Distribution and susceptibility to amphotericin B
and fluconazole of Candida spp. isolated from Taiwan
Y. L. Y A N G1, Y. A. H O2, H. H. C H E N G2, H. J. L O2*
A N D TSARY Hospitals#
1Department of Biological Science and Technology, National Chiao Tung University, Hsinchu, Taiwan, Republic of China
2Division of Clinical Research, National Health Research Institutes, Taipei, Taiwan, Republic of China
(Accepted 4 October 2004)
S U M M A R Y
Susceptibilities to amphotericin B and fluconazole of 628 clinical yeast strains collected from 22 hospitals in Taiwan were determined. A total of 53 isolates (8.4 %) were resistant to fluconazole. Each hospital had different resistance rate to fluconazole ranging from 0 % to 24 %. None of the 186 isolates from eight of the 22 hospitals was resistant to fluconazole. In contrast, isolates from nine of the remaining 14 hospitals had greater than 10 % resistance rate to fluconazole. Consistently, 88.9 % (8/9) fluconazole-resistant C. albicans isolates were from hospitals having a high resistance rate to fluconazole. The prevalence of various Candida spp. in each hospital was different. A positive association was found between the prevalence of C. tropicalis and the resistance rate to fluconazole for individual hospitals. Although only three isolates (0.5 %) were resistant to amphotericin B, a co-resistance to both amphotericin B and fluconazole was observed, which highlights the emerging problem of drug resistance.
I N T R O D U C T I O N
In the past two decades, nosocomial infections caused by fungi have been increasing significantly. On the healthy host, opportunistic pathogens including Candida spp. are commensal fungi commonly col-onizing human mucosal surfaces. Fungal pathogens cause from relatively trivial conditions such as thrush in babies and vaginal infections in women to fatal, systemic infections in immunocompromised patients [1]. The dramatic increase in the prevalence of yeast infections is probably due to the AIDS epidemic,
cancer chemotherapy, organ and bone-marrow trans-plantation, and invasive hospital procedures [2, 3]. Coinciding with the increased usage of antifungal agents, especially fluconazole, the incidence of drug resistance has increased [3]. Oropharyngeal candi-diasis due to drug-resistant fungi is a major problem for patients infected with HIV [4].
Candidaspp. have various degrees of susceptibility to common antifungal agents. C. lusitaniae is less susceptible to amphotericin B [5] and C. krusei, C. glabrata, and C. tropicalis are less susceptible to fluconazole than other Candida spp. [6–10]. The present study was carried out to determine the distri-bution and susceptibility profiles to amphotericin B and fluconazole of the 628 Candida spp. isolates collected from the 22 hospitals in the Taiwan Surveillance of Antimicrobial Resistance of Yeasts (TSARY) in 1999 [11].
* Author for correspondence : Dr Hsiu-Jung Lo, Division of Clinical Research, National Health Research Institutes, 128, Yen-Chiu-Yuan Road, Section 2, Taipei, 11529, Taiwan, ROC. (Email : hjlo@nhri.org.tw)
# The hospitals participating in the Taiwan Surveillance of Antimicrobial Resistance of Yeasts (TSARY) are listed in the Appendix.
M E T H O D S
Organisms and medium
Yeast isolates were collected from 22 hospitals, con-sisting of six medical centres and 16 regional hospi-tals, in Taiwan. Each hospital was asked to submit up to 50 clinically significant yeast isolates including 10 C. albicansand 40 non-albicans Candida spp. during the collection period, from 15 April to 15 June in 1999 [11]. One isolate was accepted from each episode of infection. A total of 660 isolates were collected orig-inally. However, there were 24 non-Candida spp. and eight Candida spp. failed to grow on RPMI medium 1640 (31800-022 ; Gibco, Paisley, Scotland, UK). The remaining 628 isolates were analysed for their susceptibility to amphotericin B and fluconazole.
After being collected, isolates were stored frozen at x70 xC in bead-containing Microbank cryovials (Pro-Lab Diagnostics, Austin, TX, USA) in every hospital. At the end of the collection period, isolates were kept frozen and transported by an express delivery company to the laboratory at the National Health Research Institutes (NHRI) within 24 h. The isolates were first subcultured on Sabouraud dex-trose agar (SDA, BBL Becton Dickinson & Co., Cockeysville, MD, USA) to check for purity and identification. Pure isolates were labelled and stored in vials containing glycerol at x70 xC for further analysis. We have described the procedure for identi-fication of Candida spp. previously [11]. In the NHRI laboratory, all isolates were first subjected to the germ tube test. Subsequently, Vitek yeast biochemical card (YBC, bioMe´rieux, St. Louis, MO, USA) and API-32C (bioMe´rieux) were used to identify isolates failing to form germ tubes in the test.
Antifungal susceptibility testing
The minimum inhibitory concentration (MIC) to amphotericin B and fluconazole of each yeast was determined by in vitro antifungal susceptibility testing according to the guidelines of the National Com-mittee of Clinical Laboratory Standards (NCCLS) as described previously [12]. The RPMI medium was used for dilution and growth of yeast culture. The final growth of each isolate was measured by a Spectra max Plus (Molecular Devices Corp., Sunny-vale, CA, US) after incubation at 35 xC for 48 h.
The interpretation of MICs was according to the guidelines of the NCCLS [12]. According to these guidelines, the MIC to amphotericin B was defined as
the concentration that reduces the growth of cells down to 90 % and the MIC to fluconazole was defined as the concentration that reduces the growth of cells down to 50 %. Isolates with MIC o2 mg/ml were considered to be resistant to amphotericin B, whereas isolates with MICso64, 16–32 and f8 mg/ml were defined as resistant, susceptible-dose dependent, and susceptible to fluconazole respectively. The control strains for the study were C. albicans (ATCC 90028), C. krusei (ATCC 6258), and C. parapsilosis (ATCC 22019).
Data analysis
The information about each isolate in the database included location and type of hospital, genus and species as identified by each hospital and the NHRI laboratory, and source of the isolate (from urine, sputum, blood, wound, and others). The significance of differences in frequencies and proportions was determined by thex2
test with Yates’ correction.
R E S U L T S
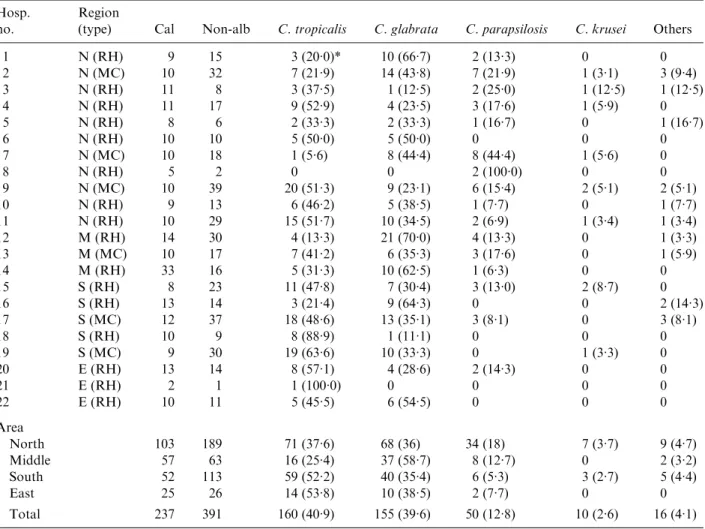
There were 11, 5, 3 and 3 hospitals participating in TSARY in 1999 located in the North, South, Middle, and East regions of Taiwan respectively. The distri-bution of tested Candida spp. is listed in Table 1. A total of 292, 165, 120, and 51 isolates were from hospitals in the North, South, Middle, and East regions respectively. Among them, 68.5, 64.7, 52.5, and 51 % isolates from hospitals in the South, North, Middle, and East regions were non-albicans Candida spp. C. tropicalis was the most frequently isolated species among non-albicans Candida spp. in the North, South, and East regions, whereas C. glabrata was the one most frequently isolated in the Middle region. Interestingly, only 25.4 % of isolates from the Middle region were C. tropicalis, which was significantly lower than other regions and also below the national average of 40.9 %.
Although all hospitals were asked to submit 10 C. albicansisolates, the final number of isolates from each hospital varied. Three hospitals (nos. 5, 8 and 21) submitted less than 10 C. albicans isolates which may be due to a low prevalence of fungal infections in those hospitals. Some isolates from certain hospitals (nos. 1, 10, 13, 15 and 19) failed to grow on the RPMI medium. Nine hospitals had more than 10 C. albicans isolates for testing since their submission of non-albicans Candida isolates were C. non-albicans. The prevalence of non-albicans Candida spp. in different
hospitals varied. The range was from 0 % to 70 % for C. glabrata, from 0 % to 100 % for C. parapsilosis, and from 0 % to 100 % for C. tropicalis.
Among the 628 isolates tested for susceptibility, 530 (84.4 %), 45 (7.2 %), and 53 (8.4 %) isolates were susceptible, susceptible-dose dependent, and resistant to fluconazole respectively (Table 2). A total of 21.6, 12.1, 6.5 and 2.5 % of the isolates from hospitals in the East, South, North, and Middle regions were resistant to fluconazole. The resistance rate to fluco-nazole of each hospital varied, ranging from 0 % to 23.8 %. Worthy of note is that none of the isolates from eight of the hospitals, out of the total of 186 isolates, was resistant to fluconazole. The localities of those hospitals were five in the North region, two in the Middle region, and one in the East region. In contrast, isolates from nine of the remaining 14 hospitals had resistance rates to fluconazole greater than 10 %. Their locations were three in the South
region, three in the North region, two in the East region, and one in the Middle region.
Hospitals without fluconazole-resistant isolates have contributed 94 non-albicans Candida spp. while those with resistant isolates have contributed 297 non-albicans Candida spp. (Table 1). Of the eight hospitals without fluconazole-resistant isolates, C. glabrata (50 %) was the most frequently isolated species followed by C. tropicalis (23.4 %), C. para-psilosis(20.2 %), C. krusei (2.1 %), and others (4.3 %). Of the remaining 14 hospitals with fluconazole-resistant isolates, C. tropicalis (48.5 %) was the most frequently isolated species followed by C. glabrata (36.4 %), C. parapsilosis (10.4 %), C. krusei (2.7 %), and others (4 %). The prevalence of C. parapsilosis and resistance rate to fluconazole had a negative association, whereas the prevalence of C. tropicalis and resistance rate to fluconazole had a positive association (P<0.05).
Table 1. The distribution of tested Candida spp. (n=628) Hosp.
no.
Region
(type) Cal Non-alb C. tropicalis C. glabrata C. parapsilosis C. krusei Others
1 N (RH) 9 15 3 (20.0)* 10 (66.7) 2 (13.3) 0 0 2 N (MC) 10 32 7 (21.9) 14 (43.8) 7 (21.9) 1 (3.1) 3 (9.4) 3 N (RH) 11 8 3 (37.5) 1 (12.5) 2 (25.0) 1 (12.5) 1 (12.5) 4 N (RH) 11 17 9 (52.9) 4 (23.5) 3 (17.6) 1 (5.9) 0 5 N (RH) 8 6 2 (33.3) 2 (33.3) 1 (16.7) 0 1 (16.7) 6 N (RH) 10 10 5 (50.0) 5 (50.0) 0 0 0 7 N (MC) 10 18 1 (5.6) 8 (44.4) 8 (44.4) 1 (5.6) 0 8 N (RH) 5 2 0 0 2 (100.0) 0 0 9 N (MC) 10 39 20 (51.3) 9 (23.1) 6 (15.4) 2 (5.1) 2 (5.1) 10 N (RH) 9 13 6 (46.2) 5 (38.5) 1 (7.7) 0 1 (7.7) 11 N (RH) 10 29 15 (51.7) 10 (34.5) 2 (6.9) 1 (3.4) 1 (3.4) 12 M (RH) 14 30 4 (13.3) 21 (70.0) 4 (13.3) 0 1 (3.3) 13 M (MC) 10 17 7 (41.2) 6 (35.3) 3 (17.6) 0 1 (5.9) 14 M (RH) 33 16 5 (31.3) 10 (62.5) 1 (6.3) 0 0 15 S (RH) 8 23 11 (47.8) 7 (30.4) 3 (13.0) 2 (8.7) 0 16 S (RH) 13 14 3 (21.4) 9 (64.3) 0 0 2 (14.3) 17 S (MC) 12 37 18 (48.6) 13 (35.1) 3 (8.1) 0 3 (8.1) 18 S (RH) 10 9 8 (88.9) 1 (11.1) 0 0 0 19 S (MC) 9 30 19 (63.6) 10 (33.3) 0 1 (3.3) 0 20 E (RH) 13 14 8 (57.1) 4 (28.6) 2 (14.3) 0 0 21 E (RH) 2 1 1 (100.0) 0 0 0 0 22 E (RH) 10 11 5 (45.5) 6 (54.5) 0 0 0 Area North 103 189 71 (37.6) 68 (36) 34 (18) 7 (3.7) 9 (4.7) Middle 57 63 16 (25.4) 37 (58.7) 8 (12.7) 0 2 (3.2) South 52 113 59 (52.2) 40 (35.4) 6 (5.3) 3 (2.7) 5 (4.4) East 25 26 14 (53.8) 10 (38.5) 2 (7.7) 0 0 Total 237 391 160 (40.9) 155 (39.6) 50 (12.8) 10 (2.6) 16 (4.1)
N, North ; M, middle ; S, south ; E, east ; RH, regional hospital ; MC, medical centre ; Cal, C. albicans ; Non-alb, non-albicans. * Number of isolates (percentage).
Of the 53 fluconazole-resistant isolates, 25, 15, 5, 2 and 6 isolates were from urine, sputum, blood, wound, and others respectively (Table 3). A total of 24 C. tropicalis, 13 C. glabrata, 9 C. albicans, and 7 C. kruseiisolates were resistant to fluconazole. Of the nine fluconazole-resistant C. albicans isolates, only one was from a hospital (no. 18) having a resistance rate to fluconazole of less than 10 % (Table 2) while three out of the seven fluconazole-resistant C. krusei isolates were from hospitals with resistance rates greater than 10 %.
Of the 628 isolates, 88 (14.0 %), 372 (59.2 %), 165 (26.3 %), and 3 (0.5 %) isolates had a MIC for amphotericin B (MICs f0.25, 0.5, 1 and 2 mg/ml respectively) (Table 4). The resistant isolates were one each of C. famata, C. krusei, and C. tropicalis. There were 43.3 % (23/53) of fluconazole-resistant isolates whose MICs to amphotericin B wereo1 mg/ml, only
24.9 % (132/530) of the susceptible isolates had MICs for amphotericin Bo1 mg/ml (P<0.05).
D I S C U S S I O N
C. krusei, C. glabrata and C. tropicalis were less sus-ceptible to fluconazole than other Candida spp. [6, 7, 9]. Furthermore, these non-albicans Candida spp. are often associated with diseases rather than colon-ization as commensal-like C. albicans [13]. On the other hand, though we requested clinically significant isolates, fluconazole-resistant isolates from non-sterile sites such as sputum and urine could be argued as being commensal. Even so, it is still noteworthy that approximately 10 % of the common Candida spp. were resistant to fluconazole [9]. Those colonization strains would cause diseases when opportunity arises [14–16]. Since we requested 10 C. albicans isolates during the collection period, the prevalence of C. albicansin the present study may be underestimated.
Isolates from the Middle (2.5 %) and North (6.5 %) regions have a lower ratio of resistance to fluconazole than isolates from the South (11.8 %) and East (21.6 %) regions [9, 10]. There are significant differences in the prevalence of C. parapsilosis and C. tropicalis between hospitals without and with fluconazole-resistant isolates. Hospitals having more C. tropicalisand less C. parapsilosis had a tendency to have more fluconazole-resistant isolates. Although C. glabratahas been recognized as less susceptible to fluconazole than other Candida spp. [17], there was no relationship between the prevalence of C. glabrata and the resistance rate to fluconazole. The resistance rate to fluconazole of C. glabrata isolates is lower in the present study than reported elsewhere (8 % vs. from 18 % to 50 %) [18]. Due to the difference in antifungal practices and infection control strategies, there are some variations of distribution of species and susceptibility to fluconazole among different institutions, localities, and countries [19, 20]. In order to understand the spectrum of Candida spp. involved and the emergence of antifungal resistance, it is necessary to further the study to investigate factors involved in the resistance rate to fluconazole in individual hospitals.
According to the in vitro antifungal susceptibility testing, 0.5 % and 8.4 % of isolates were considered to be resistant to amphotericin B and fluconazole respectively. The difference in resistance rates between fluconazole and amphotericin B may be the result of different mechanisms for antifungal activity Table 2. The distribution of susceptibility to
fluconazole Hosp. no. Region (type) Susceptible Susceptible-dose dependent Resistant 1 N (RH) 19 (79.2)* 2 (8.3) 3 (12.5) 2 N (MC) 37 (88.1) 2 (4.8) 3 (7.1) 3 N (RH) 18 (94.7) 1 (5.3) 0 4 N (RH) 26 (92.9) 0 2 (7.1) 5 N (RH) 14 (100) 0 0 6 N (RH) 15 (75.0) 3 (15.0) 2 (10.0) 7 N (MC) 25 (89.3) 3 (10.7) 0 8 N (RH) 7 (100) 0 0 9 N (MC) 41 (83.7) 3 (6.1) 5 (10.2) 10 N (RH) 22 (100) 0 0 11 N (RH) 31 (79.5) 4 (10.3) 4 (10.3) 12 M (RH) 42 (95.5) 2 (4.5) 0 13 M (MC) 22 (81.5) 2 (7.4) 3 (11.1) 14 M (RH) 49 (100) 0 0 15 S (RH) 27 (87.1) 2 (6.5) 2 (6.5) 16 S (RH) 21(77.8) 3 (11.1) 3 (11.1) 17 S (MC) 33 (67.3) 6 (12.2) 10 (20.4) 18 S (RH) 16 (84.2) 2 (10.5) 1 (5.3) 19 S (MC) 30 (76.9) 5 (12.8) 4 (10.3) 20 E (RH) 20 (74.1) 1 (3.7) 6 (22.2) 21 E (RH) 3 (100) 0 0 22 E (RH) 12 (57.1) 4 (19.0) 5 (23.8) Area North 255 (87.3) 18 (6.2) 19 (6.5) Middle 113 (94.2) 4 (3.3) 3 (2.5) South 127 (77) 18 (19.9) 20 (12.1) East 35 (68.6) 5 (9.8) 11 (21.6) Total 530 (84.4) 45 (7.2) 53 (8.4)
N, North ; M, middle ; S, south ; E, east ; RH, regional hospital ; MC, medical centre.
(fungistatic vs. fungicidal) [4, 21], frequency of usage, and/or different molecular mechanisms of drug resistance [2, 22]. Although there were only three isolates with a MIC to amphotericin B at 2mg/ml, there were 165 (26.3 %) isolates with MICs to amphotericin B at 1mg/ml. Since there is a trend of co-resistance between amphotericin B and flucon-azole, it is advisable that public health authorities as well as clinicians should be alerted to the possibility of emerging amphotericin B resistance, especially for multidrug-resistant isolates.
A C K N O W L E D G E M E N T S
We thank Drs Y.-C. Chen, M. Ho, L. L. L. Yeh, and Ms. T. L. Lauderdale for their helpful suggestions. We also thank Bristol–Myers Squibb and Pfizer for supplying the amphotericin B and fluconazole respect-ively.
A P P E N D I X . Taiwan Surveillance of Antimicrobial Resistance of Yeasts (TSARY) hospitals
We express our appreciation to all 22 TSARY partici-pating hospitals for providing the isolates and in-formation related to these isolates. They were : Chang Gung Memorial Hospital at Linkou, Chang Gung Memorial Hospital at Keelung, Hsin-Chu Hospital, Lo-Tung Poh Ai Hospital, St. Mary Hospital, Taipei Municipal Yang-Ming Hospital, Taipei Municipal Zen Ai Hospital, Tao-Yuan General Hospital, Taiwan Adventist Hospital, Koo Foundation Sun Yat-sen Cancer Center, Tri Service General Hospital, Kuan-Tien General Hospital, Veterans General Hospital-Taichung, Zen Ai General Hospital, Chi Mei Hospital, Kaohsiung Medical College Chung-Ho Memorial Hospital, Kaohsiung Military Hospital, Tai-nan Municipal Hospital, Veterans General Hospital-Kaohsiung, Buddhist Tzu-Chi General Hospital, Table 3. Sources of fluconazole-resistant Candida spp. (n=53)
Hosp. no. Resistance rate Region (type)
Urine Sputum Blood Wound Others
Total Cal Cgl Ckr Ctr Cal Cgl Ckr Ctr Ckr Ctr Cal Cal Cgl Ckr Ctr
1 12.5 N (RH) 1 1 1 3 2 7.1 N (MC) 1 1 1 3 4 7.1 N (RH) 1 1 2 6 10 N (RH) 1 1 2 9 10.2 N (MC) 2 2 1 5 11 10.3 N (RH) 2 1 1 4 13 11.1 M (MC) 2 1 3 15 6.5 S (RH) 2 2 16 11.1 S (RH) 1 1 1 3 17 20.4 S (MC) 2 2 1 2 2 1 10 18 5.3 S (RH) 1 1 19 10.3 S (MC) 1 1 1 1 4 20 22.2 E (RH) 4 1 1 6 22 23.8 E (RH) 2 2 1 5 Total 8.4 1 8 3 13 5 3 1 6 1 4 2 1 2 2 1 53
Cal, C. albicans ; Ctr, C. tropicalis ; Cgl, C. glabrata ; Cpa, C. parapsilosis ; Ckr, C. krusei. N, North ; M, middle ; S, south ; E, east ; RH, regional hospital ; MC, medical centre. Table 4. Susceptibility to both amphotericin B and fluconazole
Fluconazole Amphotericin B (mg/ml) Total 0.06 0.125 0.25 0.5 1 2 Susceptible 2 (0.4)* 4 (0.8) 76 (14.3) 316 (59.6) 131 (24.7) 1 (0.2) 530 Susceptible-dose dependent 0 0 4 (8.9) 29 (64.4) 12 (26.7) 0 45 Resistant 0 0 2 (3.8) 28 (52.8) 21 (39.6) 2 (3.8) 53 Total 2 (0.3) 4 (0.6) 82 (13.1) 373 (59.4) 164 (26.1) 3 (0.5) 628
Hua-Lien Hospital, and Mackay Memorial Hospital Taitung Branch.
R E F E R E N C E S
1. Odds FC, Gow NA, Brown AJ. Fungal virulence studies come of age. Genome Biol 2001 ; 2 : 1009.1–1009.4.
2. White TC, Marr KA, Bowden RA. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin Microbiol Rev 1998 ; 11 : 382–402. 3. Yang YL, Lo H-J. Mechanisms of antifungal agent resistance. J Microbiol Immunol Infect 2001 ; 34 : 79–86.
4. Vanden Bossche H, Marichal P, Odds FC. Molecular mechanisms of drug resistance in fungi. Trends Micro-biol 1994 ; 2 : 393–400.
5. Hadfield TL, Smith MB, Winn RE, Rinaldi MG, Guerra C. Mycoses caused by Candida lusitaniae. Rev Infect Dis 1987 ; 9 : 1006–1012.
6. Orozco AS, Higginbotham LM, Hitchcock CA, et al. Mechanism of fluconazole resistance in Candida krusei. Antimicrob Agents Chemother 1998 ; 42 : 2645–2649. 7. Piemonte P, Conte G, Flores C, et al. Emergency of
fluconazole-resistant infections by Candida krusei and Candida glabratain neutropenic patients. Rev Med Chil 1996 September ; 124 : 1149.
8. Akova M, Akalin HE, Uzun O, Gur D. Emergence of Candida krusei infections after therapy of oropharyn-geal candidiasis with fluconazole. Eur J Clin Microbiol Infect Dis 1991 ; 10 : 598–599.
9. Yang YL, Ho YA, Cheng HH, Lo H-J. Susceptibilities of Candida species to amphotericin B and fluconazole : the emergence of fluconazole resistance in Candida tropicalis. Infect Con Hosp Epi 2004 ; 25 : 60–64. 10. Yang YL, Ho YA, Cheng HH, Hsaio CF, Lo H-J.
Fluconazole resistance rate of Candida species from different region and type of hospitals in Taiwan. J Microbiol Immunol Infect 2003 ; 36 : 187–191.
11. Lo HJ, Ho AH, Ho M. Factors accounting for mis-identification of Candida species. J Microbiol Immunol Infect 2001 ; 34 : 171–177.
12. National Committee of Clinical Laboratory Standards. Reference method for broth dilution antifungal
susceptibility testing of yeasts ; M27 approved standard. Wayne, Pennsylvania NCCLS ; 1997.
13. Wingard JR, Merz WG, Saral R. Candida tropicalis : a major pathogen in immunocompromised patients. Ann Intern Med 1979 ; 91 : 539–543.
14. Pittet D, Monod M, Filthuth I, Frenk E, Suter PM, Auckenthaler R. Contour-clamped homogeneous elec-tric field gel electrophoresis as a powerful epidemiologic tool in yeast infections. Am J Med 1991 ; 91 : 256S–263S.
15. Reagan DR, Pfaller MA, Hollis RJ, Wenzel RP. Characterization of the sequence of colonization and nosocomial candidemia using DNA fingerprinting and a DNA probe. J Clin Microbiol 1990 ; 28 : 2733–2738. 16. Voss A, Hollis RJ, Pfaller MA, Wenzel RP, Doebbeling
BN. Investigation of the sequence of colonization and candidemia in nonneutropenic patients. J Clin Micro-biol 1994 ; 32 : 975–980.
17. Pfaller MA, Messer SA, Hollis RJ, Jones RN, Diekema DJ. In vitro activities of ravuconazole and voriconazole compared with those of four approved systemic anti-fungal agents against 6,970 clinical isolates of Candida spp. Antimicrob Agents Chemother 2002 ; 46 : 1723– 1727.
18. Pfaller MA, Messer SA, Boyken L, Tendolkar S, Hollis RJ, Diekema DJ. Variation in susceptibility of blood-stream isolates of Candida glabrata to fluconazole according to patient age and geographic location. J Clin Microbiol 2003 ; 41 : 2176–2179.
19. Sanglard D, Odds FC. Resistance of Candida species to antifungal agents : molecular mechanisms and clinical consequences. Lancet Infect Dis 2002 ; 2 : 73–85. 20. St Germain G, Laverdiere M, Pelletier R, et al.
Prevalence and antifungal susceptibility of 442 Candida isolates from blood and other normally sterile sites : results of a 2-year (1996 to 1998) multicenter surveil-lance study in Quebec, Canada. J Clin Microbiol 2001 ; 39 : 949–953.
21. Hitchcock CA. Cytochrome P-450-dependent 14 alpha-sterol demethylase of Candida albicans and its inter-action with azole antifungals. Biochem Soc Trans 1991 ; 19 : 782–787.
22. Vanden Bossche H, Warnock DW, Dupont B, et al. Mechanisms and clinical impact of antifungal drug re-sistance. J Med Vet Mycol 1994 ; 32 (Suppl 1) : 189–202.