Contents lists available atScienceDirect
Sensors and Actuators B: Chemical
j o u r n a l h o m e p a g e :w w w . e l s e v i e r . c o m / l o c a t e / s n bFabrication and carbon monoxide sensing characteristics of mesostructured
carbon gas sensors
Chueh-Yang Liu
a,∗, Chia-Fu Chen
b, Jih-Perng Leu
a, Chih-Cheng Lu
c, Kuan-Hsun Liao
c aDepartment of Materials Science and Engineering, National Chiao-Tung University, 1001 University Road, Hsinchu 300, Taiwan, ROCbDepartment of Materials Science and Engineering, MingDao University, ChangHua 52345, Taiwan, ROC cInstitute of Mechatronic Engineering, National Taipei University of Technology, Taipei, Taiwan, ROC
a r t i c l e i n f o
Article history:Received 29 August 2008
Received in revised form 30 June 2009 Accepted 11 August 2009
Available online 19 August 2009 Keywords: Gas sensor DEP Mesoporous CO Ordered Pore
a b s t r a c t
We demonstrated a micro-chemoresistive gas sensing device using mesoporous carbon as the sensitive film and immobilized by dielectrophoresis (DEP) process. The mesoporous materials were characterized by X-ray diffraction (XRD) patterns, N2adsorption–desorption isotherms, scanning electron microscopy
(SEM), and transmission electron microscopy (TEM). N2adsorption–desorption isotherms shows the
specific surface area of 1200 m2/g, total pore volume is 1.2 cm3/g of the mesoporous carbon, and the
pore size distribution of mesoporous carbon yields an average pore size of 3 nm. SEM images show a rod-like morphology and TEM images show a highly ordered pore channel with linear arrays. CO gas sensor properties were measured and the resulting sensitivities were 5.7, 6.8, 8.8, 11.6, and 37.6% for concentrations of 30, 45, 60, 75, and 90 ppm, respectively. Additionally, this sensor also exhibited high sensitivity and fast response times for CO gas.
Crown Copyright © 2009 Published by Elsevier B.V. All rights reserved.
1. Introduction
In recent years, mesoporous carbons have been widely used in applications that require catalysts[1], adsorption[2], and energy storage[3]. This phenomenon is largely due to their high surface areas, high pore volumes, uniform pore sizes, and high mechani-cal stability. In general, porous carbon is fabricated using zeolites, alumina, opal, and silica sols as templates[4–6]. However, these materials have limited applications because of their inadequate porosity and disordered pore systems. Recently, Ryoo et al. reported the fabrication of well-ordered porous carbon using structured porous silica as a template[7]. These materials have been used in new applications in the fields of optics and nanotechnology. Chemoresistive gas sensors have been developed and widely used in industrial applications and environmental control for several decades[8–10]. Various gas-sensitive materials such as ZnO, SnO2,
TiO2 and carbon nanotubes (CNTs) have been shown to detect
hazardous and combustible gases [11–13]. Undoubtedly, along with the widespread applications nanostructured materials such as nanotubes, nanoparticles, nanobelts, and nanowires, we expect that smaller dimensions and greater surface areas could dramati-cally improve sensitivity and response time. Recently, there have been reports of micro-gas sensors fabricated by SOI technology
∗ Corresponding author. Tel.: +886 3 5712121x55381; fax: +886 3 5724727. E-mail address:Jason.liu1107@msa.hinet.net(C.-Y. Liu).
that are fully CMOS-compatible [14–16]. These gas sensors that are based on SOI micro-hotplates, can meet the requirements of high-temperature applications. Studies that used a CVD or a drop coating method have been reported [17]; however, these solu-tions are not fully CMOS-compatible. We have recently developed a method that combines nanostructured materials (such as CNTs) and a dielectrophoresis process with micro-hotplate gas sensors in our laboratory [11]. Additionally, the carbon-based gas sen-sors, which are based on the change of electrical conductance, demonstrate that conductivity of the carbon, which adsorbed oxi-dizers, is modified by the charge transfer between the carbon surface and adsorbed gas molecules. We used mesoporous car-bon in place of CNTs as electrodes for micro-chemoresistive gas sensors because of its higher surface area (∼1200 m2/g), greater
pore volume (∼1.2 cm3/g), and uniform pore size (∼3.0 nm). In our
research, we discovered a new nanostructured material, referred to as mesoporous carbon, with dimensions of several nanometers, and we investigated its attractive properties. We utilize this promising material to evaluate the possibility of gas detection using our novel sensor platform. Carbon-based materials behave as p-type semi-conductors. In the presence of oxygen, the p-type behavior has been proposed to arise from the removal of electrons from the carbon by chemisorbed oxygen, thereby creating hole carriers. Carbon pos-sess remarkable adsorptive capacity due to their increased surface area and hence the interest in these materials for gas and vapor detection. For instance, interactions with CO have been shown to alter the electrical behavior of carbon [18]. Mesoporous carbon 0925-4005/$ – see front matter. Crown Copyright © 2009 Published by Elsevier B.V. All rights reserved.
nanoparticles are aligned and immobilized between electrodes over a thin membrane by dielectrophoresis, which is a convenient technique for manipulating dielectric substances in a liquid. To the best of our knowledge, the present study is the first to demonstrate a novel class of micro-gas sensors using mesoporous carbon as the sensitive film.
2. Experimental 2.1. Synthesis
The silica template was obtained as given in the literature[19]. Then, 1 g of SBA-15 was added to a solution that was created by dissolving 1.25 g of sucrose and 0.14 g of H2SO4in 5 g of H2O. The
mixture was placed in an oven for 6 h at 100◦C, and subsequently, the oven temperature was increased to 160◦C and maintained for another 6 h. The sample turned dark brown or black during treatment in the oven. The silica sample containing partially poly-merized and carbonized sucrose was then treated with a solution made up of 0.8 g of sucrose, 0.09 g of H2SO4, and 5 g of H2O. Then, the
sample was treated again at 100 and 160◦C using the same drying oven as before. The carbonization was completed by pyrolysis with heating typically to 900◦C in a vacuum. The carbon–silica compos-ite obtained after pyrolysis was washed with 5 wt% hydrofluoric acid (HF) at room temperature, to remove the silica template. On the other hand, we removed the HF treatment in Section3. The template-free carbon product thus obtained was filtered, washed with ethanol, and dried at 120◦C. The basic concept of the meso-porous carbon sensor is shown inFig. 1. The sensor features a thin membrane of a micro-hotplate structure, including films of silicon dioxide and silicon nitride, with polysilicon or metal micro-heaters and electrodes. The mesostructured carbon was suspended in ethanol (1g ml−1final concentration) and ultra-sonicated for
60 min. Large particles were removed by centrifuging. The equip-ment was based on the dielectrophoretic system, which has been developed for electrical inspection of micro-organisms. Each elec-trode finger had a 20m length and 5 m minimum clearances. The mesostructured carbon suspension was continuously fed into the microelectrode chamber from a reservoir by a pump at a flow rate of 0.5 ml min−1. The DEP-trapping of mesostructured carbon on the microelectrode was performed with an ac voltage of 1 MHz and 10 V amplitude (peak-to-peak) was chosen to enable the mesoporous carbons to be deposited onto the electrode gap by using dielec-trophoresis forces. The ac voltage was also used to simultaneously measure the electrode impedance by using a lock-in amplifer con-trolled by a PC. After a period of time, the DEP process was stopped
Fig. 1. Schematic design of a mesoporous carbon gas sensor with the micro-hotplate membrane via DEP process.
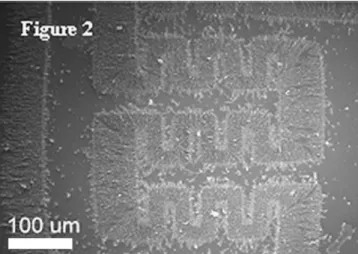
Fig. 2. The scanning electron microscopy (SEM) image of the mesostructured carbon trapped on the microelectrode.
and the ethanol was gently evaporated at room temperature. The scanning electron microscopy (SEM) images of the mesostructured carbon trapped on the microelectrode are shown inFig. 2. 2.2. Characterization of material
X-ray diffraction (XRD) patterns were obtained using a Bede/D1 diffractometer with Cu K␣ radiation (1.543 Å) at a voltage of 40 kV and current of 40 mA. SEM was performed using a JOEL 6700F electron microscope at an acceleration voltage of 3 kV. Nitrogen adsorption–desorption isotherms were measured at−196◦C using
a NOVA 1000e system in static measurement mode. The sam-ples were degassed at 200◦C for 3 h before measurement. Specific surface areas were determined by the Brunauer–Emmett–Teller (BET) method based on the adsorption branches. Pore diameter and pore size distribution (PSD) were measured from the desorption branches obtained by the Barrett–Joyner–Halenda (BJH) method [20]. Transmission electron microscopy (TEM) was used to eluci-date the structure of mesoporous silica SBA-15 using a JEOL-2010 electron microscope at 200 kV. Gas sensing properties were char-acterized using a computer-controlled gas sensing characterization system. The test gas was 30–90 ppm CO in dry air, and it was injected into the chamber at a total flow rate of 100 sccm. After cer-tain duration, the chamber was purged with air and the experiment was repeated. The electrical resistance response during testing was monitored by using a precision analyzer (Keithely 2400). Sen-sor response (S) was defined as follows: S = (Rco− Rair/Rair)×100%,
where Rairand Rcorepresent the resistances of the sensor in air and
in CO gas, respectively. The response time to CO gas was defined as the time required for 90% of total resistance in the gas.
3. Results and discussions
The low-angle XRD pattern of porous carbon is shown inFig. 3, which depicts the structural ordering of mesoporous carbon.Fig. 3 indicates that the sample has a clearly defined diffraction peak located at∼1.0◦, which can be attributed to the (1 0 0) reflections of
the hexagonal groups. Moreover, the patterns are consistent with the 2D hexagonal symmetry derived from SBA-15 structural order. The cell parameter of mesoporous carbon is 10.35 nm. It is note-worthy that the cell parameter of porous carbon is reduced by 9% as compared to the silica template. Various authors have previously reported structural shrinkage that occurred in the porous silica template[21,22]. When spatial dimensions of the silica template were unconfirmed, the reaction would accelerate the pore
shrink-Fig. 3. Low-angle XRD patterns of the mesoporous carbon.
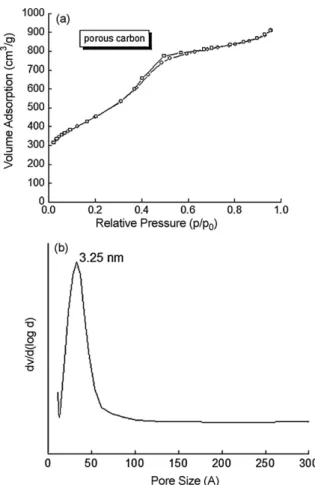
age. The nitrogen adsorption isotherm and the relative pore size distribution of porous carbon are given inFig. 4(a) and (b). The result demonstrates that the porous carbon belongs to type IV accord-ing to the IUPAC classification. The sorption isotherms showed a large increase in the relative pressure (p/p0) ranging from 0.4 to
0.6 for mesoporous carbon. As the relative pressure increases, the material undergoes a sharp step capillary condensation of nitrogen within uniform mesopores, where the p/p0position of the
inflec-tion can be correlated with the diameter of the mesopores. The sharpness of the inflection step reflected the uniform pore size
dis-Fig. 4. (a) Nitrogen sorption isotherms of mesoporous carbon and (b) pore size distribution of the mesoporous carbon.
Fig. 5. The (a) SEM and (b) TEM images of the silica template.
tribution, and p/p0position corresponded to the pore diameters in
the mesopore range. The specific surface area is 1200 m2/g, total
pore volume is 1.2 cm3/g of the mesoporous carbon, and the pore
size distribution of mesoporous carbon calculated by BJH yields an average pore size of 3 nm. This large surface area enhances the accessibility of porosity. To show the morphology of the SBA-15 template, an SEM image of silica template is provided in Fig. 5. A rod-like structure with a 2-m length and 400-m diameter can be seen and some aggregate of SBA-15 can also be observed. The micelle of SBA-15 was reconstructed and a rod-like structure formed. The structural ordering of the mesostructured carbons was influenced by the nature of the SBA-15 templates. The mesostruc-tured carbons bear rod morphology. The SEM and TEM images of the mesostructured carbon were shown inFig. 6(a) and (b). This is an interesting observation, and it suggests that the morphology of the silica templates directs the morphology of the mesostruc-tured carbons toward the formation of rod-shaped particles. The morphology of porous carbon reflects typical porous silica tem-plate morphology, indicating true replication. The TEM image of the mesoporous carbon shows the ordered stripe. Regions of high electronic densities are denoted by various shades of grey, while
Fig. 6. (a) SEM image and (b) TEM image of the mesoporous carbon, as a replica of mesoporous silica template.
the dark regions represent the pore interior, whereas the white regions are pore size. The TEM image of the mesostructured carbon revealed that the carbon materials are composed of 2D ordered nanorod array. The mesostructured carbon is 7 nm in diameter and the centers of adjacent rods are 10 nm. The carbon nanorods are interconnected by spacer, which are constituted by the car-bon that filled the channel-interconnecting micropores within the SBA-15 wall. Therefore, the mesoporous carbon clearly remained a highly ordered channel with linear arrays, originating from the silica framework that would correct into the mesopores of the resultant carbon. The mesostructured carbon particles have exactly the same morphology as that of the SBA-15 silica particles. The above results are consistent with the result of the XRD pattern and N2adsorption–desorption isotherms and the morphology proved
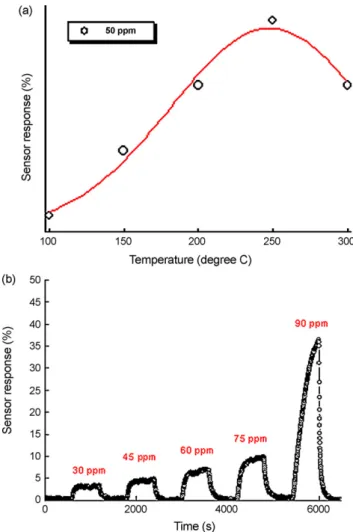
that the mesostructure remained. The working temperature plays an important role in sensitivity of the mesoporous carbon sensor. Fig. 7(a) shows the effect of the working temperature on sensitivity of mesoporous carbon sensor for CO as a function of temperature when exposed to 50 ppm CO gas. At low temperatures, the low sen-sor response should be attributed to the fact that CO molecules do not have enough thermal energy to react with the surface-adsorbed oxygen species. On the other hand, the reduction in sensor response
Fig. 7. (a) Variation of sensor response of mesoporous carbon sensor with tem-perature (b) response of the mesoporous carbon sensor measured with various CO concentrations.
above 250◦C should be attributed to the difficulty in exothermic CO gas adsorption. In general, the reactive oxygen species such as O2−, O2−and O−are first adsorbed on the ZnO surface at elevated
tem-peratures. It should be noted that the chemisorbed oxygen species depend strongly on temperature. Similar temperature dependence has also been observed[23]. It was found that measured responses were 0.7, 1.3, 3.3, 5.2, 7.7 and 5.2% when the sensor was operated at room temperature, 100, 150, 200, 250 and 300◦C, respectively. In other words, sensor response increased first, reached a maximum at 250◦C and then started to decrease as the operational tempera-ture increased to 300◦C. The sensor response increase to maximum at 250◦C would be the threshold temperature for CO gas sensing. The corresponding response to concentration curve was shown in Fig. 7(b). The sensor response increases with increasing ambient CO gas concentration. The optimum sensitivities of this sensor were 5.7, 6.8, 8.8, 11.6, and 37.6% for concentrations of 30, 45, 60, 75, and 90 ppm, respectively. Since CO is an electron donating gas the meso-porous carbon sensor behaves like a p-type semiconductor due to the increasing resistance of mesoporous carbon. The response behavior of this sensor was similar to that of the carbon nanotubes [24]. Furthermore, the highly porous structure enabled the gas to access the entire surface of the mesoporous carbon. In our case, the mesoporous carbon could contribute to the chemical reactions at the surface. Hence, this resulted in high sensitivity at optimal oper-ating temperatures. The properties of CO were determined using a CNTs-based sensor at various CO concentrations. Previous reports have shown that the CNTs-based sensors can detect various gases,
but the response and recovery times, defined as the time within 10% of the final value, are fairly long[25]. This is hardly acceptable in the case of CNTs-based sensors. The mesoporous carbon sensor showed fast responses at various CO concentrations. The average response times were approximately 115 s, and the recovery times were 211 s. The response and recovery times of the sensor are deter-mined by the adsorption kinetics. The bonding between the gas and sensor is very strong. The fast response can be explained by the changes in the schottky junction formed between mesoporous carbon and electrodes[26]. The electron transfer in the porous sys-tem by the forward bias-induced barrier lowering may enhance the gas reaction response. The longer recovery time became longer than the response time because of the residual gas molecules that had been adsorbed on the mesoporous carbon bundles and not entirely removed. Mesoporous carbon shows less grain boundaries that could offer additional sensing area for gas adsorption. This favors a fast electrical charge transduction through the material, which could justify the short response time observed. By increas-ing the number of transferred electrons in porous material systems, more electrons might be captured by CO molecules resulting in the need for a longer recovery time. These results indicate that both the response and stability of the mesoporous carbon sensor are good. Additionally, the presence of the nano-sized mesostructured carbon film can be another important reason for the CO response. The gas sensing of semiconducting metal oxides is due to the bar-rier dependence on the environment work function[27]. It was deduced that the dependence of barrier-height on the environment work function increased as the surface charge density decreased. Therefore, the CO response could be enhanced with a reduction of the surface charge accumulation induced by the decrease of grain size. Williams and Cole well agreed with the model[28].
4. Conclusions
A new mesoporous carbon gas sensor has been successfully developed with good response and recovery times in the detec-tion of CO gas at optimal temperature. The well sensitivity of the mesoporous carbon sensor strongly depends on its large surface area (1200 m2/g), uniform pore size (3 nm) and the microstructure.
The optimum sensitivities of this sensor were 5.7, 6.8, 8.8, 11.6, and 37.6% for concentrations of 30, 45, 60, 75, and 90 ppm, respectively. Acknowledgements
The authors would like to thank the National Science Council of the Republic of China, Taiwan, for financially supporting this research under Contract No. NSC 96-2221-E-451-013. The authors would also like to thank the Center for Nano Science and Technol-ogy of the National Chiao-Tung University for helping with the TEM and XRD characterizations.
References
[1] Y. Tian, G.-D. Li, Q. Gao, Y. Xiu, X.-H. Li, J.-S. Chen, A facile route to mesoporous carbon catalyst support modified with magnetic nanoparticles, Chem. Lett. 36 (2007) 422–442.
[2] K. Ariga, A. Vinu, Q. Ji, O. Ohmori, J.P. Hill, S. Acharya, J. Koike, S. Shiratori, A layered mesoporous carbon sensor based on nanopore-filling cooperative adsorption in the liquid phase, Angew. Chem. Int. Ed. 47 (2008) 7254–7257. [3] J. Lee, S. Yoon, T. Hyeon, S.M. Oh, K.B. Kim, Synthesis of a new mesoporous
carbon and its application to electrochemical double-layer capacitors, Chem. Commun. (1999) 2177–2178.
[4] T. Kyotani, T. Nagai, S. Inoue, A. Tomita, Formation of new type of porous carbon by carbonization in zeolite nanochannels, Chem. Mater. 9 (1997) 609–615. [5] C. Lin, J.A. Ritter, Interlayer interaction of two graphene sheets as a model of
double-layer carbon nanotubes, Carbon 35 (1997) 121–125.
[6] T. Kyotani, L.F. Tsai, A. Tomita, Preparation of ultrafine carbon tubes in nanochannels of an anodic aluminum oxide film, Chem. Mater. 8 (1996) 2109–2113.
[7] R. Ryoo, S.H. Joo, S. Jun, Synthesis of highly ordered carbon molecular sieves via template-mediated structural transformation, J. Phys. Chem. B 103 (1999) 7743–7746.
[8] D. Briand, B. Van der Schoot, N.F. de Rooij, H. Sundgren, I. Lundstrom, A low-power micromachined MOSFET gas sensor, J. MEMS 9 (2000) 303–308. [9] H. Baltes, O. Brand, Proc. of ESSDERC’99, 1999.
[10] J.W. Gardner, A. Pike, N.F. De Rooij, M. Koudelka-Hep, P.A. Clerc, A. Hierle-mann, W. Göpel, Integrated array sensor for detecting organic solvents, Sens. Actuators B: Chem. 26 (1995) 135–139.
[11] C.C. Lu, Proc. Eurosensors, M3A, 2006.
[12] O. Wurzinger, G. Reinhardt, CO sensing properties of doped SnO2sensors in H2-rich gases, Sens. Actuators B: Chem. 103 (2004) 104–110.
[13] H.M. Lin, Proc. Nano Structure Materials, PII 00161, 1997. [14] C.C. Lu, Proc. EuroSensors, vol. XVI, 2002, p. 1143. [15] F. Udrea, J.W. Gardner, UK and World Patent (1996).
[16] F. Udrea, J.W. Gardner, D. Setiadi, J.A. Covington, T. Dogaru, C.C. Lu, W.I. Milne, Design and simulations of SOI CMOS micro-hotplate gas sensors, Sens. Actua-tors B: Chem. 78 (2001) 180–190.
[17] R. Ionescu, E.H. Espinosa, E. Sotter, E. Llobet, X. Vilanova, X. Correig, A. Felten, C. Bittencourt, G. Van Lier, J.C. Charlier, Oxygen functionalisation of MWNT and their use as gas sensitive thick-film layers, Sens. Actuators B: Chem. 113 (2005) 36.
[18] J.C. Obirai, G. Hunter, P.K. Dutta, Multi-walled carbon nanotubes as high tem-perature carbon monoxide sensors, Sens. Actuators B 134 (2008) 640. [19] S. Jun, S.H. Joo, R. Ryoo, M. Kruk, M. Jaroniec, Z. Liu, T. Ohsuna, O. Terasaki,
Synthesis of new, nanoporous carbon with hexagonally ordered mesostructure, J. Am. Chem. Soc. 122 (2000) 10712–10713.
[20] T.W. Kim, F. Kleitz, B. Paul, R. Ryoo, MCM-48-like large mesoporous silicas with tailored pore structure: facile synthesis domain in a ternary triblock copolymer–butanol–water system, J. Am. Chem. Soc. 127 (2005) 7601–7610. [21] A.B. Fuertes, Synthesis of ordered nanoporous carbons of tunable mesopore size
by templating SBA-15 silica materials, Micropor. Mesopor. Mater. 67 (2004) 273–281.
[22] A.B. Fuertes, D.M. Nevskaia, Control of mesoporous structure of carbons syn-thesised using a mesostructured silica as template, Micropor. Mesopor. Mater. 62 (2003) 177–190.
[23] J.F. Chang, H.H. Kuo, I.C. Leu, M.H. Hong, The effects of thickness and operation temperature on ZnO:Al thin film CO gas sensor, Sens. Actuators B 84 (2002) 258.
[24] J. Li, Y. Lu, Q. Ye, M. Cinke, J. Han, M. Meyyappan, Carbon nanotube sensors for gas and organic vapor detection, Nano Lett. 3 (2003) 929–933.
[25] T. Ueda, M.M.H. Bhuiyan, H. Norimatsu, S. Katsuki, T. Ikegami, F. Mitsugi, Devel-opment of carbon nanotube-based gas sensors for NOxgas detection working at low temperature, Physica E 40 (2008) 2272–2277.
[26] J.H. Lee, J. Kim, H.W. Seo, J.W. Song, E.S. Lee, M. Won, C.S. Han, Bias modulated highly sensitive NO2gas detection using carbon nanotubes, Sens. Actuators B: Chem. 129 (2008) 628.
[27] C. Malagù, V. Guidi, M. Stefancich, M.C. Carotta, G. Martinelli, Model for Scottky barrier and surface states in nanostructured n-type semiconductors, J. Appl. Phys. 91 (2002) 808–814.
[28] G. Williams, G.S.V. Coles, The gas-sensing potential of nanocrystalline tin diox-ide produced by a laser ablation technique, MRS Bull. 24 (1999) 25–29. Biographies
Chueh-Yang Liu received his MS degree in Materials Science and Engineering, National Chung Hsing University, Taiwan in 2004. He entered the PhD course in 2004. He is interested in the field of fabrication, characterization, and gas sensing properties of semiconducting porous sensing materials.
Chia Fu Chen received his PhD degree in Electrical and Electronic Engineering, Osaka University, Japan in 1977. He has been a professor in Materials Science and Engi-neering, National Chiao Tung University, Taiwan since 1980. His research interests cover diamond, CNTs, and gas sensors.
Jih Perng Leu received his PhD degree in Chemical Engineering, from University of Minnesota, USA in 1990. He worked at Intel in the area of advanced materi-als designs from 1990 to 2004. In 2005, he has been an associate professor in Materials science and Engineering, National Chiao Tung University, Taiwan. His research interests are understanding the fundamentals of thin film behavior and its structure–property relation, thermo-mechanical integrity of copper/low-k inter-connect and die/package interaction, novel low-k materials relevant processes and integration in semiconductor, optoelectronics and photovolatics applications. Chih-Cheng Lu received his BS and MS degrees in mechanical engineering from National Sun Yat-Sen University and National Chung-Hsing University, Taiwan, in 1989–1994. He was an assistant researcher working on precision engineering and MEMS at Precision Instrument Development Centre (PIDC), Taiwan, from 1994 to 1998, and was awarded Taiwan Government Scholarship in 1997. He is currently a PhD student at Engineering Department, Cambridge University, UK and Study in the fields of SOI gas sensors, micromachining and MEMs.
Kuan-Hsun Liao received his MS degree in Institute of Mechatronic Engineering, National Taipei University of Technology, Taiwan in 2008. His research interests are in the field of solid-state gas sensors.