NSC 88-2314-B-002-333
The Study of Dyslipidemia and the Cardiac Electromechanical
Dysfunction
血脂異常對心臟電器生理及機械功能之影響
個人基本資料: 姓名 吳造中 WU CHAU-CHUNG 聯絡地址 100 台北巿中山南路 7 號 國立台灣大學醫學院內科 聯絡電話 (公)02-23970800-5438 傳真號碼 02-23959911 E-MAIL cwu@ha.mc.ntu.edu.tw 執行機關 國立台灣大學醫學院內科中文摘要: 關鍵詞:血脂異常, 心室性心律不整, 射出分率, 心肌梗塞, 許多動物實驗及人類研究結果顯示出, 高膽固醇血症可以經由非動脈硬化的病生理機制 來增加心肌缺氧時的損害, 例如改變心肌細胞膜上的鈉或鈣離子孔道, 影響血小板的活性等 等。通常冠狀動脈粥狀硬化的過程需要數年的時間, 而血脂異常所造成的心肌細胞功能上之 失全或 “ 易感性 ” 早在這以前便已發生。因此, 臨床實驗所觀察到的降血脂藥物可以很快 的降低冠心病之併發症, 其大部份的機轉應該是經由改善這些血脂異常所造成的非動脈粥狀 硬化因素而來。過去的研究顯示在誘發致死性的心室性纖維顫動時, 一個 “ 易感的 ” 心肌 可能與 “ 刺激 ( 比如心肌缺氧 ) ” 或危險因子的存在同樣重要。心肌細胞膜的生理機制在 誘發心律不整的電氣生理機轉中佔有中心性之地位, 因此改變心肌細胞膜的功能亦可改變心 肌的 “ 易感度 ” 。過去已有許多的研究顯示生物細胞膜的脂質成分改變是造成細胞功能變 化的基礎。我們過去的研究也顯示高膽固醇血症會降低心肌細胞膜上鈉離子流的活性。 由於原三年期之研究計劃被刪減為一年之經費,故本年度主要完成原第一年度欲達成之目 標。本研究選取 58 位因急性心肌梗塞(AMI)住入本院加護病房中而在第一天併發心室性心律 不整(VT/VF)之病患(A 組) , 及另 58 位性別、年齡及血栓溶解治療相稱的 AMI 病患(B 組) 。 吾人登錄其冠心病之危險因子,血壓,心跳及檢測其一週內及三個月後的血中總膽固醇(TC)、 高密度膽固醇(HDL-C)、低密度膽固醇(LDL-C)、三酸甘油酯(TG)含量。吾人發現,病人住院當 中的血中脂值濃度均較出院後三個月低。在急性期, A 組病人之 LDL-C 較 B 組高(137.6±35.6 對 122.5±31.3mg/dl, p=0.002), 其平均血壓較低(89.0±18.7 對 99.8±17.4mmHg), 而 Killip 分類為Ⅲ或Ⅳ者亦較多(16 對 5%, p=0.127) 。在三個月之慢性期, A 組病患之 TC (224.6±42.2 對 198.2±33.2mg/dl, p<0.001), LDL-C (159.4±38.1 對 128.3±30.0mg/dl, p<0.0001)及 TG (179.8±102.3 對 136.6±68.4mg/dl, p=0.009) 均較 B 組為高。以多變數分析法發現, 慢性 期 之 LDL-C 值 (p=0.0009), 急 性 期 之 平 均 血 壓 (p=0.0029), 慢 性 及 急 性 期 之 TG 值 差 (p=0.0466) 與急性期病患是否會發生 VT/VF 非常有關, 為獨立之危險因子。 因此,吾人可據以結論, 血脂異常可使急性心肌梗塞病患較易發生心室性心律不整。它可 部份解釋為何近年來發表的大型藥物研究報告中, 降血脂藥物可以降低冠心病的猝死率。
Abstr act
Background Ventricular tachycardia/fibrillation (VT/VF) developing in the acute stage
of myocardial infarction is a primary factor of sudden cardiac death that still remains as a major problem in contemporary cardiology. Several animal studies have shown that dyslipidemia can alter the lipid composition of myocardial membrane and subsequently increases the susceptibility of cardiac arrhythmia. To the best of our knowledge, there have been no previous human studies providing the longitudinal evidence of a relationship between plasma dyslipidemia and arrhythmia. The aim of this study is to determinate whether dyslipidemia is a predisposing factor of the occurrence of VT/VF during the acute stage of myocardial infarction.
Methods and Results A total of 58 patients experiencing VT/VF within 24 hours after
the onset of chest pain were matched to 58 patients without such clinical events. Sex (104 males), age (58+10 years), and the use of thrombolytic therapy (n=82) were required to matched in both groups initially. To diagnose a patient with dyslipidemia, the lipid profiles including total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and triglyceride (TG) were measured during patients’ hospitalization period (day 1 and day 7) and the outpatient follow up at the 3rd month after the index event. Other coronary risk factors, as well as clinical, hemodynamic, and angiographic characteristics were also included in our assessment. The mean serum levels of TC, HDL-C, LDL-C, and TG measured during hospitalization were all lower than those measured 3 months later (-9%, P=0.0003; -6%, P=0.08; -10%, P=0.004; and –9%, P=0.16, respectively). A unique and intriguing pattern was observed in the patients with incidence of VT/VF. During the acute stage, they had a higher level of LDL-C (137.6+35.6 vs. 122.5+31.3 mg/dl, P=0.02), a lower mean blood pressure (89.0±18.7 vs. 99.8±17.4 mmHg, P=0.002), and a higher percentage with Killip class III or IV (16 vs. 5%, P=0.127) on the initial arrival. At the 3-month follow up, these patients with experience of VT/VF had a higher level of TC (224.6+42.2 vs. 198.2+33.2 mg/dl, P<0.001), LDL-C (159.4+38.1 vs. 128.3+30.0 mg/dl, P<0.0001), and TG (179.8+102.3 vs. 136.6+68.4 mg/dl, P=0.009). In multivariate analysis, the LDL cholesterol (P=0.0009) at the 3-month follow up, the mean blood pressure on arrival (P=0.0025), and the difference in level of triglyceride between the first week and the third month (P=0.0466) were all accounted the independent predictors for the occurrence of VT/VF in the acute stage of myocardial infarction.
Conclusions This study suggested that dyslipidemia imposed a higher risk of
developing VT/VF in the acute phase of myocardial infarction. It might partly explain the reason why the current treatment with lipid-lowering drugs could reduce the risk of
major coronary events, including sudden cardiac death, in recently published large-scale study trials.
1. Intr oduction
Dyslipidemia is regarded by many as the main cause of coronary atherosclerosis.1 Several recently published clinical trials have provided the evidence that lipid-lowering interventions are associated with reduced both coronary events and total mortality.2, 3, 4,
5
In the Scandinavian Simvastatin Survival Study (4S),2the reduction in the risk of coronary death was 42% in the simvastatin group and the improvement in survival was more significant in the group with definite acute myocardial infarction (MI) and death within 24 hours. These findings suggest that dyslipidemia seem to have a detrimental impact on the evolution of MI, especially in the acute stage. Furthermore, a significant number of studies have discussed the fact that the risk of coronary occlusion is not proportional to the prior severity of coronary stenoses.6 Several angiographic studies have shown that a majority of patients who subsequently developed acute coronary syndromes appears to have only mild to moderate coronary stenoses of the infarct related artery.7, 8, 9 In addition to coronary atherosclerosis, the stability of plaques and the endothelial function have been examined in this regard.10, 11, 12, 13, 14, 15The change in plaque vulnerability by dyslipidemia develops much earlier than the change in the severity of coronary atherosclerosis, which usually takes years to occur. A number of animal and human studies have also shown that hypercholesterolemia can increase the susceptibility of myocardium to ischemic insults by various nonatherosclerotic mechanisms such as altering the movement of ions through specific channels or pumps in cardiac cell membranes,16, 17, 18, 19 influence of platelet activities,20, 21 and so on. Our previous study revealed that the dyslipidemia had adverse effect on the left ventricular function in acute MI.22 It was also interesting to study whether dyslipidemia also increased the susceptibility of cardiac arrhythmia during the ischemic stress. However, there is few clinical evidence to suggest that serum lipids, lipoproteins or cholesterol play any significant role in the development of either ischemic or reperfusion arrhythmia. The aim of this study was to examine the hypothesis that dyslipidemia could actually aggravate myocardial vulnerability of cardiac arrhythmia in the clinical setting of acute MI and that this deleterious effect was not entirely due to the coronary atherosclerosis. To the best of our knowledge, this is the first longitudinal study to determinate the relationship between plasma dyslipidemia and cardiac arrhythmia.
2. Mater ials and methods
2.1 Study Patients
This study included 58 consecutive patients (group A) who met the following criteria: 1) chest pain ≧30 min in duration; 2) electrocardiographic (ECG) ST segment elevation
≧0.1 mV in two or more leads in the same vascular territory; 3) elevated creatine kinase-MB (CK-MB) isoenzymes within 24 hours of chest pain; 4) ventricular arrhythmias (tachycardia and/or fibrillation) recorded on EKG monitor within 24 hours after the onset of chest pain; 5) the measurement of coronary angiography, radionuclide ventriculography, and serum lipid profile at the defined points in time. Another 58 patient with matching age, sex, and use of thrombolytic therapy were enrolled as control groups (group B) if they met the previous criteria except criteria #4. Patients who were excluded from the study were those with any of following conditions: 1) died within 3 months after MI; 2) could not receive the serial examinations because of disagreement or unstable condition; 3) had taken lipid-lowering medications, diuretics, or beta-blockers before admission; 4) had a previous diagnosis of hypothyroidism, chronic liver disease, nephrotic syndrome, or chronic renal failure; or 5) had a previous MI or were in New York Heart Association functional class ≧II before admission. We decided to exclude those patients to minimize the differences in baseline LVEF among all subjects.
All patients were closely monitored in the coronary care unit (CCU) for at least 3 days. Records of baseline characteristics, past medical history, clinical features, electrocardiographic, and hemodynamic data of each patient were collected. Episodes of non- or sustained ventricular tachycardia and/or fibrillation (VT/VF) were recorded automatically with arrhythmia monitoring system (Hewlett Packard 78510B and 78572B recorder). However, all data were required to be checked by the attending staffs and physicians in the CCU. All patients, if they were admitted within 6 hours after the onset of MI and without any absolute contraindications, would receive 100mg of intravenous recombinant tissue-type plasminogen activator in a 3-hour infusion. Intravenous heparin was given simultaneously as a 5,000-unit bolus and followed by a continuous infusion at 1,000U/hr for at least 2 days. The use of aspirin, beta-blockers, calcium channel antagonists, intravenous lidocaine, angiotensin-converting enzyme inhibitors, and nitrate preparations was left to the discretion of the attending physicians. Lipid-lowering medications were prohibited to all patients in the first 3 months of MI regardless of their initial serum cholesterol levels. The use of lipid-lowering medications, after patients having their lipid profiles checked 3 months after the acute episode of MI, was also left to the discretion of the attending physician.
2.2 Determination of Serum Lipid Profiles
Blood samples for measurements of lipids were drawn after a 14-hour fast. All patients had their lipid profiles checked ≧2 times both during the first week and 3 months after the acute episode of MI. Total cholesterol and triglyceride serum levels were measured with an automatic multichannel chemical analyzer (Hitachi 7450, Hitachi Corp., Tokyo, Japan) by enzymatic methods. High-density lipoprotein cholesterol (HDL-C) levels
were determined after precipitation of chylomicrons. Low-density lipoprotein cholesterol (LDL-C) concentration was computed by the Friedewald formula23 if the triglyceride (TG) level was <400mg/dl.
2.3 Enzymes
Blood samples for measuring CK and CK-MB levels were taken on admission and every 6 hours for the first 48 hours of hospitalization. In our central laboratory, the upper limits of normal range were 160 and 10 IU/L for plasma CK and CK-MB, respectively. 2.4 Definition of Coronary Risk Factors
Patients were classified as non-smokers if they had never smoked or if they had stopped smoking at least 1 year prior to the MI episode. All other patients were classified as smokers. Hypertension was defined as a casual systolic blood pressure >160 mmHg and/or diastolic pressure >95 mmHg with more than two readings, or for those who had been taking antihypertensive medication. Diabetes mellitus was defined according to World Health Organization criteria.24 Body mass index was calculated as body weight in kilograms divided by squared body length in meters. A positive family history was defined if at least one of the patient’s parents or siblings had documented coronary artery disease or sudden death under the age of 60 years.
2.5 Radionuclide Ventriculography
Rest equilibrium-gated blood pool scans were obtained in the anterior and 45o of left anterior oblique projections one week after infarction to minimize the effects of myocardial stunning. The left ventricular ejection fraction (LVEF) was calculated from the 45o left anterior oblique projection.
2.6 Coronary Angiography
Coronary angiography was performed following radionuclide ventriculography, on average of the tenth day of hospitalization. All cine films were reviewed by 2 experienced cardiologists who did not know the patients’ clinical information. Difference of opinions was resolved by a third independent cardiologist. The infarct-related artery was identified by correlating the coronary anatomy with the site of ST-segment elevation on the admission ECG and the distribution of impairment of contractility in the left ventriculogram. The infarct-related artery was classified as patent if the Thrombolysis in Myocardial Infarction (TIMI)25 perfusion grade was 2 or 3 or occluded otherwise. The location of the culprit lesion (the lesion associated with evidence of thrombus or the most proximal severe stenosis) was identified according to the 15-segment model recommended by the American Heart Association.26 Stenoses occluding more than 70% of the arterial diameter were regarded as significant. The number of diseased arteries was determined accordingly.
Mean values ±SD were calculated for continuous variables. Absolute and relative frequencies were measured for discrete variables. Between-group comparisons of lipid profiles, clinical, hemodynamic, and angiographic data were done by Student’s t test for continuous variables and by the chi-square test for categorical variables. The lipid profiles measured in the acute phase of MI and 3 months later were compared by the paired t test. Using multiple logistic regression with stepwise selection, two different models were examined to determine the independent determinants of the occurrence of ventricular arrhythmias within 24 hours after the onset of chest pain. In the model A, the following variables were examined: the age; the sex; the history of smoking, hypertension, and diabetes; the Killip classification; the mean blood pressure on admission; the LVEF measured a week after infarction, and the 3-month post-MI serum lipid profiles. In the model B, the difference between the 1-week and 3 month post-MI serum lipid profiles were also examined in addition to all the variables in the model A. Data were analyzed with the SAS statistical software package, version 6.12. Statistical significance was assumed for P<0.05.
3. Results
3.1 Baseline Characteristics of Patients
In this case-control study, 58 patients experiencing VT/VF within 24 hours after the onset of chest pain were matched to 58 patients without such clinical events. Both groups were matched for sex (104 males), age (58+10 years), and the use of thrombolytic therapy (n=82). The body mass index, risk factors for coronary artery disease, and the frequency of previous angina, and stroke were comparable in these 2 groups (Table 1).
3.2 Clinical Features of Myocardial Infarction
The hemodynamic data and clinical features of myocardial infarction on admission are summarized in Table 2. There were no significant differences in the prevalence of Q wave MI, anterior wall involvement, use of thrombolytic therapy, and peak CK level in these 2 groups. However, patients in group A had a significantly lower systolic (122.0± 26.9 vs. 134.6±24.6, P=0.01), diastolic (72.6±16.3 vs. 82.4±15.7, P=0.001), mean (89.0±18.7 vs. 99.8±17.4, P=0.002) blood pressure on arrival, and a slightly but insignificantly higher Killip class (III or IV) at admission (16 vs. 5%, P=0.127) than group B.
3.3 Serum Lipid Profiles
The mean serum levels of total cholesterol, HDL cholesterol, LDL cholesterol, and triglyceride measured during admission were all lower than those measured 3 months
later (-9%, P=0.0003; -6%, P=0.08; -11%, P=0.004; and –10%, P=0.16, respectively). The patients experiencing VT/VF had higher levels of total cholesterol (224.6+42.2 vs. 198.2+33.2 mg/dl, P<0.001), LDL cholesterol (159.4+38.1 vs. 128.3+30.0 mg/dl, P<0.0001) and triglyceride (179.8+102.3 vs. 136.6+68.4 mg/dl, P=0.009) measured 3 months after myocardial infarction, and a higher level of LDL cholesterol (137.6+35.6 vs. 122.5+31.3 mg/dl, P=0.02) during admission (Table 3).
3.4 Radionuclide Ventriculography and Angiographic Findings
Table 4 shows the radionuclide ventriculography and angiographic findings of these two groups. The patency rate of infarct-related artery and mean number of diseased coronary vessels were similar among these two groups. The locations of the culprit lesions were similar in distribution between these two groups as well. There was no significant difference in LVEF, which was measured one week after infarction among the study groups.
3.5 Influence of Dyslipidemia and Other Nonlipid Variables on the Occurrence of Ventricular Arrhythmias
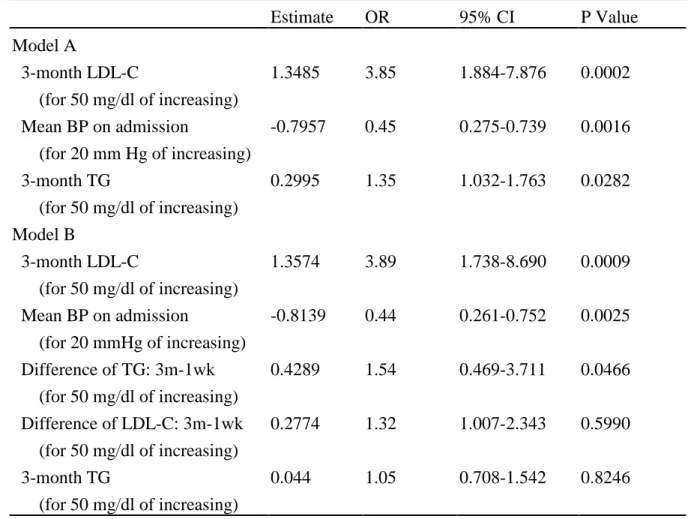
Multiple logistic regression analysis with stepwise selection was performed to assess the independent predictors for the occurrence of VT/VF in the acute stage of MI. Variables of total cholesterol levels were not included in multivariate analysis because of their significant correlation with serum LDL cholesterol levels. Multivariate analysis in model A demonstrated a significant correlation between the occurrence of VT/VF and the 3-month LDL cholesterol (P=0.0002), mean blood pressure on admission (P=0.0016), and 3-month triglyceride (P=0.0282) (Table 5). The 3-month serum LDL cholesterol was the most significant independent predictor of the occurrence of VT/VF during the first day of acute MI. The relative risk of occurrence of VT/VF was 3.85 for each increasing in 50 mg/dl of LDL cholesterol (95% CI: 1.88-7.88), 1.35 for each increasing in 50 mg/dl of triglyceride (95% CI: 1.03-1.76), and 0.45 for each increasing in 20 mmHg of mean blood pressure (95% CI: 0.28-0.74). The measures of association for accessing the predictive ability in this model were Somer’D=0.656, Goodman and Krukal’s gamma=0.657, Kendall’s tau-a=0.331, and c=0.828. All the other lipid and nonlipid variables, including the 3-month serum HDL cholesterol, the ratio of total cholesterol to HDL cholesterol, as well as the age, sex, coronary risk factors, Killip classification, and LVEF measured at 1 week were not independent predictors for the occurrence of VT/VF at the acute stage of MI. We repeated the logistic regression in the model B to access the effect of the lipid changes in acute stage on the occurrence of VT/VF. In this case, the 3-month LDL cholesterol (P=0.0009), the mean blood pressure on admission (P=0.0025), and the difference between 1-week and 3-month triglyceride (P=0.0466) were independent predictors after adjustment for other variables. The
relative risk of occurrence of VT/VF was 3.89 for each increasing in 50 mg/dl of LDL cholesterol (95% CI: 1.74-8.69), 1.54 for each increasing in 50 mg/dl of the difference between 1-week and 3-month triglyceride (95% CI: 1.01-2.34), and 0.44 for each increasing in 20 mmHg of mean blood pressure (95% CI: 0.26-0.75). The measures of association for accessing the predictive ability in this model were Somer’D=0.695, Goodman and Krukal’s gamma=0.696, Kendall’s tau-a=0.350, and c=0.847. No multicollinearity among variables was found in the estimated correlation matrix and the covariance matrix.
4. Discussion
The data in this study suggested that not only the chronic dyslipidemia but also the change of triglyceride level during the acute stage precipitated the occurrence of ventricular arrhythmias of AMI. Acute myocardial infarction has been proven to be associated with profound alterations in the serum lipid profiles, and these perturbations in lipid levels persist for about 2 months.27 We therefore used the measurement of the post-MI serum lipid profile at 3rd month as a surrogate for preinfarction baseline levels. In this study, the 3-month (baseline) LDL cholesterol and the acute change of TG levels were significantly associated with an increased risk of the occurrence of VT/VF in AMI patients. Hypotension, which is a contributor for poor myocardial perfusion, was also concluded as another independent predictor for the arrhythmic events during the acute ischemic episodes.
As there is little doubt that the myocardial membrane mediated process is central to the electrophysiological events which induce arrhythmias. Chronic dyslipidemia could induce the lipid component changes of the myocardial membrane, sequentially disturb the electrophysilogical balance, and arrthymias could occur. This is especially so as it is now known that the membrane function can be influenced by the alteration in dietary lipid intake in both human and animal experiments. Further evidence of level of free fatty acid influencing on membrane function has been demonstrated in vitro, in the culture medium in which isolated cardiac myocytes were grown.28, 29 Considerable evidence have also suggested that the changes in the lipid composition of biological membranes were fundamental to alterations of membrane function.16, 30 In the heart, dietary lipids induced changes in the fatty acid composition of sarcolemma were closely associated with the development of arrhythmia and the extent of ischemic damage.31, 32 In general, three major mechanisms have been proposed to account for these effects. First of all, an alteration in membrane fluidity could affect the function of the membrane–bound enzymes and receptors.33, 33, 33 It has been shown that human
erythrocyte membrane in primary hyperlipidemia revealed an increase in the membrane fluidity and decrease in the Ca2+- Mg2+ ATPase activity.34 Secondly, it has been demonstrated that the modification in dietary lipid could induce an alteration in the availability of fatty acid substrates or inhibitors for myocardial eicosanoid production.34 Specifically dietary fatty acids could change the balance of myocardial thromboxane A2
and prostacyclin. Thirdly, a direct lipid-protein interaction might affect the function of the ion channels and enzymes.33, 35, 36 In our previous study performed on rabbits, we have shown that the density of sodium current on the hyperlipidemic cardiac sarcolemma was significantly lower than that of the normolipidemic one.16Manipulation of the cholesterol content of isolated membranes from a variety of tissues has caused alterations in the activities of Na+-K+ATPase and Ca2+-Mg2+ ATPase.37 Incorporation of cholesterol into the isolated cardiac sarcolemmal vesicles also stimulated the Na+-Ca2+ exchange activity.38 All of these observations suggest that dyslipidemia per se might be arrhythmogenic. Our study demonstrated for the first time this pathophyslogical phenomenon in the clinical setting of acute coronary syndrome.
Moreover, change of triglyceride levels in the acute stage had significant influence on the occurrence of VT/VF. The greater difference between the 1-week and 3-month triglyceride, the greater risk of VT/VF occurrence was noted. After adjusted for the 3-month (baseline) triglyceride, it could be thought that the difference of TG levels might represent the activity of lipolysis. Lipolysis results in an increase in the plasma free fatty acids and glycerol, and is frequently promoted by acute stress such as acute MI. An excess amount of free fatty acids could increase the severity of ischemic damage and possibly arrhythmogenic in the experimental animals. These early findings lead to the views that provision of glucose is “good” and a raised circulating free fatty acid concentration is “bad” for the ischemic myocardium.39 However, the pathophysiologic role of free fatty acid at the myocardial level is still controversial in clinical setting. Paolisso et al.40reported that in non-ischemic patients with NIDDM, plasma free fatty acid concentration was associated with an increased frequency of ventricular premature complexes after intravenous infusion of fat emulsions. However, Fiaccadori et al.41 stated that no arrhythmogenicity was demonstrated during the fat emulsion infusion in patients following valvular heart surgery. The mechanisms of the fatty acid induced arrhythmogenicity seem complex and ambiguous. There is an accumulation of intracellular acylcarnitine and acyl-CoA during myocardial ischemia. Acylcarnitine inhibits the Ca2+ pump of sarcoplasmic reticulum, as well as the sarcolemmal Na+-Ca2+ exchanger and Na+-K+ pump.42 Accumulation of acylcarnitine can directly activate the Ca2+ channels, precipitating arrhythmias via Ca2+ overloading.43 Furthermore, an accumulation of tissue free fatty acid can open an abnormal K+ channel in the late stage
of ischemia.44 Therefore, we suspected that a wasteful turnover of the endogenous triglyceride pool might contribute to the dyslipidemia-related arrhythmogenicity. However, another possible explanation for the association of the acute TG level change and the occurrence of VT/VF was the surge of catecholamine pre se during myocardial ischemia. The activity of lipase was promoted by the adrenergic stimulation. The lipolytic activity might only reflect the sympathetic activity, which was a well-known triggering factor for VT/VF.
An issue needed for further discussion is the left ventricle ejection fraction. The systolic function of left ventricle is the most important predictor for the arrhythmic events in post-MI patients. However, the LVEF was not an independent predictor in this study. Possible explanations are: firstly, the LVEF measured in our study was about 1 week after the onset of MI, not right at the onset of arrhythmia. Secondly, the arrhythmic events analyzed in this study occurred within 24 hours after the onset of MI, which might have different clinical characteristics as compared with those occurring 1 week after MI. This variance could be a significant factor, which required further studies to verify and confirm the significance of acute LVEF change in trigging VT/VF for AMI patients. There is another limitation in this study. In order to obtain serum lipid profiles that could reflect the baseline lipid status, patients who died within 3 months or could not be followed up for at least 3 months after the occurrence of MI were excluded form this study. This would inevitably lead to selection bias and exclusion of patients with more severe LV dysfunction. However, since numerous studies have shown that dyslipidemias were associated with worse outcome after coronary events, the exclusion of patients with higher risk should theoretically lead to underestimation of the possible association between serum lipid profiles and arrhythmic events.
In conclusion, dyslipidemia per se imposed patients a higher risk of developing VT/VF in the acute phase of MI, and this effect was not entirely through the mechanisms of dyslipidemia-associated atherosclerosis. Not only the baseline serum lipid profiles but also the triglyceride level change during ischemic stress has been shown to play a significant role in the arrhythmogenesis. This might partly explain why treatment with lipid-lowering drugs could reduce the risk of major coronary events, including sudden cardiac death, in recently published large-scale trials.
Acknowledgments
This study was supported by NSC grant NSC88-2314-B-002-333 to Wu CC. We specially thank to Sonia Chen, Pharm D., from the National Taiwan University Hospital, for her assistance in preparing the manuscript.
REFERENCES
2. Gotto AM Jr, LaRosa JC, Hunninghake D et al. The cholesterol facts. A summary of the evidence relating dietary fats, serum cholesterol, and coronary heart disease. Circulation 1990; 81: 1721-1733.
3. Scandinavian Simvastatin Survival Study Group. Randomized trial of cholesterol lowering therapy in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344:1383-1389.
4. Shepherd J, Cobbe SM, Isles CG, Lorimer AR, MacFarlane PN, Mckillop JH, Packard CJ. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. The West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333:1301-1307.
5. Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, Brown L, Warnica JW, Arnold JMO, Wun CC, Davis BR, Braunwald E, for the Cholesterol and Recurrent Events Trial Investigators. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl Med. 1996;335:1001-1009.
6. Brown G, Albers JJ, Fisher LD, Sheafer SAM, Lin JT, Kaplan C, Zhao XQ, Bisson BD Fitzpatrick VF, Dodge HT. Regression of coronary artery disease as a result of intensive lipid lowering therapy in men with high levels of apolipoprotein B. N Engl J Med. 1990;323:1289-1298.
7. Ambrose JA, Fuster V. The risk of coronary occlusion is not proportional to the prior severity of coronary stenoses. Heart 1998; 79:3-4.
8. Ambrose JA, Tannenbaum MA, Alexopoulos D, et al. Angiographic progression of coronary artery disease and the development of myocardial infarction. J Am Coll Cardiol 1998; 12:56-52.
9. Little WC, Constantinsecu MS, Applegate RJ, et al. Can coronary angiography predict the site of subsequent myocardial infarction in patients with mild to moderate coronary disease? Circulation 1988; 78:1157-1166.
10. Brown BG, Gallery CA, Badger RS, et al. Incomplete lysis of thrombus in the moderate underlying atherosclerotic lesion during intracoronary infusion of streptokinase for acute myocardial infarction: quantitative angiographic observations. Circulation 1986; 73:653-61
11. Ganz P, Creager MA, Fang JC, McConnell MV, Lee RT, Libby P, Selwyn AP,. Pathogenetic mechanisms of atherosclerosis: Effect of lipid lowering on the biology of atherosclerosis. Am J Med. 1996;101(suppl 4A):10S-16S.
12. Vita JA, Treasure CB, Nabel EG, McLenachan JM, Fish RD, Yeung AC, Vekshtein VI, Selwyn AP, Ganz P. Coronary vasomotor response to acetylcholine
relates to risk factors for coronary artery disease. Circulation. 1990;81:491-497. 13. Zeiher AM, Schachlinger V, Hohnloser ‘SH, Saurbier B, Just H. Coronary
atherosclerotic wall thickening and vascular reactivity in humans. Elevated high-density lipoprotein levels ameliorate abnormal vasoconstriction in early atherosclerosis. Circulation. 1994;89:2525-2532.
14. Yokohama M, Hirata K, Miyake R, Akita H, Ishikawa Y, Fukuzari H. Lysophosphatidylcholine: essential role in the inhibition of endothelium-dependent vasorelaxation by oxidized low density lipoprotein. Biochem Biophys Res Commun. 1990;169:301-308.
15. Anderson TJ, Meredith IT, Yeung AC, Frei B, Selwyn AP, Ganz P. The effect of cholesterol-lowering and antioxidant therapy on endothelium-dependent coronary vasomotion. N Engl J Med. 1995;332:488-493.
16. Anderson TJ, Meredith IT, Yeung AC, Charbonneau F, Selwyn AP, Ganz P. Endothelium-dependent coronary vasomotion relates to the susceptibility of LDL to oxidation in humans. Circulation. 1996;93:1647-1650.
17. Wu CC, Su MJ, Chi JF, Chen WJ, Hsu HC, Lee YT. The effect of hypercholesterolemia on the sodium inward currents in cardiac myocyte. J Mol Cell Cardiol 1995;27:1263-1269.
18. Kutryk MJB, Pierce GN. Stimulation of sodium-calcium exchange by cholesterol incorporation into isolated cardiac sarcolemmal vesicles. J Biol Chem. 1988;263:13167-13172.
19. Lars Bastiaanse EM, Atsma DE, Kuijpers MMC, Van der Laarse A. The effect of sarcolemmal cholesterol content on intracellular calcium ion concentration in cultured cardiomyocytes. Arch Biochem Biophys. 1994;313:58-63.
20. Liu K, Massaeli H, Pierce GN. The action of oxidized low density lipoprotein on calcium transients in isolated rabbit cardiomyocytes. J Biol Chem. 1993;268:4145-4151.
21. Surya I, Akkerman J-W. The influence of lipoproteins on blood platelets. Am Heart J. 1993;125:272-275.
22. Beitz J, Block HU, Beitz A, Muller G, Winkler L, Dargel R, Mest HJ. Endogenous lipoproteins modify the thromboxane formation capacity of platelets. Atherosclerosis. 1996;60:95-99.
23. Wang TD, Wu CC, Chen WJ, Lee CM, Chen MF, Liau CS, Sung FC, Lee YT> Dyslipidemias have a detrimental effect on the left ventricular systolic function in patients with a first acute myocardial infarction. Am J Cradiol 1998;81:531-537. 24. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of
ultracentrifuge. Clin Chem. 1972;18:499-502.
25. National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1983;28:1039-1057.
26. The TIMI Study Group. The thrombolysis on myocardial infarction trial. N Engl J Med. 1985;312:932-936.
27. Austen WG, Edwards JE, Frye RL, Gensini GG, Gott VL, Griffith LSC. A reporting system on patients evaluated for coronary artery disease. Circulation. 1975;51(suppl):5-40.
28. Rosenson RS. Myocardial injury: the acute phase response and lipoprotein metabolism. J Am Coll Cardiol. 1993;22:933-940.
29. Spector AA, Yorek MA. Membrane lipid composition and cellular function. J Lipid Res 1985;26:1015-1035.
30. Hallaq H, Smith TW, Leaf A. Modulation of dihydropyridine-sensitive calcium channels in heart cells by fish oil fatty acids. Proc Natl Acad Sci USA 1992;89:1760-1764.
31. King ME, Spector AA. Effect of specific fatty acyl enrichments on membrane physical properties detected with a spin label probe. J Biol Chem 1978;253:6493-6501.
32. Dhalla NS, Ziegelhoffer A, Harrow JA. Regulatory role of membrane systems in heart function. Can J Physiol Pharmacol 1977;55:1211-1234.
33. Katz AM, Messineo FC. Lipid-membrane interactions and the pathogenesis of ischemic damage in the myocardium. Circ Res1981;48:1-16.
34. Muzulu SI, Bing RF, Norman RI. Human erythrocyte membrane fluidity and calcium pump activity in primary combined hyperlipidaemia. Clin Sci 1995; 88: 307-310.
35. Charnock JS, McLennan PL, Abeywardena MY. Dietary modulation of lipid metabolism and mechanical performance of the heart. Mol Cell Biochem 1992;116:19-25.
36. McLennan PL, Abeywardena MY, Charnock JS, McMurchie EJ. Dietary lipid modulation of myocardial β-adrenergic mechanisms, Ca2+-depenent automaticity, and arrhythmogenesis in the marmoset. J Cardiovasc Pharmacol 1987;10:293-300. 37. Kang JX, Leaf A. Evidence that free polyunsaturated fatty acids modify Na+
channels by directly binding to the channel protein. Proc. Natl. Acad. Sci. USA 1996; 93: 3542-3546.
38. Warren GB, Housley MD, Metcalfe JC, Birdsalt NJM. Cholesterol is excluded from the phospholipid annulus surrounding an active calcium transport protein. Nature 1975;255:684-687.
39. Kurtryk MJB, Pierce GN. Stimulation of sodium-calcium exchange by cholesterol incorporation into sarcolemmal vesicles. J Biol Chem 1988;263:13167-13172.
40. Oliver MF, Opie LH. Effect of glucose and fatty acids on myocardial ischemia and arrhythmias. Lancet 1994; 343:155-58.
41. Paolisso G, Gualdiero P, Manzella D, Rizzo MR, Tagliamonte MR, Gambardella A, Verza M, Gentile S, Varricchio M. Association of fasting plasma free fatty acid concentration and frequency of ventricular premature complexes in non-ischemic non-insulin-dependent diabetic patients. Am J Cardiol 1997; 80(7):932-7.
42. Fiaccadori E, Tortorella C, Gonzi G, Pincolini S, Belli L, Albertini D, Beghi C, Avogar A. Hemodynamic, respiratory, and metabolic effects of medium-chain triglyceride-enriched lipid emulsions following valvular heart surgery. Chest 1994; 106:1660-67.
43. Huang JM-C, Xian H, Bacaner M. Long-chain fatty acids activate calcium channels in ventricular myocytes. Proc Natl acad Sci USA 1992; 89:6452-6456.
44. Fischbach PS, Corr PB, Yamada KA. Long-chain acylcarnitine increases intracellular Ca2+ and induces afterdepolarization in adult ventricular myocytes. Circulation 1992: 189-200.
45. Kim D, Duff R. Regulation of K+ channels in cardiac myocytes by free fatty acids. Circ Res 1990; 67:104-46.
TABLES
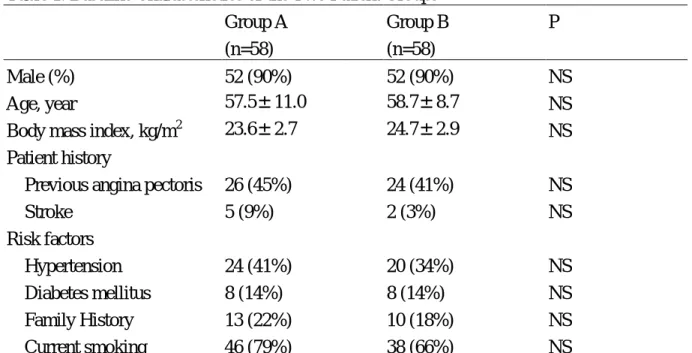
Table 1. Baseline Characteristics of the Two Patient Groups Group A (n=58) Group B (n=58) P Male (%) 52 (90%) 52 (90%) NS Age, year 57.5±11.0 58.7±8.7 NS
Body mass index, kg/m2 23.6±2.7 24.7±2.9 NS
Patient history
Previous angina pectoris 26 (45%) 24 (41%) NS
Stroke 5 (9%) 2 (3%) NS Risk factors Hypertension 24 (41%) 20 (34%) NS Diabetes mellitus 8 (14%) 8 (14%) NS Family History 13 (22%) 10 (18%) NS Current smoking 46 (79%) 38 (66%) NS
Table 2. Clinical Characteristics of the Two Patient Groups Group A (n=58) Group B (n=58) P Hemodynamic Data*
Heart rate, beats per minute 76.4 ±21.1 78.5 ±16.3 NS
Blood pressure, mmHg Systolic 122.0 ±26.9 134.6 ±24.6 0.01 Diastolic 72.6 ±16.3 82.4 ±15.7 0.001 Mean 89.0 ±18.7 99.8 ±17.4 0.002 Clinical Features of MI Infarction Location Anterior 29 (50%) 33 (57%) NS Other 29 (50%) 25 (43%) Q wave MI 52 (90%) 53 (91%) NS
Worst Killip class > III 9 (16%) 3 (5%) 0.127
Thrombolytic therapy 41 (71%) 41 (71%) NS
Peak CK, IU/L 2622.6 ±2022.2 2319.3 ±1689.6 NS
* Hemodynamic data was recorded on admission; CK indicates creatine kinase; MI, myocardial infarction.
Table 3. Serum Lipid Profiles at Studies All cases (n=116) Group A (n=58) Group B (n=58) P Total Cholesterol, mg/dl 1 week 193.4+34.9* 197.1+34.6 189.6+35.1 NS 3 month 211.4+40.1 224.6+42.2 198.2+33.2 <0.001 HDL Cholesterol, mg/dl 1 week 36.8+8.8 35.9+9.7 37.7+7.9 NS 3 month 38.9+9.2 38.1+10.0 39.7+8.3 NS LDL Cholesterol, mg/dl 1 week 130.1+34.2# 137.6+35.6 122.5+31.3 0.02 3 month 143.9+37.5 159.4+38.1 128.3+30.0 <0.0001 Triglyceride, mg/dl 1 week 143.9+64.0 144.4+66.2 143.4+62.2 NS 3 month 158.2+89.3 179.8+102.3 136.6+68.4 0.009
HDL indicates high-density lipoprotein and LDL, low-density lipoprotein.
*, P<0.001 for the comparison with the corresponding 3-month values; #, P<0.01 for the comparison with the corresponding 3-month values.
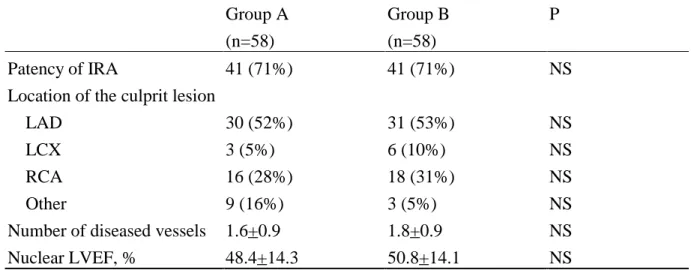
Table 4. Radionuclide Ventriculography and Angiographic Data of the Two Patient Groups Group A (n=58) Group B (n=58) P Patency of IRA 41 (71%) 41 (71%) NS
Location of the culprit lesion
LAD 30 (52%) 31 (53%) NS
LCX 3 (5%) 6 (10%) NS
RCA 16 (28%) 18 (31%) NS
Other 9 (16%) 3 (5%) NS
Number of diseased vessels 1.6+0.9 1.8+0.9 NS
Nuclear LVEF, % 48.4+14.3 50.8+14.1 NS
IRA indicates infract-related artery; LAD left anterior descending artery; LCX, left circumflex artery; RCA, right coronary artery; and LVEF, left ventricular ejection fraction.
Table 5. Variables Significantly Related to the Occurrence of Ventricular Arrhythmia by Multiple Logistic Regression Analysis
Estimate OR 95% CI P Value Model A 3-month LDL-C (for 50 mg/dl of increasing) 1.3485 3.85 1.884-7.876 0.0002 Mean BP on admission (for 20 mm Hg of increasing) -0.7957 0.45 0.275-0.739 0.0016 3-month TG (for 50 mg/dl of increasing) 0.2995 1.35 1.032-1.763 0.0282 Model B 3-month LDL-C (for 50 mg/dl of increasing) 1.3574 3.89 1.738-8.690 0.0009 Mean BP on admission (for 20 mmHg of increasing) -0.8139 0.44 0.261-0.752 0.0025 Difference of TG: 3m-1wk (for 50 mg/dl of increasing) Difference of LDL-C: 3m-1wk (for 50 mg/dl of increasing) 3-month TG (for 50 mg/dl of increasing) 0.4289 0.2774 0.044 1.54 1.32 1.05 0.469-3.711 1.007-2.343 0.708-1.542 0.0466 0.5990 0.8246
BP, blood pressure; LDL-C, low-density lipoprotein-cholesterol; TG, triglyceride; OR, Odds ratio; and 95%CI, 95% confidence interval.
1. Gotto AM Jr, LaRosa JC, Hunninghake D et al. The cholesterol facts. A summary of the evidence relating dietary fats, serum cholesterol, and coronary heart disease. Circulation 1990; 81: 1721-1733.
2. Scandinavian Simvastatin Survival Study Group. Randomized trial of cholesterol lowering therapy in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344:1383-1389.
3. Shepherd J, Cobbe SM, Isles CG, Lorimer AR, MacFarlane PN, Mckillop JH, Packard CJ. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. The West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333:1301-1307.
4. Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, Brown L, Warnica JW, Arnold JMO, Wun CC, Davis BR, Braunwald E, for the Cholesterol and Recurrent Events Trial Investigators. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl Med. 1996;335:1001-1009.
5. Brown G, Albers JJ, Fisher LD, Sheafer SAM, Lin JT, Kaplan C, Zhao XQ, Bisson BD Fitzpatrick VF, Dodge HT. Regression of coronary artery disease as a result of intensive lipid lowering therapy in men with high levels of apolipoprotein B. N Engl J Med. 1990;323:1289-1298.
6. Ambrose JA, Fuster V. The risk of coronary occlusion is not proportional to the prior severity of coronary stenoses. Heart 1998; 79:3-4.
7. Ambrose JA, Tannenbaum MA, Alexopoulos D, et al. Angiographic progression of coronary artery disease and the development of myocardial infarction. J Am Coll Cardiol 1998; 12:56-52.
8. Little WC, Constantinsecu MS, Applegate RJ, et al. Can coronary angiography predict the site of subsequent myocardial infarction in patients with mild to moderate coronary disease? Circulation 1988; 78:1157-1166.
9. Brown BG, Gallery CA, Badger RS, et al. Incomplete lysis of thrombus in the moderate underlying atherosclerotic lesion during intracoronary infusion of streptokinase for acute myocardial infarction: quantitative angiographic observations. Circulation 1986; 73:653-61
10. Ganz P, Creager MA, Fang JC, McConnell MV, Lee RT, Libby P, Selwyn AP,. Pathogenetic mechanisms of atherosclerosis: Effect of lipid lowering on the biology of atherosclerosis. Am J Med. 1996;101(suppl 4A):10S-16S.
11. Vita JA, Treasure CB, Nabel EG, McLenachan JM, Fish RD, Yeung AC, Vekshtein VI, Selwyn AP, Ganz P. Coronary vasomotor response to acetylcholine relates to risk factors for coronary artery disease. Circulation. 1990;81:491-497.
12. Zeiher AM, Schachlinger V, Hohnloser ‘SH, Saurbier B, Just H. Coronary atherosclerotic wall thickening and vascular reactivity in humans. Elevated high-density lipoprotein levels ameliorate abnormal vasoconstriction in early atherosclerosis. Circulation. 1994;89:2525-2532.
13. Yokohama M, Hirata K, Miyake R, Akita H, Ishikawa Y, Fukuzari H. Lysophosphatidylcholine: essential role in the inhibition of endothelium-dependent vasorelaxation by oxidized low density lipoprotein. Biochem Biophys Res Commun.
1990;169:301-308.
14. Anderson TJ, Meredith IT, Yeung AC, Frei B, Selwyn AP, Ganz P. The effect of cholesterol-lowering and antioxidant therapy on endothelium-dependent coronary vasomotion. N Engl J Med. 1995;332:488-493.
15. Anderson TJ, Meredith IT, Yeung AC, Charbonneau F, Selwyn AP, Ganz P. Endothelium-dependent coronary vasomotion relates to the susceptibility of LDL to oxidation in humans. Circulation. 1996;93:1647-1650.
16. Wu CC, Su MJ, Chi JF, Chen WJ, Hsu HC, Lee YT. The effect of hypercholesterolemia on the sodium inward currents in cardiac myocyte. J Mol Cell Cardiol 1995;27:1263-1269.
17. Kutryk MJB, Pierce GN. Stimulation of sodium-calcium exchange by cholesterol incorporation into isolated cardiac sarcolemmal vesicles. J Biol Chem. 1988;263:13167-13172.
18. Lars Bastiaanse EM, Atsma DE, Kuijpers MMC, Van der Laarse A. The effect of sarcolemmal cholesterol content on intracellular calcium ion concentration in cultured cardiomyocytes. Arch Biochem Biophys. 1994;313:58-63.
19. Liu K, Massaeli H, Pierce GN. The action of oxidized low density lipoprotein on calcium transients in isolated rabbit cardiomyocytes. J Biol Chem. 1993;268:4145-4151.
20. Surya I, Akkerman J-W. The influence of lipoproteins on blood platelets. Am Heart J. 1993;125:272-275.
21. Beitz J, Block HU, Beitz A, Muller G, Winkler L, Dargel R, Mest HJ. Endogenous lipoproteins modify the thromboxane formation capacity of platelets. Atherosclerosis. 1996;60:95-99.
22. Wang TD, Wu CC, Chen WJ, Lee CM, Chen MF, Liau CS, Sung FC, Lee YT> Dyslipidemias have a detrimental effect on the left ventricular systolic function in patients with a first acute myocardial infarction. Am J Cradiol 1998;81:531-537. 23. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of
low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499-502.
24. National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1983;28:1039-1057.
25. The TIMI Study Group. The thrombolysis on myocardial infarction trial. N Engl J Med. 1985;312:932-936.
26. Austen WG, Edwards JE, Frye RL, Gensini GG, Gott VL, Griffith LSC. A reporting system on patients evaluated for coronary artery disease. Circulation. 1975;51(suppl):5-40.
27. Rosenson RS. Myocardial injury: the acute phase response and lipoprotein metabolism. J Am Coll Cardiol. 1993;22:933-940.
28. Spector AA, Yorek MA. Membrane lipid composition and cellular function. J Lipid Res 1985;26:1015-1035.
29. Hallaq H, Smith TW, Leaf A. Modulation of dihydropyridine-sensitive calcium channels in heart cells by fish oil fatty acids. Proc Natl Acad Sci USA 1992;89:1760-1764.
30. King ME, Spector AA. Effect of specific fatty acyl enrichments on membrane physical properties detected with a spin label probe. J Biol Chem
6501.
31. Dhalla NS, Ziegelhoffer A, Harrow JA. Regulatory role of membrane systems in heart function. Can J Physiol Pharmacol 1977;55:1211-1234.
32. Katz AM, Messineo FC. Lipid-membrane interactions and the pathogenesis of ischemic damage in the myocardium. Circ Res1981;48:1-16.
33. Muzulu SI, Bing RF, Norman RI. Human erythrocyte membrane fluidity and calcium pump activity in primary combined hyperlipidaemia. Clin Sci 1995; 88: 307-310.
34. Charnock JS, McLennan PL, Abeywardena MY. Dietary modulation of lipid metabolism and mechanical performance of the heart. Mol Cell Biochem 1992;116:19-25.
35. McLennan PL, Abeywardena MY, Charnock JS, McMurchie EJ. Dietary lipid modulation of myocardial β-adrenergic mechanisms, Ca2+-depenent automaticity, and arrhythmogenesis in the marmoset. J Cardiovasc Pharmacol 1987;10:293-300. 36. Kang JX, Leaf A. Evidence that free polyunsaturated fatty acids modify Na+
channels by directly binding to the channel protein. Proc. Natl. Acad. Sci. USA 1996; 93: 3542-3546.
37. Warren GB, Housley MD, Metcalfe JC, Birdsalt NJM. Cholesterol is excluded from the phospholipid annulus surrounding an active calcium transport protein. Nature 1975;255:684-687.
38. Kurtryk MJB, Pierce GN. Stimulation of sodium-calcium exchange by cholesterol incorporation into sarcolemmal vesicles. J Biol Chem 1988;263:13167-13172. 39. Oliver MF, Opie LH. Effect of glucose and fatty acids on myocardial ischemia and
arrhythmias. Lancet 1994; 343:155-58.
40. Paolisso G, Gualdiero P, Manzella D, Rizzo MR, Tagliamonte MR, Gambardella A, Verza M, Gentile S, Varricchio M. Association of fasting plasma free fatty acid concentration and frequency of ventricular premature complexes in non-ischemic non-insulin-dependent diabetic patients. Am J Cardiol 1997; 80(7):932-7.
41. Fiaccadori E, Tortorella C, Gonzi G, Pincolini S, Belli L, Albertini D, Beghi C, Avogar A. Hemodynamic, respiratory, and metabolic effects of medium-chain triglyceride-enriched lipid emulsions following valvular heart surgery. Chest 1994; 106:1660-67.
42. Huang JM-C, Xian H, Bacaner M. Long-chain fatty acids activate calcium channels in ventricular myocytes. Proc Natl acad Sci USA 1992; 89:6452-6456.
43. Fischbach PS, Corr PB, Yamada KA. Long-chain acylcarnitine increases intracellular Ca2+ and induces afterdepolarization in adult ventricular myocytes. Circulation 1992: 189-200.
44. Kim D, Duff R. Regulation of K+ channels in cardiac myocytes by free fatty acids. Circ Res 1990; 67:104-46.