From the Section of Infection, Departments of Medicine, Taipei Medical University - Wan Fang Hospital, Taipei, Taiwan;
1Department of Pediatrics, National Taiwan University Hospital, Taipei, Taiwan.
Received: Mar. 6, 2007; Accepted: Aug. 2, 2007
Correspondence to: Dr. Li-Min Huang, Department of Pediatrics, National Taiwan University Hospital. 7, Jhongshan S. Rd., Jhongjheng District, Taipei City 100, Taiwan (R.O.C.) Tel.: 886-2-23123456 ext. 5139; Fax: 886-2-23934749; E-mail: lmhuang@ha.mc.ntu.edu.tw
The Transforming Streptococcus Pneumoniae
in the 21st Century
Yu-Chia Hsieh, MD, PhD; Wen-Sen Lee, MD; Pei-Lan Shao
1, MD;
Luan-Yin Chang
1, MD, PhD; Li-Min Huang
1, MD, PhD
Streptococcus pneumoniae, an important pathogen causing sepsis, sinusitis, otitis media, bacterial meningitis and bacterial pneumonia, results in global morbidity and mortality each year. The burden of pneumococcal disease is highest in chil-dren and the elderly. Treatment of pneumococcal infection has been hampered by the complexity of the host immune response. In recent decades, the increase of S. pneumoniae strains’ resistance to `-lactam antibiotics and other classes of antimicrobials has made treatment even more complicated. Fortunately, the advent of heptavalent conjugate vaccine con-fers a high degree of protection against pneumococcal disease and colonization caused by vaccine serotype strains. After the introduction of conjugate pneumococcal vaccine, invasive pneumococcal disease caused by vaccine serotypes and antibi-otic-resistant isolates has been reduced. However, naturally
transformable pneumococci may escape vaccine-induced immunity by switching their cap-sular genes to non-vaccine serotypes. Development of cheaper, serotype-independent vac-cines based on a combination of protein antigens should be pursued. (Chang Gung Med J 2008;31:117-24)
Key words: Streptococcus pneumoniae, conjugate vaccine, transformable
S
treptococcus pneumoniae, a pathogen discoveredmore than one hundred years ago, remains a lead-ing cause of bacteremia, sinusitis, otitis media, bac-terial meningitis and pneumonia. This bacterium is present worldwide, and is associated with substantial illnesses and deaths in humans.(1) Historically, study
of the biology of S. pneumoniae led to the identifica-tion of the nature of genetic material, the phenome-non of quorum sensing, the use of polysaccharide-based vaccine and the recognition of bacterial
resis-tance to antimicrobial drugs.(2,3) Since the complete
genome of S. pneumoniae was decoded in 1997, much has been discovered about the bacterial pro-teins involved in pneumococcal disease, the regula-tion of virulence and the regularegula-tion of DNA uptake.(4)
Recently, the landscape of pneumococcal infection has been changed by two major events, namely, availability of conjugate pneumococcal vaccine and more aggressive behavior of pneumococcal pneumo-nia.(5,6)It is now a good time to review our
standing of the biology and clinical behavior of S.
pneumoniae.
S. pneumoniae virulence factors
Capsule
Polysaccharide capsule is the earliest known S.
pneumoniae virulence factor, and serves as a
para-digm for studies of immune responses and polysac-charide biochemistry. Capsular polysacpolysac-charide is composed of multiple sugars that help pneumococci fight against phagocytosis. The amount of capsule expression in the microbe changes during replication in the host, a phenomenon known as phase varia-tion.(7)Reduced capsule expression (transparent
vari-ant) in the nasopharynx is instrumental in exposing the adhesins necessary for colonization, whereas increase in capsule expression (opaque variant) is essential for avoiding complement-mediated opsonophagocytosis during invasive disease. Several factors such as BOX elements, capsule locus A (CpsA), CpsB, CpsC and CpsD, and spontaneous sequence duplication contribute to the complex regu-lation of capsule synthesis.(8-12)
Choline-binding proteins
S. pneumoniae possesses several
choline-bind-ing proteins on its surface that serve as a way of attaching it to the cell surface. The most well-known choline-binding proteins in pneumococci are autolysin, pneumococcal surface protein C (PspC) and pneumococcal surface protein A (PspA). Autolysin (LytA amidase) degrades peptidoglycan of the pneumococcal cell wall and separates daughter cells. Lysis of pneumococci by autolysin leads to release of the pneumococcal cell wall and pneu-molysin, which in turn induce inflammatory respons-es and cause tissue damage.(13) PspA is a protective
antigen of S. pneumoniae, and is able to inhibit com-plement deposition and activation.(14,15)It contributes
to pneumococcal virulence in both bacteremia and sepsis models.(16) PspC, also referred to as
choline-binding protein A (CbpA), acts as an adhesin, and interacts with the polymeric immunoglobulin recep-tor (pIgR) on mucosal epithelial cells to facilitate adhesion and invasion.(17)
Pneumolysin and other virulence factors
The role of pneumolysin in pneumococcal
infec-tion has been well studied. Pneumolysin belongs to the family of pore-forming toxins, which can lyse cell membranes containing cholesterol. This toxin also activates the complement system, induces the production of proinflammatory mediators, recruits inflammatory cells and causes cell apoptosis.(18,19)
Other proteins, including LPXTG-anchored protein (hyaluronidase, neuraminidase and serine protease), lipoprotein, hydrogen peroxide, superoxide dismu-tase, NADH (nicotinamide adenine dinucleotide, reduced form) oxidase, as well as zinc metallopro-tease (immunoglobulin A prometallopro-tease, ZmpB and ZmpC), also contribute to the virulence of S.
pneu-moniae. A pneumococcal pilus encoded by the rlrA
pathogenicity islet, consisting of LPXTG-containing surface proteins and sortases, enhances adherence and stimulates the host inflammatory response.(20)
Pneumococ-cal neuraminidases cleave sialic acid-containing substrates. Neuraminidase A and B both have essential roles in respiratory tract infection and sepsis. Neuraminidase C may contribute to the abili-ty of pneumococci to cause meningitis.(21)
Capsular type or clonal type determine the invasive capacity of S. pneumoniae
S. pneumoniae can be divided into more than 91
distinct types according to capsular polysaccharides but only 20 to 30 types are associated with human diseases. Hence, there is an association between serotype and the potential of pneumococci to cause invasive disease. Certain serotypes, such as serotype 1 are highly invasive, mostly due to the specific chemical composition of their capsules. Serotype 3 can evade the immune system, readily resulting in a fatal disease.(22) Further studies of the population
biology of S. pneumoniae found that, even within the same serotype, some individual clones (such as ST9 and ST124) were significantly overrepresented in invasive diseases compared with carriage.(23) So far,
the exact mechanisms of why some serotypes can go beyond colonization to cause invasive disease remain unclear but it appears that the capsule is not suffi-cient to determine invasive potential or inflammatory response.(24,25)The genetic background of the host, in
addition to the capsule, also plays a critical role in dictating virulence. Understanding the underlying mechanism of virulent genotypes becomes a priority in the era of the pneumococcal conjugate vaccine.
Innate immunity
S. pneumoniae infection is countered by a robust
inflammatory reaction in the host. Complement, C-reactive proteins (CRP), surfactant protein, Toll-like receptors (TLR) and T cells comprise the major com-ponents of the immune response against S.
pneumo-niae. Studies using mice deficient in specific genes
indicated that both the classical and alternative com-plement pathways were vital in host defense against pneumococcal infection.(26)CRP specifically binds to
phosphocholine residues of C-polysaccharide (PnC) in the cell wall of S. pneumoniae to activate the clas-sical pathway of complement in human serum.(27)
Lung surfactant protein-D (SP-D) facilitated the early clearance of pneumococci in a murine model of bronchopneumonia and bacteremia.(28) TLR2
recog-nizes pneumococcal lipoteichoic acid (LTA) and cell wall peptidoglycan to initiate an inflammatory response. TLR2 also had a protective role in sys-temic infection and nasopharyngeal colonization in a murine model.(29,30) TLR4 recognizes pneumococcal
pneumolysin to limit pneumococcal proliferation in the nasopharynx.(31) TLR4 also interacts with
pneu-molysin to induce mammalian cell apoptosis against pneumococcal infection.(32) CD4 (cluster of
differen-tiation 4) T cells were found to contribute to early protective immunity to S. pneumoniae based on stud-ies using mice lacking the major histocompatibility complex II (MHCII) gene.(33)However, how CD4+ T
cells function in this aspect remains unclear.
In addition, Nod1 and Nod2 are cytosolic pro-teins of the pathogen recognition receptor within host cells that respond to pneumococci.(34) The
myeloid differentiation factor (MyD88) is an adaptor molecule in the signaling of the host inflammatory cascade against pneumococcal infection.(35)
Pneumococcal colonization
The first step leading to pneumococcal disease is nasopharyngeal colonization. S. pneumoniae spreads through respiratory droplets. Following exposure, the pathogen may establish itself in the nasopharynx of the new host. The human nasophar-ynx is the only known natural reservoir for S.
pneu-moniae. Invasive pneumococcal disease occurs when
pneumococci gain access into deep human tissues, which might be facilitated by prior virus infection, especially influenza virus infection.(36)S. pneumoniae
invades human nasopharyngeal epithelial cells
through a process termed reverse endocytosis medi-ated by pIgR. Nasopharyngeal colonization is dynamic, and influenced by overcrowding, smoking, ethnicity and socioeconomic status.(37) Colonization
rates vary from 3% to 70% in healthy children in dif-ferent countries and gradually decline with age up to adulthood.(38-40)One way to reduce invasive
pneumo-coccal disease is prevention of colonization. However, this may lead to replacement by other bac-terial species in the nasopharynx, such as
Staphylococcus aureus and Haemophilus influen-zae.(41)Hence, a protein-based pneumococcal vaccine
to prevent the invasive disease without disturbing the bacterial ecology in the nasopharynx may be consid-ered for controlling pneumococcal disease.(41)
Evolution of S. pneumoniae
S. pneumoniae was the first pathogen to
demon-strate the phenomenon of transformation. In 1944, Avery et al. proved that the genetic material in bacte-rial cells was DNA by using a transformation model in S. pneumoniae.(42)Natural competence for genetic
transformation in S. pneumoniae is mediated by a quorum sensing-regulated system. CSP, a heptade-capeptide pheromone, induces competence in grow-ing cells at a critical cell density by activatgrow-ing the 2-component signal transduction system comDE.(3)Due
to the ability to undergo horizontal gene transfer, S.
pneumoniae easily adapts to environmental changes,
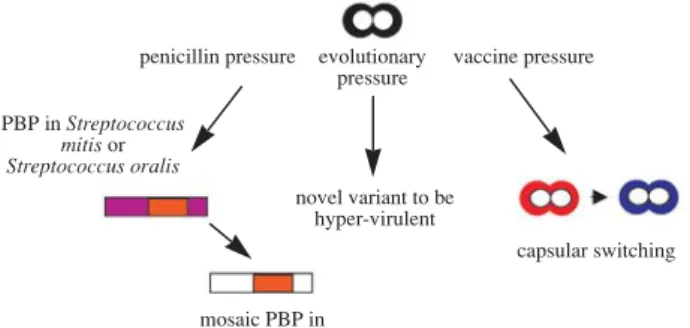
which leads to substantial genetic heterogeneity as well as genomic plasticity (Fig. 1). The first example is the presence of divergent mosaic blocks in peni-cillin binding protein (PBP) genes in penipeni-cillin-resis- penicillin-resis-tant S. pneumoniae under the selective pressure of
Fig. 1 Evolution of naturally transformable Streptococcus
pneumoniae.
penicillin pressure evolutionary vaccine pressure pressure PBP in Streptococcus mitis or Streptococcus oralis novel variant to be hyper-virulent capsular switching mosaic PBP in S. pneumoniae
penicillin. Mosaic PBP genes evolve to be penicillin-resistant via acquiring PBP from other Streptococcus species.(43) The second example is the evolution to
greater virulence via recombination. Serotype 6B causes more invasive diseases than serotype 6A. By using multilocus sequence typing, serotype 6B clones evolved almost exclusively by recombination, whereas serotype 6A evolved by mutation.(44) The
third example is capsular switching under a large-scale vaccination program.(45) Although the current
introduction of conjugate pneumococcal vaccine has successfully reduced invasive pneumococcal disease caused by the vaccine serotypes and effectively decreased the spread of antimicrobial drug-resistant isolates, pneumococcal infection remains a major issue. At least two consequences have been noted since the use of heptavalent conjugate vaccine. First, serotypes not covered by the conjugate vaccine have increased both in nasopharyngeal colonization and clinical illness.(45) Second, serotype switching can
occur through recombination in naturally trans-formable clones and result in the acquisition of a non-vaccine capsule to escape vaccine-induced immunity.(45) Furthermore, the ability of different
serotypes to be transformed affected the evolutionary biology and genetic diversity of each serotype. Serotype competence accounts for why the reported serotypes that underwent in vivo capsular transfor-mation were also antibiotic-resistant. Gene transfer has been a powerful tool in the evolution of S.
pneu-moniae.
Emerging disease: complicated pneumonia
S. pneumoniae is the most common pathogen of
pyogenic pneumonia in children. Previous studies have shown that the lungs return to normal after pneumococcal pneumonia, regardless of the severity at the peak stage of the disease. This is for two rea-sons. First, S. pneumoniae usually induces granulo-cyte apoptosis, which tends to limit tissue injury and promotes the complete resolution of pneumonia.(46)
Second, S. pneumoniae produces few exotoxins capable of inducing lung damage, in contrast to other organisms such as Staphylococcus aureus and
Streptococcus pyogenes, which produce a variety of
tissue-damaging substances causing lung necrosis and destructive lung injury.(47)Since the advent of the
use of penicillin, S. pneumoniae infection has rarely developed into empyema or lung necrosis.
However, an increase of complicated pneumo-coccal pneumonia, including necrotizing pneumonia, lung abscess and empyema, has been observed in children since the 1990s(5,48,49) (Table 1). The
occur-rence of complicated pneumonia was associated with longer durations of fever, longer oxygen requirement and longer hospital stays.(5,48) Older age, white race,
presence of immature polymorphonuclear leukocytes in peripheral blood, high CRP levels, no underlying disease or chest pain on presentation were predictors of lung necrosis and/or abscess.(5,48) This increase of
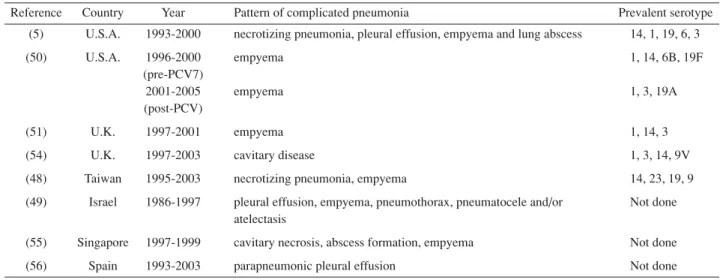
Table 1. Studies of an Increase in Complicated Pneumococcal Pneumonia
Reference Country Year Pattern of complicated pneumonia Prevalent serotype (5) U.S.A. 1993-2000 necrotizing pneumonia, pleural effusion, empyema and lung abscess 14, 1, 19, 6, 3
(50) U.S.A. 1996-2000 empyema 1, 14, 6B, 19F
(pre-PCV7)
2001-2005 empyema 1, 3, 19A
(post-PCV)
(51) U.K. 1997-2001 empyema 1, 14, 3
(54) U.K. 1997-2003 cavitary disease 1, 3, 14, 9V
(48) Taiwan 1995-2003 necrotizing pneumonia, empyema 14, 23, 19, 9 (49) Israel 1986-1997 pleural effusion, empyema, pneumothorax, pneumatocele and/or Not done
atelectasis
(55) Singapore 1997-1999 cavitary necrosis, abscess formation, empyema Not done
(56) Spain 1993-2003 parapneumonic pleural effusion Not done
complicated pneumonia is not directly related to the increase in penicillin-resistant S. pneumoniae.(5,48,49)In
the U.S., serotype 14 was the most common serotype causing complicated pneumonia, whereas serotype 1 and serotype 3 significantly caused complicated pneumonia compared to those serotypes causing lobar pneumonia in children before the widespread use of heptavalent pneumococcal conjugate vac-cine.(5) After the utilization of conjugate vaccine,
serotype 1 remained prevalent, and serotypes 3 and 19A were increasingly detected.(50) In the U.K.,
serotype 1 was also the dominant serotype causing pneumococcal empyema.(51) Clonal spread of
pneu-mococcal serotype 1 is speculated to contribute to the increased complicated pneumonia in the U.S. and U.K. Interestingly, serotype 1 S. pneumoniae was rare in the nasopharynx but had a high clinical inci-dence. This serotype was common in both Northern Europe and North America in the early 20th century, and now has become more prevalent in developing countries such as Rwanda, Egypt and Africa. Poverty, overcrowding and decreased availability of antibiotics all contribute to the spread of serotype 1.(52) In view of the rare carriage of serotype 1 S.
pneumoniae, it is mysterious as to how it is
transmit-ted among humans. In most cases of culture-negative parapneumonic pleural effusion or empyema, serotype 1 was the frequent etiology.(51,53)
Surprisingly, several studies failed to identify serotype 1 in clinical samples in Taiwan. Instead, the major clone associated with complicated pneumonia in Taiwan was serotype 14.(48)Since serotype 1 is
dif-ficult to culture, whether there is real serotype differ-ence in complicated pneumonia is worth further study in Taiwan.
Conclusion
Given the proclivity of horizontal gene transfer, current advances in antimicrobial therapy and serotype-limited conjugate vaccine are inadequate to combat pneumococcal diseases. In the future, better understanding of molecular interaction at the cellular level could provide insight into the development of protein vaccine and new modulation therapy.
REFERENCES
1. Hausdorff WP, Bryant J, Paradiso PR, Siber GR. Which
pneumococcal serogroups cause the most invasive dis-ease: implications for conjugate vaccine formulation and use, part I. Clin Infect Dis 2000;30:100-21.
2. Austrian R. Pneumococcus: the first one hundred years. Rev Infect Dis 1981;3:183-9.
3. Havarstein LS, Coomaraswamy G, Morrison DA. An unmodified heptadecapeptide pheromone induces compe-tence for genetic transformation in Streptococcus
pneumo-niae. Proc Natl Acad Sci USA 1995;92:11140-4.
4. Campbell EA, Choi SY, Masure HR. A competence regu-lon in Streptococcus pneumoniae revealed by genomic analysis. Mol Microbiol 1998;27:929-39.
5. Tan TQ, Mason EO Jr, Wald ER, Barson WJ, Schutze GE, Bradley JS, Givner LB, Yogev R, Kim KS, Kaplan SL. Clinical characteristics of children with complicated pneumonia caused by Streptococcus pneumoniae. Pediatrics 2002;110:1-6.
6. Whitney CG, Farley MM, Hadler J, Harrison LH, Bennett NM, Lynfield R, Reingold A, Cieslak PR, Pilishvili T, Jackson D, Facklam RR, Jorgensen JH, Schuchat A; Active Bacterial Core Surveillance of the Emerging Infections Program Network. Decline in invasive pneu-mococcal disease after the introduction of protein-poly-saccharide conjugate vaccine. N Engl J Med 2003;348:1737-46.
7. Weiser JN, Markiewicz Z, Tuomanen EI, Wani JH. Relationship between phase variation in colony morphol-ogy, intrastrain variation in cell wall physiolmorphol-ogy, and nasopharyngeal colonization by Streptococcus
pneumoni-ae. Infect Immun 1996;64:2240-5.
8. Morona JK, Paton JC, Miller DC, Morona R. Tyrosine phosphorylation of CpsD negatively regulates capsular polysaccharide biosynthesis in Streptococcus
pneumoniae. Mol Microbiol 2000;35:1431-42.
9. Saluja SK, Weiser JN. The genetic basis of colony opacity in Streptococcus pneumoniae: evidence for the effect of box elements on the frequency of phenotypic variation. Mol Microbiol 1995;16:215-27.
10. Waite RD, Struthers JK, Dowson CG. Spontaneous
sequence duplication within an open reading frame of the
pneumococcal type 3 capsule locus causes high-frequency phase variation. Mol Microbiol 2001;42:1223-32. 11. Guidolin A, Morona JK, Morona R, Hansman D, Paton
JC. Nucleotide sequence analysis of genes essential for capsular polysaccharide biosynthesis in Streptococcus
pneumoniae type 19F. Infect Immun 1994;62:5384-96.
12. Bender MH, Yother J. CpsB is a modulator of capsule-associated tyrosine kinase activity in Streptococcus
pneu-moniae. J Biol Chem 2001;276:47966-74.
13. Lock RA, Hansman D, Paton JC. Comparative efficacy of autolysin and pneumolysin as immunogens protecting mice against infection by Streptococcus pneumoniae. Microb Pathog 1992;12:137-43.
14. Ren B, Szalai AJ, Thomas O, Hollingshead SK, Briles DE. Both family 1 and family 2 PspA proteins can inhibit
complement deposition and confer virulence to a capsular serotype 3 strain of Streptococcus pneumoniae. Infect Immun 2003;71:75-85.
15. Tu AH, Fulgham RL, McCrory MA, Briles DE, Szalai AJ. Pneumococcal surface protein A inhibits complement activation by Streptococcus pneumoniae. Infect Immun 1999;67:4720-4.
16. McDaniel LS, Yother J, Vijayakumar M, McGarry L, Guild WR, Briles DE. Use of insertional inactivation to facilitate studies of biological properties of pneumococcal surface protein A (PspA). J Exp Med 1987;165:381-94. 17. Luo R, Mann B, Lewis WS, Rowe A, Heath R, Stewart
ML, Hamburger AE, Sivakolundu S, Lacy ER, Bjorkman PJ, Tuomanen E, Kriwacki RW. Solution structure of choline binding protein A, the major adhesin of
Streptococcus pneumoniae. EMBO J 2005;24:34-43.
18. Cockeran R, Durandt C, Feldman C, Mitchell TJ, Anderson R. Pneumolysin activates the synthesis and release of interleukin-8 by human neutrophils in vitro. J Infect Dis 2002;186:562-5.
19. Zysk G, Bejo L, Schneider-Wald BK, Nau R, Heinz H. Induction of necrosis and apoptosis of neutrophil granulo-cytes by Streptococcus pneumoniae. Clin Exp Immunol 2000;122:61-6.
20. Barocchi MA, Ries J, Zogaj X, Hemsley C, Albiger B, Kanth A, Dahlberg S, Fernebro J, Moschioni M, Masignani V, Hultenby K, Taddei AR, Beiter K, Wartha F, von Euler A, Covacci A, Holden DW, Normark S, Rappuoli R, Henriques-Normark B. A pneumococcal pilus influences virulence and host inflammatory respons-es. Proc Natl Acad Sci USA 2006;103:2857-62.
21. Pettigrew MM, Fennie KP, York MP, Daniels J, Ghaffar F. Variation in the presence of neuraminidase genes among
Streptococcus pneumoniae isolates with identical
sequence types. Infect Immun 2006;74:3360-5.
22. Ling E, Feldman G, Dagan R, Mizrachi-Nebenzahl Y. Cytokine mRNA expression in pneumococcal carriage, pneumonia, and sepsis in young mice. J Infect Dis 2003;188:1752-6.
23. Brueggemann AB, Griffiths DT, Meats E, Peto T, Crook DW, Spratt BG. Clonal relationships between invasive and carriage Streptococcus pneumoniae and serotype- and clone-specific differences in invasive disease potential. J Infect Dis 2003;187:1424-32.
24. Sa-Leao R, Tomasz A, Sanches IS, Nunes S, Alves CR, Avo AB, Saldanha J, Kristinsson KG, de Lencastre H. Genetic diversity and clonal patterns among antibiotic-susceptible and -resistant Streptococcus pneumoniae colo-nizing children: day care centers as autonomous epidemi-ological units. J Clin Microbiol 2000;38:4137-44. 25. Mohler J, Azoulay-Dupuis E, Amory-Rivier C, Mazoit
JX, Bedos JP, Rieux V, Moine P. Streptococcus
pneumoni-ae strain-dependent lung inflammatory responses in a
murine model of pneumococcal pneumonia. Intensive Care Med 2003;29:808-16.
26. Brown JS, Hussell T, Gilliland SM, Holden DW, Paton JC, Ehrenstein MR, Walport MJ, Botto M. The classical pathway is the dominant complement pathway required for innate immunity to Streptococcus pneumoniae infec-tion in mice. Proc Natl Acad Sci USA 2002;99:16969-74. 27. Kaplan MH, Volanakis JE. Interaction of C-reactive
pro-tein complexes with the complement system. I. Consumption of human complement associated with the reaction of C-reactive protein with pneumococcal C-poly-saccharide and with the choline phosphatides, lecithin and sphingomyelin. J Immunol 1974;112:2135-47.
28. Kadioglu A, Andrew PW. The innate immune response to pneumococcal lung infection: the untold story. Trends Immunol 2004;25:143-9.
29. Khan AQ, Chen Q, Wu ZQ, Paton JC, Snapper CM. Both innate immunity and type 1 humoral immunity to
Streptococcus pneumoniae are mediated by MyD88 but
differ in their relative levels of dependence on toll-like receptor 2. Infect Immun 2005;73:298-307.
30. van Rossum AM, Lysenko ES, Weiser JN. Host and bac-terial factors contributing to the clearance of colonization by Streptococcus pneumoniae in a murine model. Infect Immun 2005;73:7718-26.
31. Malley R, Henneke P, Morse SC, Cieslewicz MJ, Lipsitch M, Thompson CM, Kurt-Jones E, Paton JC, Wessels MR, Golenbock DT. Recognition of pneumolysin by Toll-like receptor 4 confers resistance to pneumococcal infection. Proc Natl Acad Sci USA 2003;100:1966-71.
32. Srivastava A, Henneke P, Visintin A, Morse SC, Martin V, Watkins C, Paton JC, Wessels MR, Golenbock DT, Malley R. The apoptotic response to pneumolysin is Toll-like receptor 4 dependent and protects against pneumo-coccal disease. Infect Immun 2005;73:6479-87.
33. Kadioglu A, Coward W, Colston MJ, Hewitt CR, Andrew PW. CD4-T-lymphocyte interactions with pneumolysin and pneumococci suggest a crucial protective role in the host response to pneumococcal infection. Infect Immun 2004;72:2689-97.
34. Philpott DJ, Girardin SE. The role of Toll-like receptors and Nod proteins in bacterial infection. Mol Immunol 2004;41:1099-108.
35. Yamamoto M, Takeda K, Akira S. TIR domain-containing adaptors define the specificity of TLR signaling. Mol Immunol 2004;40:861-8.
36. Muller HE. Lethal synergism between influenza virus and
Streptococcus pneumoniae. J Infect Dis 2003;187:1674-5.
37. Hoge CW, Reichler MR, Dominguez EA, Bremer JC, Mastro TD, Hendricks KA, Musher DM, Elliott JA, Facklam RR, Breiman RF. An epidemic of pneumococcal disease in an overcrowded, inadequately ventilated jail. N Engl J Med 1994;331:643-8.
38. Vives M, Garcia ME, Saenz P, Mora MA, Mata L, Sabharwal H, Svanborg C. Nasopharyngeal colonization in Costa Rican children during the first year of life. Pediatr Infect Dis J 1997;16:852-8.
39. Coles CL, Kanungo R, Rahmathullah L, Thulasiraj RD, Katz J, Santosham M, Tielsch JM. Pneumococcal nasopharyngeal colonization in young South Indian infants. Pediatr Infect Dis J 2001;20:289-95.
40. Chiou CC, Liu YC, Huang TS, Hwang WK, Wang JH, Lin HH, Yen MY, Hsieh KS. Extremely high prevalence of nasopharyngeal carriage of penicillin-resistant
Streptococcus pneumoniae among children in Kaohsiung,
Taiwan. J Clin Microbiol 1998;36:1933-7.
41. Bogaert D, De Groot R, Hermans PW. Streptococcus
pneumoniae colonisation: the key to pneumococcal
dis-ease. Lancet Infect Dis 2004;4:144-54.
42. Avery OT, MacLeod CM, McCarty M. Studies on the chemical nature of the substance inducing transformation of pneumococcal types. J Exp Med 1944;79:137-58. 43. Dowson CG, Hutchison A, Brannigan JA, George RC,
Hansman D, Linares J, Tomasz A, Smith JM, Spratt BG. Horizontal transfer of penicillin-binding protein genes in penicillin-resistant clinical isolates of Streptococcus
pneu-moniae. Proc Natl Acad Sci USA 1989;86:8842-6.
44. Robinson DA, Briles DE, Crain MJ, Hollingshead SK. Evolution and virulence of serogroup 6 pneumococci on a global scale. J Bacteriol 2002;184:6367-75.
45. Kaplan SL, Mason EO Jr, Wald ER, Schutze GE, Bradley JS, Tan TQ, Hoffman JA, Givner LB, Yogev R, Barson WJ. Decrease of invasive pneumococcal infections in children among 8 children’s hospitals in the United States after the introduction of the 7-valent pneumococcal conju-gate vaccine. Pediatrics 2004;113:443-9.
46. Haslett C. Granulocyte apoptosis and its role in the reso-lution and control of lung inflammation. Am J Respir Crit Care Med 1999;160:S5-11.
47. Spreer A, Lis A, Gerber J, Reinert RR, Eiffert H, Nau R. Differences in clinical manifestation of Streptococcus
pneumoniae infection are not correlated with in vitro
pro-duction and release of the virulence factors pneumolysin and lipoteichoic and teichoic acids. J Clin Microbiol 2004;42:3342-5.
48. Hsieh YC, Hsueh PR, Lu CY, Lee PI, Lee CY, Huang LM. Clinical manifestations and molecular epidemiology of necrotizing pneumonia and empyema caused by
Streptococcus pneumoniae in children in Taiwan. Clin
Infect Dis 2004;38:830-5.
49. Wexler ID, Knoll S, Picard E, Villa Y, Shoseyov D, Engelhard D, Kerem E. Clinical characteristics and out-come of complicated pneumococcal pneumonia in a pedi-atric population. Pediatr Pulmonol 2006;41:726-34. 50. Byington CL, Korgenski K, Daly J, Ampofo K, Pavia A,
Mason EO. Impact of the pneumococcal conjugate vac-cine on pneumococcal parapneumonic empyema. Pediatr Infect Dis J 2006;25:250-4.
51. Eastham KM, Freeman R, Kearns AM, Eltringham G, Clark J, Leeming J, Spencer DA. Clinical features, aetiol-ogy and outcome of empyema in children in the north east of England. Thorax 2004;59:522-5.
52. Dagan R, Gradstein S, Belmaker I, Porat N, Siton Y, Weber G, Janco J, Yagupsky P. An outbreak of
Streptococcus pneumoniae serotype 1 in a closed
commu-nity in southern Israel. Clin Infect Dis 2000;30:319-21. 53. Obando I, Arroyo LA, Sanchez-Tatay D, Moreno D,
Hausdorff WP, Brueggemann AB. Molecular typing of pneumococci causing parapneumonic empyema in Spanish children using multilocus sequence typing direct-ly on pleural fluid samples. Pediatr Infect Dis J 2006;25:962-3.
54. Ramphul N, Eastham KM, Freeman R, Eltringham G, Kearns AM, Leeming JP, Hasan A, Hamilton LJ, Spencer DA. Cavitatory lung disease complicating empyema in children. Pediatr Pulmonol 2006;41:750-3.
55. Tan Kendrick AP, Ling H, Subramaniam R, Joseph VT. The value of early CT in complicated childhood pneumo-nia. Pediatr Radiol 2002;32:16-21.
56. Deiros Bronte L, Baquero-Artigao F, Garcia-Miguel MJ, Hernandez Gonzalez N, Pena Garcia P, del Castillo Martin F. Parapneumonic pleural effusion: an 11-year review. An Pediatr (Barc) 2006;64:40-5.
1 1 1 7 ( 2008;31:117-24) 1 96 3 6 96 8 2 100 7 Tel.: (02)23123456 5139;