Synthesis and characterization of fluorinated polybenzoxazine material
with low dielectric constant
Yi-Che Su, Feng-Chih Chang*
Institute of Applied Chemistry, National Chiao-Tung University, Hsin-Chu 30050, Taiwan Received 18 June 2003; received in revised form 25 September 2003; accepted 14 October 2003
Abstract
It is well-known that low dielectric materials are used as insulating materials, and the incorporation of fluorinated substitutes into polymer is able to decrease its dielectric constant because of small dipole and the low polarizability of the C – F bond. In this study, a novel structure of the fluorinated benzoxazine (F-1 benzoxazine) has been synthesized by incorporating the trifluoromethyl groups into the monomer, and its structure has also been characterized by1H NMR,19F NMR and FT-IR. Further, we have prepared the fluorinated copolybenzoxazine (B-a/F-1 ¼ (B-a/F-1/(B-a/F-1) with substantially reduced dielectric constant at K ¼ 2:36: In addition, this fluorinated copolybenzoxazine possesses high glass transition temperature and high thermal stability, which is suitable for high temperature operation for certain special processes of interlayer dielectrics.
q2003 Published by Elsevier Ltd.
Keywords: Dielectric constant; Fluorination; Benzoxazine
1. Introduction
Low dielectric constant materials ðK , 3:0Þ have the advantage of facilitating manufacture of higher performance integrated-circuit (IC) devices with decreasing feature size of the chip[1]. However, interlevel dielectric materials must meet stringent material property requirements for successful integration into either the conventional or damascene interconnect structures. These requirements are based on electrical properties, thermal stability, thermomechanical, thermal stress properties, and moisture uptake. The desired electrical properties are low dielectric constant, low dielectric loss, and high breakdown voltage. However, the propagation delay and cross-talk are primary problems which have been concerned in dielectric materials.
Continuing improvement in device’s performance has significantly affected its dimensional requirement. These enhancements have to reduce in the wiring pitch and increase in the number of wiring leave to fulfill demands for density and performance improvement, especially at high frequency operations in the hundreds of megahertz or even
gigahertz range. As device dimensions shrink to less than 0.18 mm process (even 0.13 mm or smaller), it is necessary to reduce either the resistance of the metallization or the dielectric constant of the IMD (inter-metal dielectrics) material or both [1,2]. The time, which is related to the resulting signal delay is given by Eq. (1).
t¼ RC ð1Þ
and call the RC the time delay of signals. R is the line resistance and C is the line capacitance of the used structure. Reducing the resistance – capacitance ðRCÞ [3,4] is needed in order to avoid propagation delay, cross-talk noise, and power dissipation [3]. Eq. (2) can be used to estimate the RC delay.
RC ¼ 2r110 4L2 P2 þ L2 T2 ! ð2Þ R : total line resistance, C : total line capacitance, r: metal resistivity, 1 : dielectric constant, 10: permittivity of
free space, L : line length, P : the distance between two conducting lines, T : metal thickness.
After replacing the aluminum process by the cupper process, the metal resistivity has been reduced from 2.650 £ 1028Vm to 1.678 £ 1028Vm [2]. The most
0032-3861/$ - see front matter q 2003 Published by Elsevier Ltd. doi:10.1016/j.polymer.2003.10.026
www.elsevier.com/locate/polymer
* Corresponding author. Tel.: þ886-3-5727077; fax: þ886-3-5719507. E-mail address: muddaxac.ac87g@nctu.edu.tw (F.C. Chang), changfc@cc.nctu.edu.tw (F.C. Chang).
feasible approach is to use an insulating material possessing a lower dielectric constant without changing the copper process. In addition to the low dielectric constant, materials must also exhibit high thermal stability (Tg and Td), low
thermal expansion coefficient, low moisture uptake, good chemical stability and electric properties for practical applications[1,3].
Polybenzoxazine (PBZZ) resins were found to possess several outstanding properties such as near-zero shrinkage after curing, high thermal stability and low water absorption [5]. Furthermore, the PBZZ has high glass transition temperature even thought it has relatively low crosslinking density[6]. Several methods have been developed to lower the material dielectric constant[1 – 3]. Fluorinated polymer is a practicable and feasible approach to develop low dielectric materials. It is well-known that the incorporation of fluorinated substitutes into a polymer is able to decrease its dielectric constant because of small dipole and the low polarizability of the C – F bond [1,2,7]. Furthermore, polymer free volume is also increased by replacing methyl groups by trifluoromethyl groups[1].
In this paper, a series of fluorinated PBZZ possessing desirable properties of low dielectric constant with low RC delay time and high speed logic chip have been synthesized and characterized. 2. Experimental 2.1. Synthesis of 3-[4-(trifluoromethyl)phenol]-6-(2,2,2-trifluoro-1-(trifluoromethyl) -1-{3-[4-(trifluoromethyl) phenol]-3,4-dihydro-2H-1,3-benzoxazine-6-yl}-ethyl)-3,4-dihydro-2H-1,3-benzoxazine (F-1 benzoxazine)
The F-1 benzoxazine was prepared according toScheme 1. 0.04 mol 37% formaldehyde aqueous solution and 5 ml dioxane were fed into a three-necked flask equipped with nitrogen flow and an ice bath for 10 min. Then, 0.02 mol 4-(trifluoromethyl)aniline dissolved in 5 ml dioxane was added into the reactor slowly by a dropping funnel. The mixture was stirred continuously for 10 min, and 0.01 mol hexafluorobisphenol A in 20 ml dioxane was added. The reaction temperature was raised to 100 8C, and the mixture was allowed to reflux for 24 h. The solvent was then removed by reducing pressure, and the yellow solid product obtained. The crude product was dissolved in ethyl ether and washed with 1N NaOH and water in sequence for three times. The product solution was dried by magnesium sodium and distilled by reducing pressure, a light yellow solid product, F-1 benzoxazine, in 71% yield was obtained. 2.2. Nuclear magnetic resonance (NMR)
1H and 19F NMR spectra were recorded on a Varian
Unity Inova 500 FT NMR Spectrometer operating at
500 MHz with chemical shift reported in parts per million (ppm). Deuterium chloroform was used as solvent. 2.3. Fourier transform infrared spectroscopy (FT-IR)
FT-IR measurement was recorded on a Nicolet Avatar 320 FT-IR Spectrophotometer, 32 scans were collected with a spectral resolution of 1 cm21. Infrared spectra of the benzoxazine were obtained using the conventional NaCl method. The film used in this study was thin enough to obey the Beer – Lambert law. The sample chamber was purged with nitrogen during process of measurement in order to maintain sample film drying.
2.4. Reactants preparations
As shown inScheme 2, blends of B-a/F-1 benzoxazines with different ratio were prepared by solution blending because the mechanical strength of the F-1 PBZZ is too weak to form a film for DEA experiment. The monomer mixture was stirred and dissolved in acetone and the solution was allowed to evaporate slowly at 50 8C for 1 day and cured at 180 8C for 4 h under vacuum to ensure total curing of the co-polybenzoxazine (PBZZ). After curing, a transparent, smooth, stiff and hazel to brown film was obtained.
2.5. Dielectric analysis
Dielectric relaxation data were obtained using a TA instrument (DEA-2970), which was incorporated a parallel plate cell arrangement and a computer-controlled furnace to ensure good electrical contact between the electrodes and the sample. In order to increase the accuracy of measuring dielectric constant, the flat films are required; therefore, we used the automatic scraper to form a flat film. The experiment was conducted under a nitrogen flow 20 ml/min and the thickness of sample was controlled between 0.125 and 0.75 mm. The dielectric constant and dielectric loss were determined with a heating rate of 1 8C/min from 25 to 50 8C with scan frequencies ranging from 1 to 105Hz.
2.6. Differential scanning calorimetry (DSC)
The calorimetric measurement was performed using a TA Instruments Differential Scanning Calorimeter (DSC-2010) conducted under a nitrogen flow of 25 ml/min. The sample was preheated with a scan rate of 20 8C/min from 30 to 260 8C (or 300 8C) and maintained at 260 8C (or 300 8C) for 2 min. The measurement was made using 5 – 10 mg sample in a DSC sample cell by cooling to 30 8C quickly from the melt of the first scan. The second scan rate was 20 8C/min from 30 to 300 8C (or 350 8C) and the Tg was
taken as the midpoint of the heat capacity transition between
Y.-C. Su, F.-C. Chang / Polymer 44 (2003) 7989–7996 7990
the upper and lower points of deviation from the extrapolated liquids and glass lines.
2.7. Thermogravimetric analysis (TGA)
Thermal stability of the cured sample was investigated by a Du Pont 2050 TGA. The cured sample of 5 – 10 mg was placed in a Pt cell and heated at a heating rate of 10 8C/min from 30 to 800 8C at a nitrogen flow of 90 ml/min.
2.8. Water absorption
The cured sample was dried under vacuum at 80 8C for 12 h before placing in the environments of air or water for one day and one week. Then, the percentages of water absorption of cured sample were calculated.
3. Results and discussion
3.1. Nuclear magnetic resonance analysis
After the cyclization of oxazine, the characteristic peaks Ar – CH2– N and N – CH2– O can be observed in proton
NMR[5,6].Fig. 1shows the1H NMR (CDCl3, 500 MHz)
spectrum of F-1 benzoxazine: d 4.65 ppm (4H, Ar – CH2–
N),d5.40 ppm (4H, N – CH2– O), d6.78 – 7.52 ppm (14H,
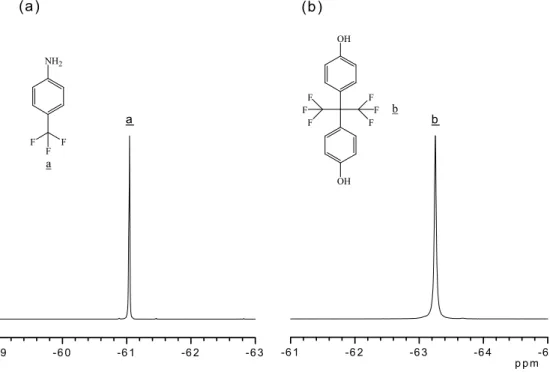
Ar).Fig. 2(a) and (b)show the19F NMR (CDCl3, 500 MHz)
spectra of monomers of 4-(trifluoromethyl)aniline and hexafluorobisphenol A: d 2 61.05 ppm (3F, NH2– Ar –
CF3) [8], d 2 63.25 ppm (6F, C – CF3). The 19F NMR
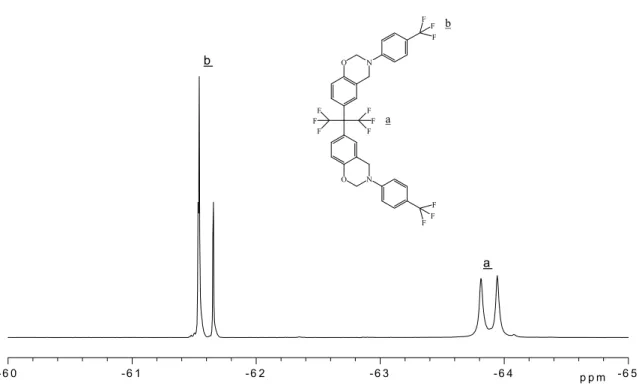
(CDCl3, 500 MHz) spectrum of F-1 benzoxazine is shown
in Fig. 3: d 2 61.54 to 2 61.66 ppm (6F, N – Ar – CF3),d
2 63.81 to 63.94 ppm (6F, C – CF3). The slight chemical
shift (within 1 ppm) observed in 19F NMR spectrum
Scheme 1. The synthesis of F-1 benzoxazine.
because the structure of the F-1 benzoxazine ring was formed.
3.2. Fourier transfer infrared spectroscopy analysis The FT-IR spectrum of F-1 benzoxazine is shown in Fig. 4. The bands at 755 and 692 cm21 (referred to
monosubstituted benzene) do not appear in comparing with the B-a type benzoxazine [9,10]. However, the same characteristic absorption bands of oxazine ring at 937 and 1521 cm21 from the trisubstituted benzene ring, and at 1242 cm21 from the CH2 wagging appear. Besides, the
absorptions at 1038 and 1228 cm21assigned to symmetric and asymmetric C – O – C bonds of the benzoxazine are both
Fig. 1. The1H NMR spectrum of F-1 benzoxazine.
Fig. 2. The19F NMR spectra of (a) 4-(trifluoromethyl)aniline, (b) hexafluorobisphenol A.
Y.-C. Su, F.-C. Chang / Polymer 44 (2003) 7989–7996 7992
present. Based on the NMR and FT-IR results, we confirmed that the F-1 benzoxazine was formed in the study.
3.3. Dielectric constant analysis
The dielectric constant K is directly related to the polarizability of a material, therefore, it is strongly dependent on its chemical structure[11]. Saturated hydro-carbons are significantly lower polarizable than species that are unsaturated, conjugated, or have polarizable phenyl
groups. This effect is demonstrated by comparing the dielectric constants of an aromatic PI with that of the semi-aliphatic PI[11]. In general, the K value can be lowered by breaking off the conjugated system or decreasing the number of phenyl group in the monomer. However, these alterations have to compromise with lower thermal stability. To fluorinate the polymer is one method to solve above-mentioned problems[12]. Fluorine substitution lowers the K value by decreasing the polarizability and the moisture absorption and by increasing the free volume. Substitution
Fig. 3. The19F NMR spectrum of F-1 benzoxazine.
of hydrogen with F or – CF3group decreases the electronic
polarizability due to strong electron-withdrawing inductive effect. The bulky – CF3 group is able to reduce efficient
molecular packing and increase the free volume. The hydrophobicity introduced by F substitution is important since the moisture, even in small concentrations, strongly affects the dielectric constant due to the large K value of water, 78.5 at 25 8C[2].
In this study, we incorporated fluorine atoms into the backbone of PBZZ.Table 1presents the dielectric constant, and dissipation factor, tand; of co-PBZZ with different B-a/F-1 ratios measured at 105Hz and 298 K. Prior to each measurement, the sample was thoroughly dried under vacuum to reduce the influence of the absorbed moisture on the dielectric constant. Comparing these co-PBZZ mixtures synthesized with different B-a/F-1 weight ratios, the incorporation of fluorinated substitution (F-1 benzox-azine) results in decreasing in the dielectric constant with minimum value of 2.36 (B-a/F-1 ¼ 1/1). In addition, the dissipation factor is also a very important property of ILD (interlayer dielectrics) materials [13 – 15] that less than 0.005 at 1 MHz is normally required. In this study, those compositions all meet the requirement of dissipation factor. Hence, the fluorination of benzoxazine reduces its dielectric constant with tolerable dissipation factor.
3.4. Thermal properties
Thermal properties of organic polymers with low K dielectrics are another primary concern in process inte-gration. The C – H, C – C, and C – N bonds of aliphatic polymers generally become unstable at temperatures above 400 8C[1 – 4]even in the nitrogen or vacuum environment. The resulting volatiles may cause delaminating or blistering in the ILD. Only organic materials composed of nonalipha-tic C – H, C – C, C – N, and C – S bonds, such as aromanonalipha-tic, crosslinking or ladder structure, is able to resist such elevated temperatures. Unfortunately, material possessing good thermal stability generally tends to have high dielectric constant. The incorporation of fluorine atoms into the benzoxazine structure is able to achieve materials with high thermal stability and low dielectric constant. Fluorination can improve thermal properties partly because the C – F bond is stronger than the C – H bond [1]. More
important, it can reduce the dielectric constant due to its lower polarizability structure.
3.4.1. Glass transition temperature analysis
The glass transition temperature is an important property of a dielectric film. Exceeding Tgthe polymer may cause a
large decrease in Young’s modulus and typically results in a shift in the dielectric properties. Hence, a polymer with Tg
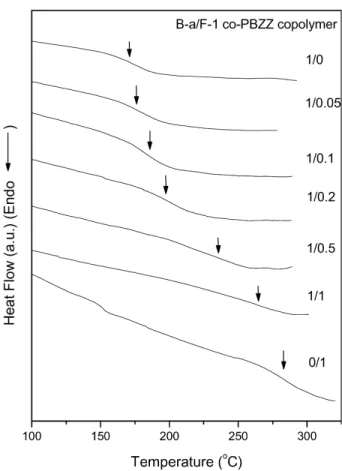
greater or equal to the highest processing temperature is desirable. All B-a/F-1 co-PBZZ were subjected to DSC measurements for the purpose of examining microscopic miscibility.Fig. 5shows the DSC thermograms of all B-a/F-1 co-PBZZ exhibiting only one Tgfrom all composition. A
single Tg strongly implies that all these B-a/F-1 co-PBZZs
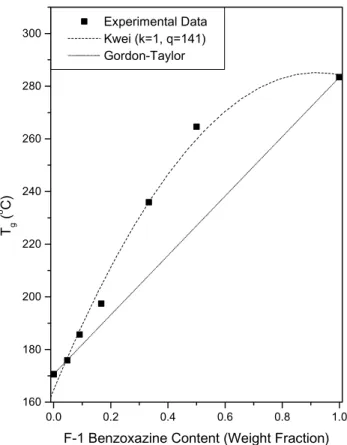
are homogenous.Fig. 6shows the dependence of the Tgon
the composition of these copolymers, increasing the F-1 benzoxzine content results in substantial Tg increase than average values. The hydrogen bonding interactions of O – H· · ·F – C [16] and O – H· · ·O – H are expected in these copolymers. Kwei equation [17] describes the effect of hydrogen bonding interaction on Tgbetween polymers or a
copolymer as shown in Eq. (3): Tg¼
W1Tg1þ kW2Tg2
ðW1þ kW2Þ
þ qW1W2 ð3Þ
where W1and W2are weight fractions of the components,
Tg1 and Tg2 represent the component glass transition
Fig. 5. The DSC scans of B-a/F-1 co-PBZZ copolymers with different composition.
Table 1
The dielectric constant and dissipation factor of B-a/F-1 co-PBZZ at 298 K and 105Hz
B-a/F-1 (weight ratio) Dielectric constant Dissipation factor (tand)
1/0 3.56 ^ 0.01 0.00206 1/0.05 3.02 ^ 0.03 0.00235 1/0.1 2.85 ^ 0.02 0.00282 1/0.2 2.76 ^ 0.02 0.00287 1/0.5 2.39 ^ 0.01 0.00387 1/1 2.36 ^ 0.01 0.00440
Y.-C. Su, F.-C. Chang / Polymer 44 (2003) 7989–7996 7994
temperatures. Both k and q are fitting constants. In general, the parameter q may be considered as a measurement of the specific interaction in a polymer blend system. When the intermolecular interaction is stronger than intramolecular interaction in a binary blend or copolymer, the value of q will be positive; otherwise, q will be negative. When the q value is larger, it represents that the interaction is stronger than the self-interaction of the blend. In case of no interaction existed between components, the q value will be equal to zero and its Tgbehavior can be described by the
Gordon – Taylor equation[18]as shown in Eq. (4).
Tg¼
W1Tg1þ kW2Tg2
ðW1þ kW2Þ
ð4Þ
As shown in Fig. 5, the B-a/F-1 co-PBZZs are completely miscible in the amorphous phase and results in positive Tg deviation. After the ‘best fitting’ by the Kwei
equation, k ¼ 1 and q ¼ 141 were obtained. In this study, a large positive q value of 141 indicates that a strong intermolecular interaction exists between B-a/F-1 co-PBZZ. This result can also explain why Tgs of these
copolymers are positively deviated as shown inFig. 6. The glass transition temperature increases about 113 8C, from 170.6 8C of the B-a benzoxazine to 283.4 8C (F-1 benzox-azine). The resulted higher Tgmakes the high temperature
applications possible.
3.4.2. Thermogravimetric analysis
For organic films, thermal stability is one of the most important requirements for new IMD materials. Unlike aluminum metallization, copper metallization can be achieved by electroplating or electroless plating (chemical reduction) besides PVD and CVD techniques [19 – 21]. These processes can be conducted at temperatures below 250 8C. Unfortunately, an annealing step is necessary to ensure void free copper deposits. Since this step is performed at temperatures in the range 400 – 450 8C for up to 1 h, any low K material must be able to withstand this temperature for several hours.
Table 2shows the weight loss of different compositions of co-PBZZ. The incorporation of fluorinated structure into the backbone of B-a benzoxazine results in noticeable change in thermal stability. The minimum weight loss occurs in the pure F-1 PBZZ structure that can resist high temperature (above 400 8C).
3.5. Water absorption
Since the water possesses large dielectric constant value (78.5 at 25 8C) [2], the water absorption is required to be below 1%. Therefore, the hydrophobic character is required in the low dielectric constant materials. The PBZZ possesses low water absorption ability[5]due to its highly crosslink density and hydrophobic property, and their percentages of water absorption are listed in Table 3. The water absorptions are all below 1 wt%, even under the water environment. The results indicate that the fluorinated co-PBZZs possess an outstanding property of resisting moisture uptake.
4. Conclusions
In this study, we have synthesized a series of co-PBZZ with different B-a/F-1 ratios. A single glass transition temperature ðTgÞ over entire compositions indicates that the
amorphous phase of the co-PBZZ is totally miscible and homogeneous. In addition, a large positive deviation based on Kwei equation in the Tg versus composition diagram,
implies that strong hydrogen bonding interactions exist
Fig. 6. Tg versus composition curved base on B-a/F-1 co-PBZZ
copolymers.
Table 2
The weight loss of B-a/F-1 co-PBZZ under N2environment
B-a/F-1 (weight ratio)
5%
(weight loss) temp. (8C)
10%
(weight loss) temp. (8C) 1/0 329.0 363.6 1/0.05 329.9 366.1 1/0.1 346.1 379.7 1/0.2 354.7 400.2 1/0.5 360.5 420.6 1/1 368.6 430.6 0/1 374.4 455.7
within B-a/F-1 co-PBZZ. Furthermore, the fluorination on PBZZ is able to reduce dielectric constant and increase thermal properties. The co-PBZZ with B-a/F-1 ¼ 1/1 gives low dielectric constant at 2.36 and tandat 0.0044 which is suitable for insulating applications. Moreover, its thermal properties are also substantially improved over the B-a type PBZZ.
References
[1] Maier G. Prog Polym Sci 2001;26:3.
[2] Nalwa HS, editor. Handbook of advanced electronic and photonic materials and devies, vol. 4. San Diego: Academic Press; 2001. [3] Treichel H, Ruhl G, Ansmann P, Wurl R, Muller CH, Dietlmeier M.
Microelectronic Engng 1998;40:1. [4] Peters L. Semicond Int 1998;21:64.
[5] Ishida H, Low HY. Macromolecules 1997;30:1099.
[6] Ning X, Ishida H. J Polym Sci, Part B: Polym Phys 1994;32:921. [7] Goodwin AA, Atkinson JR, Hay JN, Mercer FW. Polymer 1999;40:
1515.
[8] Bardin VV. J Fluorine Chem 1998;89:195.
[9] Ishida H, Sanders DP. Macromolecules 2000;33:8149. [10] Agag T, Takeichi T. Macromolecules 2001;34:7257.
[11] Hougham G, Tesoro G, Viehbeck A, Chapple-Sokol JD. Macromol-ecules 1994;27:5964.
[12] Simposon JO, Clair AKS. Thin Solid Films 1997;308– 309:480. [13] Durstock MF, Rubner MF. Langmuir 2001;17:7865.
[14] Kannurpatti AR, Bowman C. Macromolecules 1998;31:3311. [15] Carter KR, Dipietro RA, Sanchez MI, Swanson SA. Chem Mater
2001;13:213.
[16] Thalladi VR, Weiss HC, Blaser D, Boese R, Nangia A, Desiraju GR. J Am Chem Soc 1998;120:8702.
[17] Kwei TK. J Polym Sci Polym Lett Ed 1984;22:307. [18] Gondon M, Taylor JS. J Appl Chem 1952;2:493. [19] Singer P. Semicond Int 1999;22:38.
[20] Peters L. Semicond Int 2000;23:108. [21] Peters L. Semicond Int 2002;25:57. Table 3
The percentages of water absorption of B-a/F-1 co-PBZZ at room temperature Environment B-a/F-1 co-PBZZ water absorption (%)
1/0 1/0.05 1/0.1 1/0.2 1/0.5 1/1 0/1
In air 1 day , 0.01 , 0.01 , 0.01 , 0.01 , 0.01 , 0.01 , 0.01
In air 1 week , 0.01 , 0.01 , 0.01 , 0.01 , 0.01 , 0.01 , 0.01
In water 1 day 0.24 , 0.01 , 0.01 , 0.01 , 0.01 , 0.01 , 0.01
In water 1 week 0.70 0.69 0.61 0.64 0.60 0.71 0.52
Y.-C. Su, F.-C. Chang / Polymer 44 (2003) 7989–7996 7996