Sorption and Transformation of Methyl-tert-Butyl Ether
(MTBE) over Nafion, a Solid Acid Catalyst
連興隆,國立高雄大學土木與環境工程學系副教授
Wei-xian Zhang, Associate Professor, Dep. of Civil and Environ. Eng., Lehigh University, Bethlehem, PA 18015, USA
摘要
本研究利用一有機高子固體聚合物 Nafion 來進行去除水中甲基第三丁基醚 (methyl tert-butyl ether,MTBE)之研究。Nafion 是一種酸性強度與 100%硫酸近似 之固體超強酸催化劑(super-acid catalyst),已被廣泛應用於有機合成之反應。 MTBE 曾是被廣泛使用的含氧汽油添加劑,已成為地下水中常見之污染物,並造
成飲用水的污染。本研究發現,利用比表面積約 200 m2
/g 之 Nafion 處理水中之 MTBE,除了具有顯著之吸附作用外,同時有 MTBE 之降解作用發生。MTBE 被轉化成第三丁基醇(tert-butyl alcohol,TBA)與丙酮;TBA 則轉化成丙酮。丙酮 的生成應存在異丁烯(isobutene)做為中間產物,然而卻不易由 GC 分析得之。由 於異丁烯的生成,在探討 MTBE 降解的反應途徑中扮演重要角色,因此本研究 利用 tert-amyl methyl ether (TAME)做為探測分子(probe),於 GC-MS 的分析中確 認生成與異丁烯相應之 isoamylene。本研究結果顯示,整體而言,MTBE 的轉化 作 用 應 該 包 括 了 水 解 (hydrolysis) 、 脫 氫 作 用 (dehydrogenation) 與 氧 化 反 應 (oxidation)。
關鍵字:甲基第三丁基醚、固體超強酸催化劑、MTBE、Nafion、水污染
Introduction
MTBE is used primarily as a gasoline oxygenate that, when mixed with gasoline, promotes more complete combustion, thereby reducing exhaust emissions of carbon monoxide and reactive organic compounds (US EPA, 1993; 1998). The 1990 Clean Air Act Amendments require the use of oxygenates through the reformulated gasoline program (RFG) and winter oxygenated fuel program. About 30% of gasoline used in the U.S. is RFG. MTBE is used in approximately 76% of RFG at 11% by volume. The increasing use of MTBE since 1990 has turned it into one of the most
frequently detected contaminants in groundwater and surface waters in the U.S. (Squillace et al., 1996; CalEPA, 1998). Incidents of both groundwater and surface water contamination by MTBE have been widely reported (Delzer et al., 1996).
MTBE is a comparatively unreactive compound. The ether linkage is quite stable toward bases, oxidizing agents, and reducing agents. In so far as the ether linkage itself is concerned, ethers under go primarily one kind of reaction, cleavage by acids (McMurry, 1996; Morrison and Boyd, 1992):
OH R X R HX R O R− − + → − + − (1)
The cleavage reaction takes place only under quite vigorous conditions: concentrated acids and high temperature.
The use of a water-soluble strong acid such sulfuric acid would not be applicable for drinking water and wastewater treatment due to obvious obstacles such as a strongly acidic effluent and corrosion. It would be beneficial to use a water-insoluble solid acids which may serve as a catalyst for the MTBE transformation. Nafion may prove promising for this purpose. Nafion, with average molecular weight greater than 1500, is a polymeric organic acid that consists of perfluorosulfonic acid resin with terminal sulfonic acid groups shown as follows (Waller and Van Scoyoc, 1989; Sun et al., 1997):
[(CF2CF2)mCFCF2]n (OCF2CF)kCF3 O CF2CF2SO3H m = 5-15 n = ca. 1000 k = 1, 2, 3
The perfluorinated backbone gives the mechanical strength and chemical stability to the polymer. The ether linkage between the side chain and polymer backbone leads to its flexibility (Waller and Van Scoyoc, 1989). Nafion in the acid form has a terminal –CF2CF2SO3H group. The fluorocarbon portion of the polymer molecule has
high electron-withdrawing capacities, which leave the sulfonate-proton bond strongly polarized. The acid groups in Nafion have a Hammett acidity (-H0~12) similar to
100% sulfuric acid (Waller and Van Scoyoc, 1989). Accordingly, it has been often termed as a superacidic catalyst. The high acid strength and chemical inertness of the fluorocarbon make Nafion an attractive alternative for homogeneous acid catalysts (Olah et al., 1985).
An important advantage of a solid catalyst used in a packed-bed reactor is the easy separation of treated water from the catalyst. Furthermore, deactivated Nafion can be regenerated with dilute acid solutions (Waller and Van Scoyoc, 1989). Therefore, no chemical is added during the treatment run. Objective of this study is to test feasibility of Nafion as a solid catalyst for the MTBE removal from aqueous solution. Experiments are designed to determine the sorption of MTBE, rate and extent of MTBE transformation, and reaction intermediates and final products.
Experimental Sections
Materials and Chemicals. Nafion SAC-13 was obtained from Aldrich. Methyl
tert-butyl ether (MTBE), tert-butyl alcohol (TBA), tert-amyl alcohol, acetone,
methanol, and isobutene were all of HPLC grade and were purchased from Sigma-Aldrich. tert-Amyl methyl ether (TAME) from Aldrich has a purity of 97%.
Batch Experiments. The batch tests for MTBE sorption and transformation were
conducted with 100 mL serum vials with crimp top septa. 2-5 grams of Nafion SAC-13 were charged to a 50 mL MTBE solution (0.1-1000 mg/L). Then the serum vials were put in a rotator (50 rpm) and sampled at regular intervals with a gas-tight syringe.
Method of Analyses. Organic analysis was performed using a Tekmar 3000
purge-and-trap concentrator connected to a Shimadzu QP5000 GC/MS fitted with a DB-624 capillary column (30 m x 0.25 mm). A VOCARB 3000 trap column (Supelco) was installed to remove excessive water. The Tekmar default method was used except that a desorption time was shortened from 120 to 30 seconds. GC temperature was programmed at 50°C for 5 minutes and increased at a rate of 5 °C/min to 100°C. Injection and detector temperatures were set at 150 and 230 °C, respectively. Quadrupole mass spectrometer was set to scan from 20 to 150 m/z and data collection every 0.1 second.
Results and Discussion
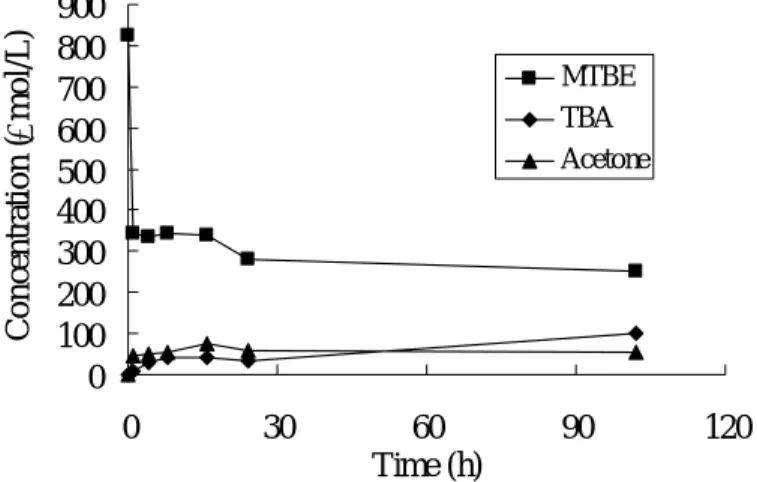
Figure 1 shows the time courses of MTBE removal and its transformational products in a 50 mL batch solution with 4 g of Nafion SAC-13. Rapid sorption and slow transformation were observed. 58% of MTBE was removed within the first hour. Acetone was detected instantly. TBA appeared in the solution after 6 hours.
Concentrations of acetone and TBA increased slowly after that. Yields of acetone and TBA after 100 hours were about 6% and 12%, respectively.
0 100 200 300 400 500 600 700 800 900 0 30 60 90 120 Time (h) C o n ce n tr atio n ( µ m o l/L ) MTBE TBA Acetone
Figure 1. Transformation of MTBE over Nafion SAC-13 (3 g/50mL).
Results for the transformation of TBA with Nafion are shown in Figure 2. TBA concentration decreased significantly within first few hours and produced acetone as the byproduct. About 55% of TBA was removed and the yield of acetone was approximately 10%. 0 100 200 300 400 500 600 0 30 60 90 120 Time (h) C o n cen tr at io n ( µ mo l/ L ) TBA Acetone
Figure 2. Transformation of TBA over Nafion SAC-13 (3 g/50mL).
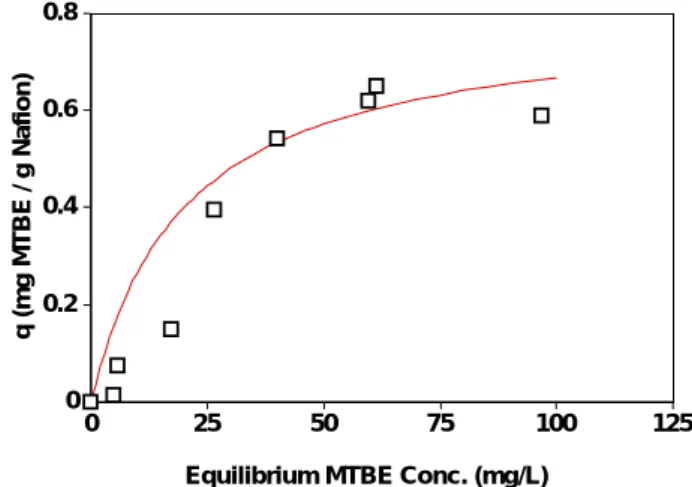
The slow production of transformational products suggests that the initial removal of MTBE was largely due to the sorption of MTBE to Nafion. The presence of ether bonds in both Nafion and MTBE may favor the sorption and accumulation of MTBE over the Nafion surface. A sorption isotherm was measured with batch
experiments under 25°C, assuming that the sorption equilibrium was achieved within 2 hours. The sorption data were fitted to the Langmuir isotherm as shown in Figure 3:
q= QbCe
1+ bCe (2)
where q is the sorption capacity (mg/g), Q is the maximum sorption capacity (mg/g), b is the sorption equilibrium constant (L/mg) and Ce is the equilibrium MTBE
concentration in the aqueous phase (mg/L). The maximum sorption capacity and the sorption equilibrium constant were determined to be 0.04 mg/g and 0.05 L/mg, respectively. 0 0.2 0.4 0.6 0.8 q (mg MTBE / g Nafion) 0 25 50 75 100 125
Equilibrium MTBE Conc. (mg/L)
Figure 3. Sorption of MTBE on Nafion SAC-13. The curve is plotted with a Langmuir isotherm.
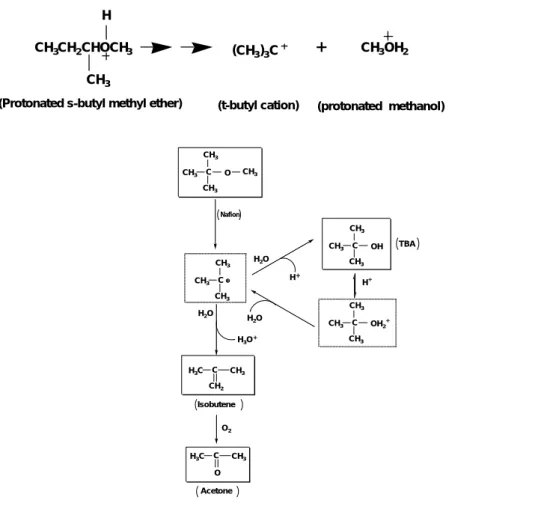
Transformational reactions occurred on the surface after the sorption. Based upon the above observations, a scheme of the MTBE transformational pathways is presented in Figure 4. Thermodynamically, MTBE could be hydrolyzed to form
tert-butyl alcohol (TBA) and methanol:
OH CH COH ) CH ( O H COCH ) CH ( 3 3 3 + 2 → 3 3 + 3 (3)
Dehydrogenation of MTBE to isobutene and methanol is also thermodynamically favorable: OH CH CH C ) CH ( COCH ) CH ( 3 3 3 → 3 2 = 2 + 3 (4)
Following the extensive work by Olah and his co-workers (Olah et al., 1986), the above two reactions involve a common intermediate: a carbocation (tert-butyl cation). For example, protonated s-butyl methyl ether can be cleaved to protonated methanol and t-butyl cation (Olah et al., 1986):
(Protonated s-butyl methyl ether) (t-butyl cation) (protonated methanol)
CH3CH2CHOCH3
CH3
H
(CH3)3C + CH3OH2
(5)
Figure 4. A scheme of the reaction pathways for the transformation of MTBE with Nafion.
The carbocation formed from MTBE is extremely unstable and undergoes hydrolysis and dehydrogenation reactions. Hydrolysis of carboncations by water is a typical nucleophilic substitution reaction (Matouq and Goto, 1993). Dehydrogenation of carbocations, on the other hand, is catalogued as an elimination reaction. For the MTBE transformation, hydrolysis of tert-butyl cation yields TBA while the dehydrogenation leads to the formation of isobutene. Subsequently, isobutene undergoes oxidation to form acetone (Nunan et al., 1993). A competition between the hydrolysis and dehydrogenation reactions in the transformation of MTBE is therefore
CH3 C CH3 CH3 CH3 O OH C CH3 CH3 CH3 OH2+ C CH3 CH3 CH3 C CH3 H3C CH2 C CH3 H3C O TBA Isobutene Acetone H2O H+ C CH3 CH3 CH3 H2O H2O H3O H O2 Nafion
According to the scheme shown in Figure 4, dehydration of TBA to isobutene may also be possible. Isobutene is a key intermediate to explain the observations and hypothesized reaction pathways. However, quantification of isobutene was restricted by the presence of water. Peaks of water and isobutene overlap with each other in the GC/MS spectrum. To overcome this limitation, tert-amyl methyl ether (TAME) was used as a probe molecule. The structural difference between MTBE and TAME is substitution of an ethyl group for a methyl group on the tertiary carbon. If isobutene is formed during MTBE transformation, then a β-isoamylene should be produced during TAME degradation.
OH CH ) CH ( CH C ) CH ( COCH ) H C ( ) CH ( 3 2 2 5 3 → 3 2 = 3 + 3 (6)
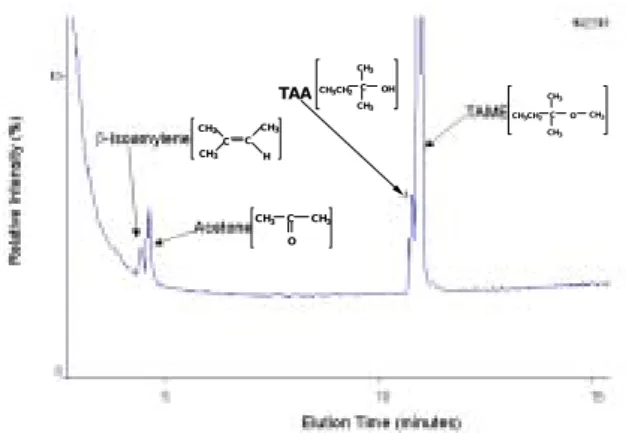
Figure 5 presents the GC/MS spectrum of TAME transformation (60 mg/L) with 2g of the Nafion in a 50 mL aqueous solution. TAA, acetone and β-isoamylene were positively identified. The appearance of β-isoamylene from TAME supports that the dehydrogenation reaction can occur in the transformation of MTBE.
C CH3 CH3 CH3 C H CH3 C CH3 CH3CH2 CH3 O OH C CH3 CH3CH2 CH3 C CH3 CH3 O TAA
Figure 5. A GC/MS chromatogram of TAME and products formed after a 12-hour contact time.
In summary, removal of MTBE over a superacidic organic polymer, Nafion was studied. Equilibria of sorption and subsequent transformation of MTBE were observed in batch experiments. MTBE was converted to tert-butyl alcohol (TBA), acetone and possibly isobutene and methanol. Transformation of TBA to isobutene and acetone was also detected. Results suggest that possible transformation pathways
may include hydrolysis, dehydrogenation and oxidation reactions. The hydrolysis reaction leads to the formation of TBA while dehydrogenation reaction yields isobutene. Dissolved oxygen is needed for the oxidation of isobutene to acetone. Current progress in our laboratory is directed toward column experiments for the removal of MTBE under continuous flow conditions and regeneration of Nafion and will be reported in due course.
References
1. Cal-EPA (California Environmental Protection Agency). (1997). Briefing paper on MTBE. Updated Septembet 3, 1998. http://www.arb.ca.gov/cbg/pub.
2. Delzer G. C., Zogorski J. S., Lopes T. J. and Bosshart R. L. (1996) Occurrence of the gasolineoxygenate MTBE and BTEX compounds in urban stormwater in the United States, 1991-95. U.S. Geological Survey; water-resources investigations report 96-4145.
3. Matouq M. and Goto S. (1993). Kinetics of liquid phase synthesis of methyl
tert-butyl ether from tert-butyl alcohol and methanol catalyzed by ion exchange
resin. Int. J. Chem. Kinet. 25: 825-831.
4. McMurry J. (1996). Organic Chemistry: 4th Edition. Brooks/Cole Publishing
Company, Pacific Grove, CA.
5. Morrison R. T. and Boyd R. N. (1992). Organic Chemistry 6th Edition. Prentic
Hall, Englewood Cliffs.
6. Nunan J. G., Klier K. and Herman R. G. (1993). Methanol and 2-methyl-1-propanol (isobutanol) coupling to ethers and dehydration over Nafion H: selectivity, kinetics, and mechanism. J. Catal. 139: 406-420.
7. Olah G. A., Lyer P. S. and Surya Prakash G. K. (1986). Perfluorinated resinsulfonic acid (Nafion-H) catalysis in synthesis. Synthesis. 513-531.
8. Squillace P. J., Zogorski J. S., Wilber W. G. and Price C. V. (1996). Preliminary assessment of the occurrence and possible sources of MTBE in groundwater in the United States, 1993-1994. Environ. Sci. Technol. 30: 1721-1730.
9. Sun Q., Harmer M. A. and Farneth W. E. (1997). An extermely active solid acid catalyst, Nafion resin/silica composite, for the Friedel-Crafts benzylation of benzene and p-xylene with benzyl alcohol. Ind. Eng. Chem. Res. 36: 5541-5544. 10. U.S. Environmental Protection Agency. (1993). Assessment of potential risks of
Office of Research and Development; November; report no. EPA/600/R-93/206. 11. U.S. Environmental Protection Agency (1998). Office of Research and
Development. Oxygenates in water: critical information and research needs. EPA/600/R-98/048. http://www.epa.gov/ncea/oxyneeds.htm.
12. Waller F. J. and Van Scoyoc R. W. (1987). Catalysis with Nafion.