doi:10.1152/ajpcell.00031.2009
296:C1133-C1139, 2009. First published 11 March 2009;
Am J Physiol Cell Physiol
Shao-Chun Lu, Hsiao-Wen Wu, Yen-Jen Lin and Shwu-Fen Chang
by trichostatin A
regulation
of iNOS in RAW 264.7 macrophages and its
The essential role of Oct-2 in LPS-induced expression
You might find this additional info useful...
42 articles, 19 of which can be accessed free at:
This article cites
http://ajpcell.physiology.org/content/296/5/C1133.full.html#ref-list-1 1 other HighWire hosted articles
This article has been cited by
[PDF] [Full Text] [Abstract]
, June , 2010; 87 (6): 1103-1114.
J Leukoc Biol
Matthias, David P. Fairlie and Matthew J. Sweet
Patrick Cao, Erica Lovelace, Robert C. Reid, Giang T. Le, David A. Hume, Katharine M. Irvine, Maria A. Halili, Melanie R. Andrews, Larisa I. Labzin, Kate Schroder, Gabriele Matthias, Chun the Toll-like receptor 4 agonist LPS
Differential effects of selective HDAC inhibitors on macrophage inflammatory responses to
including high resolution figures, can be found at:
Updated information and services
http://ajpcell.physiology.org/content/296/5/C1133.full.html
can be found at:
AJP - Cell Physiology
about
Additional material and information
http://www.the-aps.org/publications/ajpcell
This infomation is current as of May 28, 2011.
American Physiological Society. ISSN: 0363-6143, ESSN: 1522-1563. Visit our website at http://www.the-aps.org/.
a year (monthly) by the American Physiological Society, 9650 Rockville Pike, Bethesda MD 20814-3991. Copyright © 2009 by the is dedicated to innovative approaches to the study of cell and molecular physiology. It is published 12 times AJP - Cell Physiology
on May 28, 2011
ajpcell.physiology.org
The essential role of Oct-2 in LPS-induced expression of iNOS in RAW
264.7 macrophages and its regulation by trichostatin A
Shao-Chun Lu,1Hsiao-Wen Wu,1Yen-Jen Lin,2and Shwu-Fen Chang2
1Department of Biochemistry and Molecular Biology, College of Medicine, National Taiwan University, Taipei; and2Graduate Institute of Medical Sciences, Taipei Medical University, Taipei, Taiwan
Submitted 16 January 2009; accepted in final form 24 February 2009
Lu S, Wu H, Lin Y, Chang S. The essential role of Oct-2 in LPS-induced expression of iNOS in Raw 264.7 macrophages and its regulation by trichostatin A. Am J Physiol Cell Physiol 296: C1133–C1139, 2009. First published March 11, 2009; doi:10.1152/ajpcell.00031.2009.—This article reports on a study of the effect of trichostatin A (TSA), an inhibitor of histone deacetylase, on lipopolysaccharide (LPS)-induced expression of inducible nitric oxide synthase (iNOS) in RAW 264.7 macrophages and its underlying mechanisms. TSA pretreatment potently diminishes LPS-stimulated nitric oxide (NO) release and both mRNA and protein levels of iNOS in macrophages. The effects of TSA and LPS on transcription factors binding to two LPS-responsive elements within the iNOS promoter, one binding the NF-B site and the other the octamer element, were investigated. Results show that TSA did not alter the LPS-activated NF-B activity demonstrated by the nuclear translocation of p50 and p65 and by a NF-B-driven reporter gene expression system. In addition, neither TSA nor LPS changed the expression of Oct-1, a ubiquitously expressed octamer binding pro-tein. However, TSA suppressed the LPS-induced expression of Oct-2, another octamer binding protein, at both mRNA and protein levels. Chromatin immunoprecipitation assays revealed that binding of Oct-2 to the iNOS promoter was enhanced by LPS treatment; however, pretreatment with TSA resulted in loss of this binding. Moreover, forced expression of Oct-2 by transfection of pCG-Oct-2 plasmid restored the TSA-suppressed iNOS expression elevated by LPS stim-ulation, further indicating that Oct-2 activation is a crucial step for transcriptional activation of the iNOS gene in response to LPS stimulation in macrophages. This study demonstrates that TSA dimin-ishes iNOS expression in LPS-treated macrophages by inhibiting Oct-2 expression and thus reducing the production of NO.
inducible nitric oxide synthase; lipopolysaccharide; macrophage
HISTONE MODIFICATIONS, such as acetylation, methylation, and
phosphorylation, play important roles in gene expression. Acetylation is the most frequent posttranslational modification of histone. The level of histone acetylation is controlled by the opposing activities of histone acetyltransferases (HATs) and histone deacetylases (HDACs) (11). Higher levels of histone acetylation lead to relaxation of the local structure of chromatin and thus increase the accessibility of transcription factors to the DNA, subsequently enhancing gene transcription. In contrast, silent genes are usually located at nucleosomes with hy-poacetylated histone proteins (35).
Trichostatin A (TSA) is a potent and nonselective inhibitor of HDACs and is widely used to study the role of histone acetylation in genes. TSA treatment induces histone acetyla-tion in mammalian cells (25, 38). However, several studies have shown that TSA treatment changes the expression of only
about 2% of genes (24, 39). Aung et al. (1) reported that TSA enhanced LPS-induced expressions of Cox-2, Cxcl2, and Lfit2, whereas LPS-induced expressions of Ccl2, Ccl7, and Edn1 were suppressed by TSA in bone marrow-derived macro-phages. However, the mechanisms underlying the conflicting effects of TSA on gene expression remain unclear.
Inducible nitric oxide synthase (iNOS) catalyzes the produc-tion of nitric oxide (NO), one of the most critical mediators for inflammatory response in macrophages (26, 27). The iNOS expression in macrophages is induced by a number of cyto-kines, such as interferon-␥ (IFN-␥), tumor necrosis factor-␣ (TNF-␣), interleukin- (IL-1), and bacterial LPS (10, 14, 34). Although iNOS expression is under complex transcriptional and posttranscriptional control, transcriptional activation of iNOS is the main control mechanism in response to inflamma-tory stimuli. The 5⬘ flanking region of the murine iNOS gene contains two cis-regulatory elements, a NF-B site and an octamer, which are essential for LPS-induced iNOS gene expression, and mutation at either one of these elements results in a⬎95% decrease in promoter activity (37). Moreover, LPS-and INF-␥-induced transcription of iNOS requires the histone acetyltransferase activity of p300 (9). Whereas Yu et al. (41) showed that LPS- and INF-␥-induced increases of iNOS pro-moter activity and NO production in mesangial cells and RAW 264.7 cells were reduced by TSA, Larsen et al. (20) showed that IL-1-induced increase of iNOS expression and NO pro-duction in INS-1E cells were inhibited by TSA. These results suggest that histone acetylation also may be involved in regu-lating the expression of iNOS gene.
In this study, we investigated the effect of TSA on LPS-induced iNOS expression and NO production in RAW 264.7 cells. Our results show that TSA potently represses the LPS-induced NO release by decreasing the expression levels of both iNOS mRNA and protein in RAW 264.7 cells. Furthermore, the functional roles of NF-B and octamer binding proteins in TSA-suppressed LPS-induced iNOS expression were evalu-ated. The results show that suppression of LPS-stimulated iNOS expression by TSA is via blockage of Oct-2 expression.
MATERIALS AND METHODS
Reagents and cell culture. Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were obtained from GIBCO BRL (Life Technologies, Rockville, MD). LPS from Escherichia coli (serotype 0111:B4) and TSA were purchased from Sigma-Aldrich (St. Louis, MO). A Griess reagent system kit was obtained from Promega (Madison, WI). SuperFect transfection reagent was purchased from Qiagen (Hilden, Germany), and SuperScript II reverse transcriptase was purchased from Invitrogen (Carlsbad, CA). Antibodies against iNOS, Oct-1, Oct-2, acetylated histone H3 (Ac-H3), and-actin were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). An improved chemiluminescence detection system was obtained from
Address for reprint requests and other correspondence: S.-F. Chang, Grad-uate Institute of Medical Sciences, Taipei Medical Univ., 250 Wu-Hsing St., Taipei 110, Taiwan (e-mail: cmbsfc21@tmu.edu.tw).
First published March 11, 2009; doi:10.1152/ajpcell.00031.2009.
on May 28, 2011
ajpcell.physiology.org
NEN Life Science Products (Boston, MA). The pTransNF-B-Neo plasmid was purchased from Panomics (Fremont, CA), and the plasmid pCG-Oct-2 and its parent plasmid, pCG, were generous gifts from Dr. W. Herr (Cold Spring Harbor Laboratory, Cold Spring Harbor, NY) (33).
RAW 264.7 cells, a murine macrophage-like cell line, were cul-tured as described previously (21). Briefly, cells were grown at 37°C in 5% CO2in DMEM supplemented with 10% FBS, 2 mM glutamine, and 1,000 U/ml penicillin-streptomycin. In these experiments, Oct-1, Oct-2, and iNOS mRNA and protein levels were compared in un-treated cells and in cells un-treated with TSA and/or LPS. Unless otherwise specified, treatment was with 200 nM TSA for 20 min before 100 ng/ml LPS treatment for 8 h.
Quantitation of NO in culture medium. The levels of NO in the culture medium of macrophages were measured using a Griess re-agent system kit according to the manufacturer’s instructions. Briefly, 50l of culture supernatants were gently mixed with an equal volume of sulfanilamide solution (1% sulfanilamide in 5% phosphoric acid) and incubated in the dark at room temperature (RT) for 10 min. After the incubation, 50l of 0.1% naphthyletilenediamine dihydrochloride was added to the reaction and incubated in the dark at RT for another 10 min. The absorbance at 540 nm was measured in a microplate reader. Nitrite concentration, an indicator of NO production, was calculated from a NaNO2standard curve.
RNA isolation and analyses of Oct-2, Oct-1, and iNOS mRNA. Total cellular RNA was isolated from RAW 264.7 cells according to the method of Chomczynski and Sacchi (7). RNA concentrations were determined from the absorbance at 260 nm. First-strand cDNA was synthesized from total RNA using the SuperScript II reverse tran-scriptase (Invitrogen) and oligo(dT) primer. The mRNA levels of Oct-1, Oct-2, iNOS, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were determined by RT-PCR, using the specific primers described above (21). The conditions for the iNOS- and Oct-1-specific PCRs were as follows: 94°C for 3 min and 28 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 30 s, followed by a 7-min incubation at 72°C. The annealing temperature and cycling number in the PCR program was 57°C with 25 cycles and 61°C with 19 cycles for Oct-2 and GAPDH PCR, respectively. The amplified DNA fragments were sequenced to confirm their identity. Quantitative real-time PCR (qPCR) was used to verify the differential expression of Oct-2 and iNOS genes in response to LPS and TSA. A constitutive expressed gene, GAPDH, was used as internal control to verify the real-time qPCR reaction. Diethyl pyrocarbonate-treated water for the replace-ment of cDNA template was used as a negative control. The ampli-fications were carried out in an ABI Prism 7300 sequence detection system (Applied Biosystems). The reaction was performed in a 20-l mixture containing 1⫻ reaction buffer (SYBR green master mixture; Applied Biosystems) and 100 nM primers. The gene-specific primers Oct-2 (sense), 5⬘-GGGCGGAGACGCAAGAAGA-3⬘; Oct-2 (anti-sense), 5⬘-TCTCTAAGGCGAAGCGGACAT-3⬘; iNOS (sense), 5⬘-GAAACGCTTCACTTCCAATGC-3⬘; iNOS (antisense), 5⬘-GGCA-GCCTGTGAGACCTTTG-3⬘; GAPDH (sense), 5⬘-CATGGCCTTC-CGTGTTCCTA-3⬘; and GAPDH (antisense), 5⬘-GCGGCACGTCA-GATCCA-3⬘ were used to amplify the target DNA sequence. The ampli-fication conditions were as follows: 2 min at 50°C, 10 min at 95°C, and a two-step cycle at 95°C for 15 s and 60°C for 1 min for a total of 40 cycles. The specificity was verified by an additional dissociation curve. The interpolated number of cycles to reach a fixed threshold above background noise (Ct) was used to quantify amplification.
Western blot analysis. Samples of cell lysate or nuclear extract from RAW 264.7 cells were separated by SDS-PAGE on 10% gels and transferred to a polyvinylidene difluoride membrane, which was blocked overnight at 4°C with blocking buffer (10 mM Tris䡠HCl, pH 8.0, 0.15 M NaCl, 0.1% Tween 20, and 5% fat-free milk). The blots were then incubated for 1 h at room temperature with 0.5g/ml rabbit polyclonal Oct-1, Oct-2, iNOS, p50, p65, anti-Ac-H3, anti-lamin B, or anti--actin antibody and for 40 min at RT
with peroxidase-conjugated anti-rabbit IgG antibody (Amersham Pharmacia Biotech), and then bound antibodies were detected using an improved chemiluminescence detection system (NEN Life Science Products). Protein concentrations were determined using the Bradford method (DC Protein Assay; Bio-Rad Laboratories, Hercules, CA).
Transient transfection and luciferase reporter assays. These pro-cedures were performed as described in our previous study (21). Briefly, RAW 264.7 cells were plated at a density of 3 ⫻ 105 cells/well in 12-well tissue culture plates 1 day before transfection and then transiently transfected with 1.5g of pTransNF-B-Neo plasmid using the SuperFect transfection reagent according to the manufac-turer’s instructions. Twenty hours after transfection, the cells were either left untreated or treated with TSA for 20 min and then treated with or without 100 ng/ml LPS for 8 h. Luciferase assays were carried out using the Dual-Luciferase reporter assay system (Promega) ac-cording to the manufactures’ guidelines. For forced expression of Oct-2, RAW 264.7 cells were plated in 6-cm dishes, cultured over-night, and then transfected with 0.1 or 0.2 g of pCG-Oct-2 (total amount of DNA was adjusted to 7 g with the pCG vector) as described above.
Preparation of nuclear extracts. Nuclear extracts were prepared as described previously (21). Briefly, cells were washed twice with ice-cold PBS, incubated with lysis buffer (10 mM HEPES, pH 7.4, 10 mM NaCl, 1.5 mM MgCl2, 0.5 mM DTT, 0.2% Nonidet P-40, and 0.2 mM PMSF) on ice for 5 min, and collected by gentle pipetting. Nuclei were pelleted by centrifugation at 500 g for 5 min at 4°C, nuclear proteins were extracted by incubation of the nuclei with nuclear extract buffer (20 mM HEPES-KOH, pH 7.9, 25% glycerol, 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM DTT, 0.2 mM PMSF, and 1⫻ protease inhibitor cocktail) for 15 min at 4°C, and cell debris were removed by centrifugation at 12,900 g for 10 min at 4°C. The Bradford method (DC Protein Assay; Bio-Rad) was used to measure the protein concentration in the extract, which was then stored in aliquots at⫺80°C.
Chromatin immunoprecipitation assay. Chromatin immunoprecipi-tation (ChIP) assays were performed to determine the binding of Oct-2 and Ac-H3 to the promoter of iNOS. Briefly, the cells were fixed with 1% formaldehyde for 10 min at 37°C. After fixation, the cells were collected by scraping and then sonicated to fragment the chromatin. Immunoprecipitation analysis was carried out using con-trol rabbit IgG, anti-Oct-2, or anti-Ac-H3 antibodies. Cross-links were reversed at 65°C for 4 h, and proteins were digested with proteinase K for 1 h at 45°C. Immunoprecipitated DNA was recovered by phenol-chloroform extraction and ethanol precipitation and was used as a template for iNOS promoter (⫺90 to ⫹151) PCR amplification using forward (5⬘-AACTGGGGACTCTCCCTTTG-3⬘) and reverse primer (5⬘-CTACTCCGTGGAGTGAACAA-3⬘). Ten percent of the chromatin DNA used for immunoprecipitation was similarly sub-jected to PCR analysis and indicated as “input.” The number of PCR cycles was as follows: 35 PCR cycles for all the ChIP experiments and 26 PCR cycles for the input samples.
Statistical analysis. Results are means⫾ SD. Differences between mean values were evaluated using Student’s t-test and are considered significant at P⬍ 0.05.
RESULTS
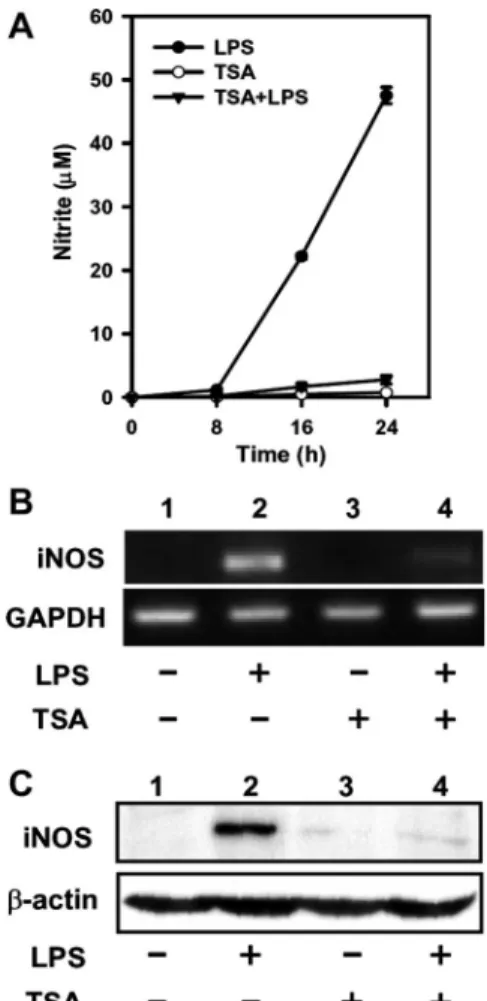
TSA reduces LPS-induced iNOS expression and NO produc-tion. The effects of TSA, a HDAC inhibitor, on the levels of iNOS mRNA and protein in LPS-stimulated macrophages were examined. RAW 264.7 cells were exposed to 200 nM TSA for 20 min before LPS treatment, and iNOS mRNA and iNOS protein were measured by RT-PCR and Western blot analysis, respectively. Results showed that NO production increased significantly at 8 h after LPS treatment; whereas pretreatment with TSA attenuated LPS-induced NO production (Fig. 1A).
C1134 OCT-2 IN THE LPS-INDUCEDINOS EXPRESSION
AJP-Cell Physiol•VOL 296 • MAY 2009 •www.ajpcell.org
on May 28, 2011
ajpcell.physiology.org
The iNOS mRNA and protein were undetectable in untreated Raw 264.7 cells, but their levels increased considerably at 8 h after LPS treatment (Fig. 1, B and C). Pretreatment of cells for 20 min with TSA lowered the LPS-induced increase in both mRNA and iNOS protein (Fig. 1, B and C). Inhibition of HDAC activities by TSA results in hyperacetylation of core histones in mammalian cells (38). To confirm the effect of TSA, we determined levels of total Ac-H3 and of Ac-H3 associated with the iNOS promoter. The results showed that LPS alone increased nuclear Ac-H3 slightly, whereas TSA significantly increased the level of Ac-H3 regardless of LPS treatment (Fig. 2A). ChIP analysis was performed to analyze the amount of Ac-H3 at the iNOS gene promoter, and the results showed that treatment with LPS resulted in a slight increase of Ac-H3 at the iNOS promoter and that the level of Ac-H3 was further enhanced by TSA treatment (Fig. 2B).
LPS-induced NF-B activation is not altered by TSA. To test whether the activation of NF-B, the main transcriptional regulator of iNOS expression, is affected by TSA treatment, both p50 and p65 in the nucleus of LPS-treated cells were assayed by Western blot analysis. Figure 3A shows that both
nuclear p50 and p65 were increased at 30 min after LPS treatment, and pretreatment with TSA did not change the levels of nuclear p50 and p65. To further evaluate NF-B activation under LPS and TSA treatment, we transfected RAW 264.7 cells with a NF-B-driven luciferase reporter plasmid, pNF-B-Neo-Luc, and treated with TSA and LPS, as described above, at 24 h after transfection. The luciferase activity was significantly upregulated by LPS treatment, whereas the lucif-erase activity in LPS-treated cells was unchanged when cells were pretreated with TSA (Fig. 3B). These results suggest that LPS-triggered NF-B activation, including the nuclear trans-location and transactivation activity, is not affected by TSA.
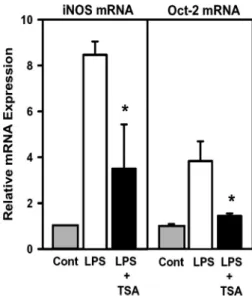
LPS-elevated Oct-2 expression is blocked by TSA. In addi-tion to the NF-B binding site, an octamer located at ⫺61 to ⫺54 of the iNOS promoter was known to be essential for transcription of the iNOS gene in response to LPS treatment (37). We then tested whether the levels of octamer binding proteins Oct-1 and Oct-2 are affected by LPS and TSA. Figure 4 shows that the levels of Oct-1 mRNA and protein in RAW 264.7 cells were unaffected in cells exposed to LPS and/or TSA; the levels of both Oct-2 mRNA and protein were significantly increased after LPS treatment, whereas pretreat-ment with TSA attenuated the LPS-induced Oct-2 expression at both mRNA and protein levels (Fig. 4, A and B). The mRNA levels of iNOS and Oct-2 were further measured by real-time qPCR. The results show that a 7.5- and 2.8-fold increase at iNOS mRNA and Oct-2 mRNA, respectively, was detected in cells treated with LPS, whereas only a 2.5-fold increase at iNOS mRNA and a 0.4-fold increase at Oct-2 mRNA were detected in cells pretreated with TSA followed by 8-h exposure to LPS (Fig. 5). The LPS-induced increase of iNOS and Oct-2 gene expression was prevented by pretreatment with poly-myxin B, a potent antibiotic that binds to and neutralizes LPS,
Fig. 1. Trichostatin A (TSA) suppressed LPS-induced NO production and inducible NO synthase (iNOS) mRNA and protein expression in RAW 264.7 cells. The RAW 264.7 cells were untreated or pretreated with TSA (200 nM) for 20 min and then treated with LPS (100 ng/ml) for 0, 8, 16, and 24 h. NO production in culture medium was determined using the Griess reagent system kit (A). Levels of iNOS mRNA (B) and protein (C) were analyzed by RT-PCR and Western blot analysis, respectively, in cells at 8 h after LPS treatment. GAPDH mRNA or-actin were used as internal controls. Similar results were obtained in at least 3 independent experiments.
Fig. 2. Effects of TSA and LPS on the levels of total acetylated histone proteins and those associated with iNOS promoter in RAW 264.7 cells. The RAW 264.7 cells were pretreated with or without TSA (200 nM) for 20 min and then treated with or without LPS at 100 ng/ml for 8 h. Cells were disrupted, and levels of acetylated histone H3 (Ac-H3) in the nuclear extracts were determined by Western blot analysis (A). Lamin B was used as a control. The level of Ac-H3 in the iNOS promoter region was analyzed by chromatin immunoprecipitation (ChIP) assay (B). Antibodies to Ac-H3 or control IgG were used for immunoprecipitation, and the targeted promoter regions of iNOS were amplified by PCR. IgG was used as a negative control. Ten percent of the chromatin DNA used for immunoprecipitation was subjected to PCR and is indicated as “input.” Similar results were obtained in 3 independent experi-ments.
on May 28, 2011
ajpcell.physiology.org
before LPS treatment, suggesting that the effect is not resulted from the contaminants in the commercial LPS (data not shown).
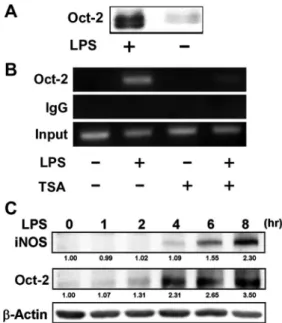
Oct-2 binds to the promoter of iNOS. To investigate whether Oct-2 is able to bind to the iNOS promoter, nuclear proteins bound to the octamer of the iNOS promoter were pulled down by a biotinylated probe and detected by Western blot analysis. Figure 6A shows that Oct-2 was able to bind to the iNOS promoter. To further investigate binding of Oct-2 to the iNOS promoter in vivo, we performed a ChIP assay using an anti-Oct-2 antibody. Results in Fig. 6B show that the iNOS pro-moter encompassing the octamer element could be immuno-precipitated with the anti-Oct-2 antibody in LPS-treated RAW 264.7 cells, whereas it could not be immunoprecipitated in untreated control cells. This result indicates that LPS induces binding of Oct-2 to the promoter of iNOS. In TSA-pretreated cells, no Oct-2 binding to the promoter of iNOS was detected regardless of LPS treatment. The results suggest that Oct-2 can bind to the promoter of the iNOS gene in LPS-treated cells and is thus involved in regulating iNOS gene expression and that the binding is abolished by TSA pretreatment. A time-course study shows that Oct-2 expression was detectable at about 2– 4 h before iNOS expression after LPS treatment (Fig. 6C), further supporting the suggestion that LPS induces expression of Oct-2, which then activates transcription of iNOS.
Forced expression of Oct-2 rescues the expression of iNOS in TSA- and LPS-treated cells. To further confirm the TSA effect on LPS-regulated iNOS and Oct-2 expression, RAW
264.7 cells were pretreated with lower concentrations of TSA (10 or 20 nM) for 20 min followed by LPS treatment for 8 h. Figure 7A shows that levels of both iNOS and Oct-2 protein were elevated by LPS, and both were downregulated by TSA in a dose-dependent manner. The result further supports the suggestion that LPS-elevated Oct-2 and iNOS expression can be attenuated by TSA. To test whether low expression of iNOS in TSA- and LPS-treated cells is due to reduced Oct-2 expres-sion, we transfected RAW 264.7 cells with an increasing amount of pCG-Oct-2 DNA, an Oct-2 expression plasmid, to
Fig. 3. Effects of TSA and LPS on nuclear translocation of NF-B and the NF-B-regulated reporter gene expression in RAW 264.7 cells. A: RAW 264.7 cells were treated with TSA and LPS as described in Fig. 2. Nuclear proteins were prepared and analyzed by Western blotting with an antibody specific to p50 and p65. Lamin B was used as a control for nuclear protein. B: luciferase reporter assay. RAW 264.7 cells were transiently transfected with pNF- B-Neo-Luc reporter plasmid. At 24 h after transfection, cells were pretreated with TSA for 20 min, followed by exposure to LPS (100 ng/ml) or no treatment. Luciferase activity and total proteins in cell lysates were measured. Luciferase activity was normalized to total protein, and results are shown as relative activity compared with that of LPS treated (relative value⫽ 100). Values are means⫾ SD of 3 independent experiments.
Fig. 4. Effects of TSA and LPS on the expression of Oct-1 and Oct-2 in RAW 264.7 cells. The RAW 264.7 cells were treated with TSA and/or LPS as described in Fig. 2. The mRNA levels of Oct-1 and Oct-2 were determined by RT-PCR (A). The protein levels of Oct-1 and Oct-2 were analyzed by Western blot analysis (B). The expression levels of GAPDH and-actin were used as loading controls. Asterisk indicates a nonspecific signal.
Fig. 5. Quantitative measurement of mRNA levels of iNOS and Oct-2 by real-time PCR. Cells were treated with TSA and/or LPS as described in Fig. 2. The mRNA levels of iNOS and Oct-2 were determined by real-time PCR and normalized to that of GAPDH, and the amount of expression was expressed relative to the expression level of untreated control cells (relative value⫽ 1). *P⬍ 0.05, compared with LPS-treated group.
C1136 OCT-2 IN THE LPS-INDUCEDINOS EXPRESSION
AJP-Cell Physiol•VOL 296 • MAY 2009 •www.ajpcell.org
on May 28, 2011
ajpcell.physiology.org
raise the level of Oct-2 protein. Figure 7B shows that forced expression of Oct-2 restored the protein levels of iNOS in TSA/LPS-treated cells in a dose-dependent manner. The result strongly suggests that TSA repression of iNOS expression in LPS-activated macrophages is due to loss of Oct-2 expression.
DISCUSSION
In this study, we have shown that LPS-induced iNOS gene expression and NO production were attenuated by TSA in RAW 264.7 cells. The results are similar to findings reported by Yu et al. (41). In their study, RAW 264.7 cells were treated with LPS and IFN-␥ for 24 h in the presence or absence of TSA, and the results showed that TSA attenuated LPS- and IFN-␥-induced nitrite production and iNOS promoter activ-ity. Moreover, the result also resembles the observations of Chakravortty et el. (4), who showed that LPS-induced iNOS gene expression in RAW 264.7 cells was repressed by butyrate, another HDAC inhibitor. In addition, Guo et al. (12) showed that TSA attenuated iNOS expression in IFN-␥-stimulated RAW 264.7 cells. TSA-inhibited inflammatory cytokine-induced expression of iNOS also has been demonstrated in pancreatic -cells (20) and mesangial cells (40). However, TSA has been shown to enhance LPS-induced expression of iNOS in microglial cells (32). It is very likely that the effect of TSA on iNOS gene expression is cell type specific, and apparently that increase in histone acetylation does not always associate with higher iNOS gene expression.
NF-B is the most important and well-studied regulator of LPS-responsive gene expression. Studies have shown that TSA prolongs NF-B activation in various cell types under stimu-lation of TNF-␣ or sodium pervanadate (5, 15). In this study, we showed that TSA has no effects on LPS-induced nuclear translocation of both p65 and p50 (Fig. 3A) and activation of luciferase reporter expression driven by NF-B (Fig. 3B). The results are in agreement with the observations of studies in macrophages and dendritic cells (3, 41). Thus it is unlikely that NF-B is responsible for the TSA-attenuated LPS-induced iNOS gene expression shown in this study, although the inhibitory effect of butyrate on LPS-induced iNOS expression was shown to be due to downregulation of NF-B activation (4). Other than the NF-B binding site, the octamer on the iNOS promoter has been shown to play a critical role in LPS-induced iNOS expression. Both Oct-1 and Oct-2 are characterized as octamer binding proteins. Oct-1 is ubiqui-tously expressed, but with some isoforms expressing in a tissue-specific manner (23, 42). Although Oct-2 is expressed predominantly in B cells (30, 31), it is also found in neuronal cells (13), T cells (18), and macrophages (21, 22, 28). Our results show that the expression of Oct-2 is in parallel with the expression of iNOS in TSA- and/or LPS-treated cells, whereas the expression level of Oct-1 remains constant regardless of TSA and LPS treatment (Fig. 4). Binding of Oct-2 to the promoter of iNOS in LPS-treated cells was demonstrated by ChIP assay (Fig. 6). Furthermore, the present study demon-strated that ectopic expression of Oct-2 recovered TSA-atten-uated LPS-induced iNOS expression. Therefore, TSA seems to inhibit Oct-2 expression and thus suppress iNOS expression in LPS-treated RAW 264.7 macrophages. Oct-2 is known to be
Fig. 6. Oct-2 binds to the promoter of iNOS gene. A: DNA affinity precipi-tation assay of Oct-2 binding to iNOS promoter. The RAW 264.7 cells were treated with LPS at 100 ng/ml for 8 h. Nuclear extracts were incubated with a biotinylated iNOS promoter probe (⫺90 to ⫺47). The DNA-protein complexes were pulled down with (left lane) or without (right lane) streptavidin-agarose beads, and Oct-2 in the complex was determined by Western blot analysis.
B: ChIP assays. After treatment with TSA and LPS as described in Fig. 2, ChIP
assays were performed as described inMATERIALS AND METHODS. The targeted promoter regions of iNOS were amplified by PCR. IgG was used as a negative control. Ten percent of the chromatin DNA used for immunoprecipitation was subjected to PCR and is indicated as “input.” C: time course of LPS-induced iNOS and Oct-2 expressions analyzed by Western blot analysis. Values below blots indicate the relative (fold) change in expression compared with untreated control (relative value⫽ 1.00).
Fig. 7. Forced expression of Oct-2 rescued TSA-suppressed LPS-induced iNOS expression. A: RAW 264.7 cells were pretreated with TSA (0, 10, and 20 nM) and/or LPS (100 ng/ml) as described in Fig. 2. Levels of iNOS, Oct-2, and -actin protein were determined by Western blot analysis. Similar results were obtained in 3 independent experiments. B: RAW 264.7 cells were transfected with 0, 0.1, or 0.2g of pCG-Oct-2, and the total amount of DNA was adjusted to 2g with pCG vector. At 24 h posttransfection, cells were pretreated with TSA (20 nM) for 20 min and then treated with LPS (100 ng/ml) for 8 h. The protein levels of iNOS and Oct-2 were determined by Western blot analysis. The protein level of-actin was measured to serve as a loading control. Similar results were obtained in 2 independent experiments.
on May 28, 2011
ajpcell.physiology.org
mainly involved in the immunoglobulin gene transcription in B cells (8), and little is known of its functions in macrophages and other cells. We (21) have demonstrated that Oct-2 is involved in LPS-induced upregulation of resistin gene expres-sion in macrophages. In this present study, we have shown that activation of Oct-2 is crucial for the transcriptional activation of the iNOS gene in LPS-stimulated macrophages.
LPS triggers expression of genes through a variety of sig-naling pathways in macrophages. Recent studies have shown that the activities of HAT and HDAC change in response to LPS stimulation in macrophages (2). Koch et al. (19) showed that HAT activity was significantly induced by LPS in human alveolar macrophages, whereas studies with BAL macrophages found that HDAC activity was inhibited by LPS (17). Aung et al. (1) demonstrated that expression of some HDACs is transiently suppressed and then induced by LPS. Thus the response of a specific gene expression to LPS stimulation may be regulated by the balance of HAT and HDAC activities in the particular promoter region. TSA treatment changes the balance of HAT and HDAC and results in genes that are attenuated or further enhanced under LPS treatment. In this study we have shown that TSA suppresses the LPS-induced expression of Oct-2 and subsequently attenuates the transcription of iNOS. As far as we know, this is the first report showing the TSA effect on the expression of Oct-2 transcription factor. It re-mains unclear how Oct-2 expression is upregulated by LPS and which is downregulated by TSA. The mechanism that under-lies TSA/LPS regulation of Oct-2 expression is currently under investigation. It is also unclear whether the effects of TSA/LPS on expressions of iNOS and Oct-2 in other macrophages, such as peritoneal macrophage or human macrophage, are similar to the results observed in this study. An interesting direction for research would be to evaluate whether Oct-2 is also induced by LPS and involved in LPS-induced iNOS gene expression.
There is considerable interest in using TSA in the treatment of cancers or inflammatory diseases, because it can reduce expression of some inflammatory genes induced by LPS; however, it enhances the expression of some other genes induced by LPS (1, 3). TSA may alter gene expression through distinct mechanisms. Other than its effect on histone acetyla-tion, it has been reported to repress DNA methylation (29) and alter the acetylation status of various transcription factors such as p53, Stat 3, Smad7, and NF-B (6, 16). In this report, our results provide evidence that emphasizes a novel immune-modulator role for Oct-2 that involves TSA attenuating LPS-induced iNOS expression. The precise mechanism of the effect of TSA on gene expression is not known at this time, and further approaches are needed to clarify the mechanism of TSA’s effects on LPS-induced gene expression in macro-phages.
GRANTS
This study was supported by National Science Council of Taiwan Grants NSC96-2320-B-038-028 and NSC 96-2320-B-002-022.
REFERENCES
1. Aung HT, Schroder K, Himes SR, Brion K, van Zuylen W, Trieu A,
Suzuki H, Hayashizaki Y, Hume DA, Sweet MJ, Ravasi T. LPS
regulates proinflammatory gene expression in macrophages by altering histone deacetylase expression. FASEB J 20: 1315–1327, 2006.
2. Barnes PJ, Adcock IM, Ito K. Histone acetylation and deacetylation: importance in inflammatory lung diseases. Eur Respir J 25: 552–563, 2005.
3. Bode KA, Schroder K, Hume DA, Ravasi T, Heeg K, Sweet MJ,
Dalpke AH. Histone deacetylase inhibitors decrease Toll-like
receptor-mediated activation of proinflammatory gene expression by impairing transcription factor recruitment. Immunology 122: 596 – 606, 2007. 4. Chakravortty D, Koide N, Kato Y, Sugiyama T, Mu MM, Yoshida T,
Yokochi T. The inhibitory action of butyrate on
lipopolysaccharide-induced nitric oxide production in RAW 264.7 murine macrophage cells.
J Endotoxin Res 6: 243–247, 2000.
5. Chen Lf Fischle W, Verdin E, Greene WC. Duration of nuclear NF-kappaB action regulated by reversible acetylation. Science 293: 1653– 1657, 2001.
6. Chen LF, Greene WC. Regulation of distinct biological activities of the NF-kappaB transcription factor complex by acetylation. J Mol Med 81: 549 –557, 2003.
7. Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156 –159, 1987.
8. Clerc RG, Corcoran LM, LeBowitz JH, Baltimore D, Sharp PA. The B-cell-specific Oct-2 protein contains POU box- and homeo box-type domains. Genes Dev 2: 1570 –1581, 1988.
9. Deng WG, Wu KK. Regulation of inducible nitric oxide synthase ex-pression by p300 and p50 acetylation. J Immunol 171: 6581– 6588, 2003. 10. Geller DA, Billiar TR. Molecular biology of nitric oxide synthases.
Cancer Metastasis Rev 17: 7–23, 1998.
11. Grunstein M. Histone acetylation in chromatin structure and transcrip-tion. Nature 389: 349 –352, 1997.
12. Guo L, Guo H, Gao C, Mi Z, Russell WB, Kuo PC. Stat1 acetylation inhibits inducible nitric oxide synthase expression in interferon-gamma-treated RAW264.7 murine macrophages. Surgery 142: 156 –162, 2007. 13. He X, Treacy MN, Simmons DM, Ingraham HA, Swanson LW,
Rosenfeld MG. Expression of a large family of POU-domain regulatory
genes in mammalian brain development. Nature 340: 35– 41, 1989. 14. Heba G, Krzemin´ski T, Porc M, Grzyb J, Dembin´ska-Kiec´ A. Relation
between expression of TNF alpha, iNOS, VEGF mRNA and development of heart failure after experimental myocardial infarction in rats. J Physiol
Pharmacol 52: 39 –52, 2001.
15. Horion J, Gloire G, El Mjiyad N, Quivy V, Vermeulen L, Vanden
Berghe W, Haegeman G, Van Lint C, Piette J, Habraken Y. Histone
deacetylase inhibitor trichostatin A sustains sodium pervanadate-induced NF-kappaB activation by delaying IkappaBalpha mRNA resynthesis: comparison with tumor necrosis factor alpha. J Biol Chem 282: 15383– 15393, 2007.
16. Imhof A, Yang XJ, Ogryzko VV, Nakatani Y, Wolffe AP, Ge H. Acetylation of general transcription factors by histone acetyltransferase.
Curr Biol 7: 689 – 692, 1997.
17. Ito K, Lim S, Caramori G, Cosio B, Chung KF, Adcock IM, Barnes
PJ. A molecular mechanism of action of theophylline: Induction of
histone deacetylase activity to decrease inflammatory gene expression.
Proc Natl Acad Sci USA 99: 8921– 8926, 2002.
18. Kang SM, Tsang W, Doll S, Scherle P, Ko HS, Tran AC, Lenardo MJ,
Staudt LM. Induction of the POU domain transcription factor Oct-2
during T-cell activation by cognate antigen. Mol Cell Biol 12: 3149 –3154, 1992.
19. Koch A, Giembycz M, Ito K, Lim S, Jazrawi E, Barnes PJ, Adcock I,
Erdmann E, Chung KF. Mitogen-activated protein kinase modulation of
nuclear factor-kappaB-induced granulocyte macrophage-colony-stimulat-ing factor release from human alveolar macrophages. Am J Respir Cell
Mol Biol 30: 342–349, 2004.
20. Larsen L, Tonnesen M, Ronn SG, Størling J, Jørgensen S, Mascagni
P, Dinarello CA, Billestrup N, Mandrup-Poulsen T. Inhibition of
histone deacetylases prevents cytokine-induced toxicity in beta cells.
Diabetologia 50: 779 –789, 2007.
21. Lu SC, Chang SF, Chen HL, Chou YY, Lan YH, Chuang CY, Yu WH,
Chen CL. A novel role for Oct-2 in the lipopolysaccharide-mediated
induction of resistin gene expression in RAW264.7 cells. Biochem J 402: 387–395, 2007.
22. Lopez-Rodriguez C, Zubiaur M, Sancho J, Concha A, Corbi AL. An Octamer element functions as a regulatory element in the differentiation-responsive CD11c integrin gene promoter: Oct-2 inducibility during myelomonocytic differentiation. J Immunol 158: 5833–5840, 1997.
C1138 OCT-2 IN THE LPS-INDUCEDINOS EXPRESSION
AJP-Cell Physiol•VOL 296 • MAY 2009 •www.ajpcell.org
on May 28, 2011
ajpcell.physiology.org
23. Luchina NN, Krivega IV, Pankratova EV. Human Oct-1L isoform has tissue-specific expression pattern similar to Oct-2. Immunol Lett 85: 237–241, 2003.
24. Marks PA, Richon VM, Breslow R, Rifkind RA. Histone deacetylase inhibitors as new cancer drugs. Curr Opin Oncol 13: 477– 483, 2001. 25. Marks PA, Richon VM, Rifkind RA. Histone deacetylase inhibitors:
inducers of differentiation or apoptosis of transformed cells. J Natl Cancer
Inst 92: 1210 –1216, 2000.
26. Marletta MA. Nitric oxide synthase: aspects concerning structure and catalysis. Cell 78: 927–930, 1994.
27. Moncada S. Nitric oxide: discovery and impact on clinical medicine. J R
Soc Med 92: 164 –169, 1999.
28. Neumann M, Fries H, Scheicher C, Keikavoussi P, Kolb-Maurer A,
Brocker E, Serfling E, Kampgen E. Differential expression of
Rel/NF-kappaB and octamer factors is a hallmark of the generation and maturation of dendritic cells. Blood 95: 277–285, 2000.
29. Selker EU. Trichostatin A causes selective loss of DNA methylation in
Neurospora. Proc Natl Acad Sci USA 95: 9430 –9435, 1998.
30. Staudt LM, Clerc RG, Singh H, LeBowitz JH, Sharp PA, Baltimore D. Cloning of a lymphoid-specific cDNA encoding a protein binding the regulatory Octamer DNA motif. Science 241: 577–580, 1988.
31. Staudt LM, Singh H, Sen R, Wirth T, Sharp PA, Baltimore D. A lymphoid-specific protein binding to the octamer motif of immunoglobu-lin genes. Nature 323: 640 – 643, 1986.
32. Suuronen T, Huuskonen J, Pihlaja R, Kyrylenko S, Salminen A. Regulation of microglial inflammatory response by histone deacetylase inhibitors. J Neurochem 87: 407– 416, 2003.
33. Tanaka M, Herr W. Differential transcriptional activation by Oct-1 and Oct-2: interdependent activation domains induce Oct-2 phosphorylation.
Cell 60: 375–386, 1990.
34. Taylor BS, Geller DA. Molecular regulation of the human inducible nitric oxide synthase (iNOS) gene. Shock 13: 413– 424, 2000.
35. Turner BM. Histone acetylation and control of gene expression. J Cell Sci 99: 13–20, 1991.
36. Van Lint C, Emiliani S, Verdin E. The expression of a small fraction of cellular genes is changed in response to histone hyperacetylation. Gene
Expr 5: 245–253, 1996.
37. Xie Q. A novel lipopolysaccharide-response element contributes to induc-tion of nitric oxide synthase. J Biol Chem 272: 14867–14872, 1997. 38. Yoshida M, Kijima M, Akita M, Beppu T. Potent and specific inhibition
of mammalian histone deacetylase both in vivo and in vitro by trichostatin A. J Biol Chem 265: 17174 –17179, 1990.
39. Yoshida M, Horinouchi S, Beppu T. Trichostatin A and trapoxin: novel chemical probes for the role of histone acetylation in chromatin structure and function. Bioessays 17: 423– 430, 1995.
40. Yu Z, Kone BC. Targeted histone H4 acetylation via phosphoinositide 3-kinase- and p70s6-kinase-dependent pathways inhibits iNOS induction in mesangial cells. Am J Physiol Renal Physiol 290: F496 –F502, 2006. 41. Yu Z, Zhang W, Kone BC. Histone deacetylases augment cytokine
induction of the iNOS gene. J Am Soc Nephrol 13: 2009 –2017, 2002. 42. Zhao FQ, Zheng Y, Dong B, Oka T. Cloning, genomic organization,
expression, and effect on beta-casein promoter activity of a novel isoform of the mouse Oct-1 transcription factor. Gene 326: 175–187, 2004.
on May 28, 2011
ajpcell.physiology.org