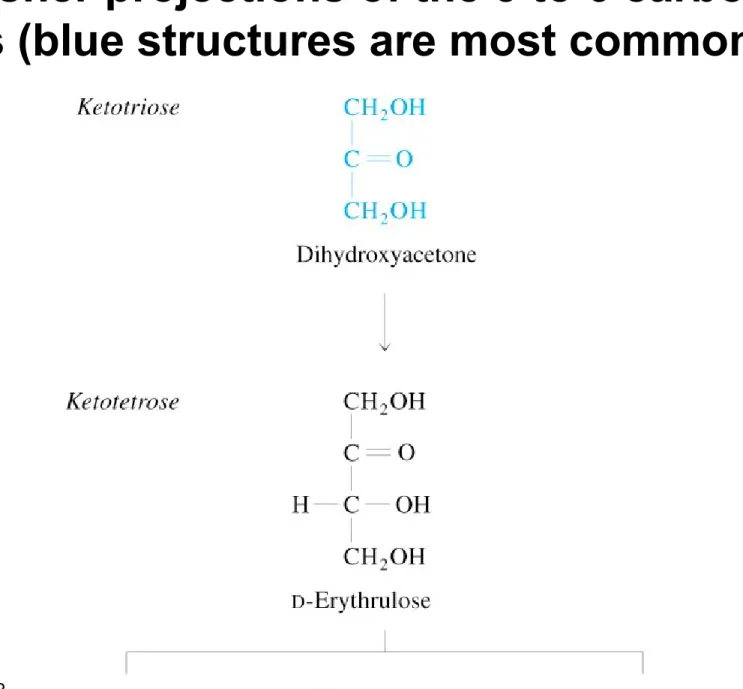
• Aldoses shown in blue (next slide) are most important in biochemistry
Fig. 8.3
Fig. 8.3 (continued)
Fig 8.3 (continued)
Enantiomers and epimers
• D-Sugars predominate in nature
• Enantiomers - pairs of D-sugars and L-sugars
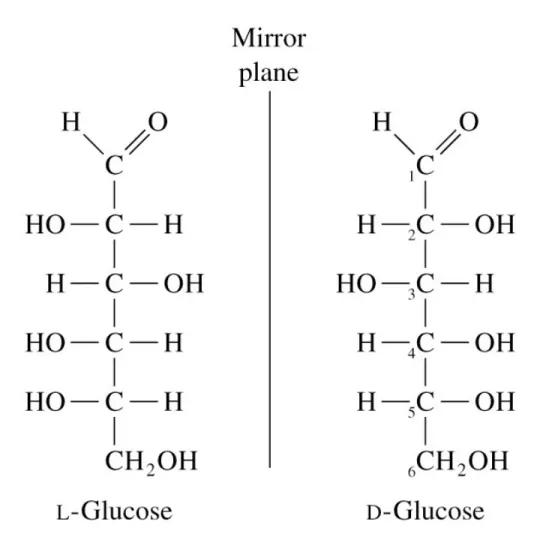
• Epimers - sugars that differ at only one of several chiral centers
• Example: D-galactose is an epimer of D-glucose at C-4
Fig 8.4 Fisher projections of L- and D-glucose
Fig 8.5 Fisher projections of the 3 to 6 carbon D-ketoses (blue structures are most common)
Fig. 8.5 (continued)
Fig 8.5 (continued)
8.2 Cyclization of Aldoses and Ketoses
Fig. 8.6 Reaction of an alcohol with:
(a) An aldehyde to form a hemiacetal (b) A ketone to form a
hemiketal
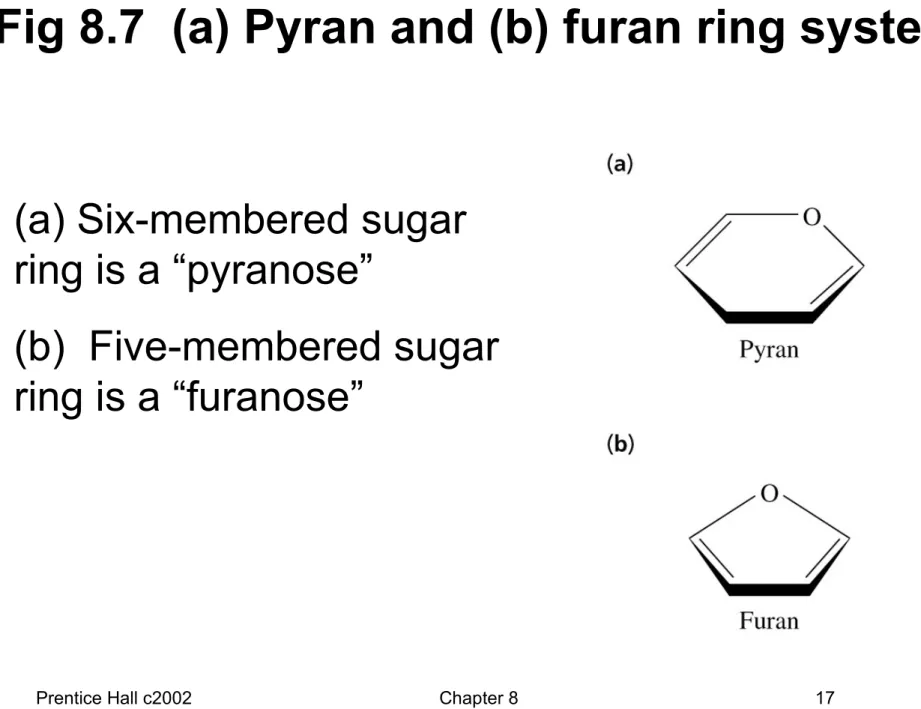
Fig 8.7 (a) Pyran and (b) furan ring systems
• (a) Six-membered sugar ring is a “pyranose”
• (b) Five-membered sugar ring is a “furanose”
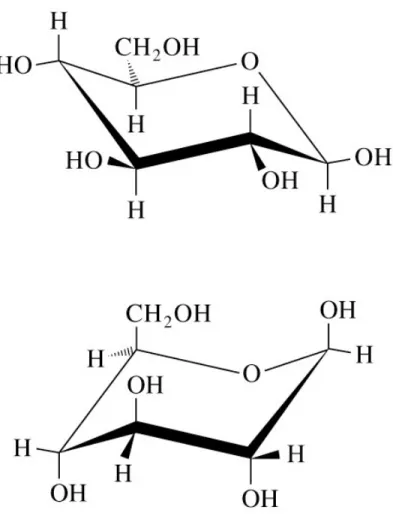
Fig 8.8 Cyclization of D-glucose to form glycopyranose
• Fischer projection (top left)
• Three-
dimensional figure (top right)
• C-5 hydroxyl close to aldehylde group (lower left)
Fig. 8.8 (continued)
• Reaction of C-5 hydroxyl with one side of C-1 gives , reaction with the
other side gives
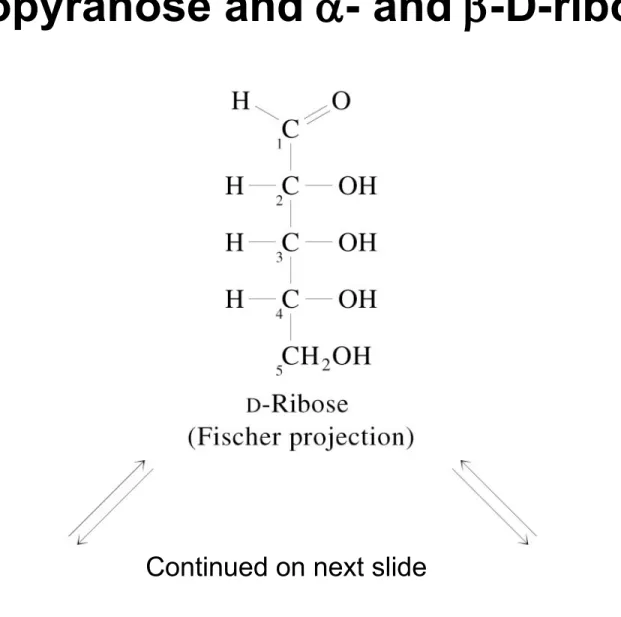
Fig 8.9 Cyclization of D-ribose to form - and
-D-ribopyranose and - and -D-ribofuranose
Continued on next slide
Fig. 8.9 (continued)
Continued next slide
Fig 8.9 (continued)
8.3 Conformations of Monosaccharides
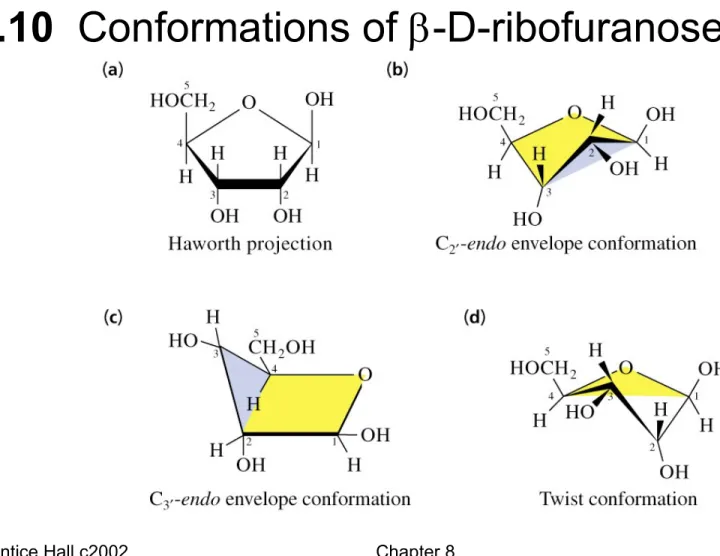
Fig. 8.10 Conformations of -D-ribofuranose
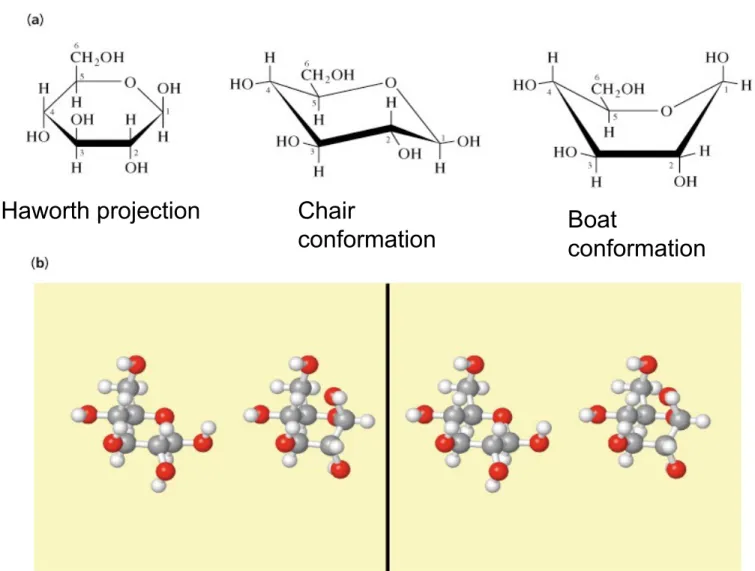
Fig 8.11 Conformations of -D-glucopyranose
(b) Stereo view of
chair (left), boat (right)
Haworth projection Chair
conformation Boat
conformation
Fig 8.12 Conformations of -D-glucopyranose
• Top conformer is more stable because it has the bulky hydroxyl
substituents in
equatorial positions (less steric strain)
8.4 Derivatives of Monosaccharides
• Many sugar derivatives are found in biological systems
• Some are part of monosaccharides, oligosaccharides or polysaccharides
• These include sugar phosphates, deoxy and amino sugars, sugar alcohols and acids
Table 8.1
A. Sugar Phosphates
Fig 8.13 Some important sugar phosphates
B. Deoxy Sugars
• In deoxy sugars an H replaces an OH Fig 8.14 Deoxy sugars
C. Amino Sugars
• An amino group replaces a monosaccharide OH
• Amino group is sometimes acetylated
• Amino sugars of glucose and galactose occur commonly in glycoconjugates
Fig 8.15 Several amino sugars
• Amino and acetylamino groups are shown in red
Fig. 8.15 (continued)
D. Sugar Alcohols (polyhydroxy alcohols)
• Sugar alcohols: carbonyl oxygen is reduced Fig 8.16 Several sugar alcohols
E. Sugar Acids
• Sugar acids are carboxylic acids
• Produced from aldoses by:
(1) Oxidation of C-1 to yield an aldonic acid (2) Oxidation of the highest-numbered carbon
to an alduronic acid
Fig 8.17 Sugar acids derived from glucose
Fig. 8.17 (continued)
F. Ascorbic Acid (Vitamin C)
• L-Ascorbic acid is derived from D-glucuronate Fig 8.18 L-Ascorbic acid
8.5 Disaccharides and Other Glycosides
• Glycosidic bond - primary structural linkage in all polymers of monosaccharides
• An acetal linkage - the anomeric sugar carbon is condensed with an alcohol, amine or thiol
• Glucosides - glucose provides the anomeric carbon
Fig 8.19 Glucopyranose + methanol yields a glycoside
A. Structures of Disaccharides
Fig 8.20 Structures of (a) maltose, (b) cellobiose
Fig. 8.20 (continued)
Structures of (c) lactose, (d) sucrose
B. Reducing and Nonreducing Sugars
• Monosaccharides and most disaccharides are hemiacetals (contain a reactive carbonyl group)
• Called reducing sugars because they can reduce metal ions (Cu2+, Ag+)
• Examples: glucose, maltose, cellobiose, lactose
C. Nucleosides and Other Glycosides
• Anomeric carbons of sugars can form glycosidic linkages with alcohols, amines and thiols
• Aglycones are the groups attached to the anomeric sugar carbon
• N-Glycosides - nucleosides attached via a ring nitrogen in a glycosidic linkage
Fig 8.21 Structures of three glycosides
8.6 Polysaccharides
• Homoglycans - homopolysaccharides
containing only one type of monosaccharide
• Heteroglycans - heteropolysaccharides
containing residues of more than one type of monosaccharide
• Lengths and compositions of a polysaccharide may vary within a population of these molecules
A. Starch and Glycogen
• D-Glucose is stored intracellularly in polymeric forms
• Plants and fungi - starch
• Animals - glycogen
• Starch is a mixture of amylose (unbranched) and amylopectin (branched)
Fig 8.22 Structure of amylose
(a) Amylose is a linear
polymer
(b) Assumes a left-handed helical
conformation in water
Fig 8.23 Structure of amylopectin
Fig 8.24 Action of - and -amylase on amylopectin
• -Amylase
cleaves random internal -(1-4) glucosidic bonds
• -Amylase acts on nonreducing ends
B. Cellulose and Chitin
Fig 8.25 Structure of cellulose
(a) Chair
conformation (b) Haworth projection
Fig 8.26 Stereo view of cellulose fibrils
• Intra- and interchain H-bonding gives strength
Fig 8.27 Structure of chitin
• Repeating units of -(1-4)GlcNAc residues
8.7 Glycoconjugates
• Heteroglycans appear in three types of glycoconjugates:
Proteoglycans Peptidoglycans Glycoproteins
A. Proteoglycans
• Proteoglycans - glycosaminoglycan-protein complexes
• Glycosaminoglycans - unbranched
heteroglycans of repeating disaccharides (many sulfated hydroxyl and amino groups)
• Disaccharide components include: (1) amino sugar (D-galactosamine or D-glucosamine), (2) an alduronic acid
Fig 8.28 Repeating disaccharide of hyaluronic acid
• GlcUA =
D-glucuronate
• GlcNAc=
N-acetylglucosamine
Fig 8.29 Proteoglycan aggregate of cartilage
B. Peptidoglycans
• Peptidoglycans - heteroglycan chains linked to peptides
• Major component of bacterial cell walls
• Heteroglycan composed of alternating GlcNAc and N-acetylmuramic acid (MurNAc)
• -(1 4) linkages connect the units
Fig 8.30 Glycan moiety of peptidoglycan
Fig 8.31 Structure of the peptidoglycan of S. aureus
(a) Repeating disaccharide unit, (b) Cross-linking of the peptidoglycan macromolecule
Fig. 8.31 (continued)
(to disaccharide, previous slide)
Penicillin inhibits a transpeptidase involved in bacterial cell wall formation
• Fig 8.32 Structures of penicillin and
-D-Ala-D-Ala
• Penicillin structure resembling -D-Ala- D-Ala is shown in red
C. Glycoproteins
• Proteins that contain covalently-bound oligosaccharides
• O-Glycosidic and N-glycosidic linkages
• Oligosaccharide chains exhibit great variability in sugar sequence and composition
• Glycoforms - proteins with identical amino acid sequences but different oligosaccharide chain composition
Four subclasses of O-glycosidic linkages
(1) GalNAc-Ser/Thr (most common)
(2) 5-Hydroxylysine (Hyl) to D-galactose (unique to collagen)
(3) Gal-Gal-Xyl-Ser-core protein
(4) GlcNAc to a single serine or threonine
Fig. 8.33 O-Glycosidic and N-glycosidic linkages
Fig 8.34 Four subclasses of O-glycosidic linkages
Fig 8.35 Structures of N-linked oligosaccharides
Fig. 8.35 (continued)