1
Lidocaine for prolonged and intensified spinal anesthesia by
coadministration of propranolol in the rat
Yu-Wen Chen, Ph.D.,a,b Chin-Chen Chu, M.D., Ph.D.,b Yu-Chung Chen, M.S.,c Ching-Hsia Hung, Ph.D.,d Yung-Tsung Li, M.S.,a Jhi-Joung Wang, M.D., Ph.D.b
a
Department of Physical Therapy, China Medical University, Taichung, Taiwan
b
Department of Medical Research, Chi-Mei Medical Center, Tainan, Taiwan
c
Division of Physical Therapy, Department of Physical Medicine and Rehabilitation, Cheng Hsin General Hospital, Taipei, Taiwan
d
Institute & Department of Physical Therapy, National Cheng Kung University, Tainan, Taiwan
*Corresponding Author:
Ching-Hsia Hung, PhD. Associate Professor
Institute & Department of Physical Therapy National Cheng Kung University
No.1 Ta-Hsueh Road, Tainan, Taiwan Phone: 886-6-2353535 ext 5939 FAX: 886-6-2370411
Abstract
Although the coadministration of lidocaine with propranolol interferes with the
metabolic profile (pharmacokinetics), its pharmacodynamics is still unclear. In this
report, we investigate whether propranolol can potentiate the effect of lidocaine, a
conventional local anesthetic. After intrathecal injections of drugs in rats, three
neurobehavioral examinations (motor function, proprioception, and nocicception)
were performed. Rats received spinal anesthesia with lidocaine co-injected with
propranolol. We showed that lidocaine and propranolol elicited a spinal blockade in
motor function, proprioception, and nociception. Propranolol at the dose of 0.82 μmol/kg produced no spinal anesthesia. Co-administration of lidocaine [50% effective dose (ED50) or ED95] and propranolol (0.82 μmol/kg) produced greater spinal
anesthesia than lidocaine (ED50 or ED95), respectively. These preclinical findings
demonstrated that propranolol and lidocaine displayed spinal anesthesia. When
combined with propranolol, lidocaine elicited a supra-additive effect of spinal
anesthesia.
Propranolol, a β-adrenergic receptor antagonist, is considered to be one of the
most important contributions to pharmacology and clinical medicine in the 20th
century [29]. Indications for the treatment of propranolol are numerous, including the
therapy of angina pectoris [10, 29], cardiac arrhythmias [21], hypertension [10],
migraine [20], hyperthrophic obstructive cardiomyopathy [13], and in the treatment of
many neuropsychiatric disorders [27]. Recently, propranolol has been introduced as a
novel modality for the therapy of dental anxiety [12] and proliferating haemangiomas
[3, 22, 29]. The response of infantile haemangiomas to propranolol reported in the
New England Journal of Medicine by Léauté-Labréze et al. [17] catapulted the use of
this therapy to first-line status among physicians managing this disease [26].
It has been shown that co-injection with high concentrations of propranolol
prolonged tetrodotoxin block to 486 min in rat sciatic nerve blockade [16]. In addition,
Saranteas et al. described that the concurrent administration of lidocaine with
propranolol increases the concentration of the local anesthetic (lidocaine) in serum
[24]. We suggest that the pharmacokinetic interactions may be significant for the
effects of the pharmacodynamics (local anesthesia) of lidocaine in clinical
applications. The goal of this study was to determine the pharmacodynamic
interaction of lidocaine and propranolol. Lidocaine remains the most commonly used
infiltration, peripheral nerve block, epidural anesthesia, and topical anesthesia [2, 8].
Therefore, the spinal anesthetic effect of coadministration of lidocaine and
propranolol was compared with the same dose of lidocaine or the same dose of
propranolol alone. Our results reported that propranolol as adjuvant for lidocaine has
a significant action in improving the quality and duration of spinal anesthesia.
Eighty-eight male Sprague-Dawley rats (300-350 g) were obtained from the
National Laboratory Animal Centre in Taiwan, and then housed in groups of three in
a climate controlled room maintained at 22℃ with approximately 50% relative
humidity. Lighting was on a 12-h light/dark cycle (light on at 6:00 AM), with food
and water available ad libitum up to time of the experiment. The experimental
protocols were approved by the Institutional Animal Care and Use Committee of
China Medical University, Taiwan, and conformed to the recommendations and
policies of the International Association for the Study of Pain (IASP).
Lidocaine HCl and (±)-Propranolol HCl were purchased from Sigma-Aldrich
Chemical Co. (St. Louis, MO, USA). All drugs were freshly prepared in 5% dextrose
as solution before intrathecal injections.
Three experiments were carried out. In experiment 1, the dose-dependent effects
of lidocaine (1.54, 3.08, 6.15, 9.23 µmol/kg) and propranolol (0.82, 1.63 µmol/kg) on
2, the spinal anesthetic effect of co-administration of lidocaine at ED50 (2.92 μmol/kg)
and propranolol (0.82 μmol/kg) was compared with lidocaine (2.92 μmol/kg) alone
(n=8 rats for each dose of each drug). In experiment 3, the spinal anesthetic effect of
co-administration of lidocaine at ED95(7.46 μmol/kg;) and propranolol (0.82 μmol/kg)
was compared with lidocaine (7.46 μmol/kg) alone (n=8 rats for each dose of each
drug).
Spinal anesthesia was practiced in conscious rats. Following an optimal flexion of
the rat lumbar spine under prone position, each 50-µl of 1% lidocaine was injected
into the right and left side of paraspinal space (0.5 cm in depth) which was 0.5 cm
away from the mid-point of the longitudinal line of L4–5 intervertebral space. Two
minutes later, a 27-gauge needle attached to a 50-µL syringe (Hamilton, Reno,
Nevada) was inserted into the mid-line of the L4–5 intervertebral space and advanced
at a slightly caudal angle until a tail-flick indicated entrance into the intrathecal space.
Twenty-five microliters of drug were injected and the rat was observed for the
development of spinal blockade, indicated by paralysis of both hind limbs [4, 19].
Rats, which showed unilateral blockade, were excluded from the study and sacrificed
by using an over dose of isoflurane.
After intrathecal injection of drug, three neurobehavioral examinations, which
conducted [5, 6, 15]. For consistency, one trained examiner was responsible for
handling of all rats and behavioral evaluations. In brief, the motor function was
evaluated by measuring 'the extensor postural thrust' of the right hind limb of each rat
on a digital scale. The reduction in force, resulting from extensor muscle tone, was considered motor deficit. A force <20 g (also referred to a weight of the ‘flaccid limb’) was considered absence of extensor postural thrust or 100% motor block or 100%
maximal possible effect (MPE). Nociception was evaluated using the withdrawal
reflex or vocalization elicited by pinching a skin fold on each rat's back at 1 cm from
the proximal part of the tail, the lateral metatarsus of both hind limbs, and the dorsal
part of the mid-tail. Nociceptive block was graded as 0 (absent or 100% MPE), 1
(75% MPE), 2 (50% MPE), 3 (25% MPE), and 4 (normal or 0% MPE) [11]. Proprioception was based on the resting posture and postural reactions (‘tactile placing’ and ‘hopping’). The functional deficit was graded as 3 (normal or 0% MPE),
2 (slightly impaired or 33% MPE), 1 (severely impaired or 67% MPE), and 0
(completely impaired or 100% MPE).
After injecting rats with four doses of lidocaine (n = 8 for each dose of each drug)
intrathecally, the dose—response curve was constructed by the % MPE of each dose
of lidocaine. The curve was then fitted using SAS NLIN Procedures (SAS Institute
50% and 95% spinal anesthesia, respectively, were obtained [5, 6, 14].
The complete block duration of drug was defined as the interval between times of
100% blockade (100% MPE) of drug. The full recovery time of each blockade,
defined as the interval from drug injection to full recovery, was measured and
compared. In addition, the AUCs of spinal blockades of drugs were estimated by
Kinetica version 2.0.1 (InnaPhase Corporation, Philadelphia, PA).
Experimental data are presented as mean ± SEM or ED50 values with 95%
confidence interval (95% CI). All data were evaluated by 2-sided Student t test with
unequal variances. A statistical software, SPSS for Windows (version 17.0, SPSS, Inc,
Chicago, IL, USA), was used, and a P value less than 0.05 was considered statistically
significant.
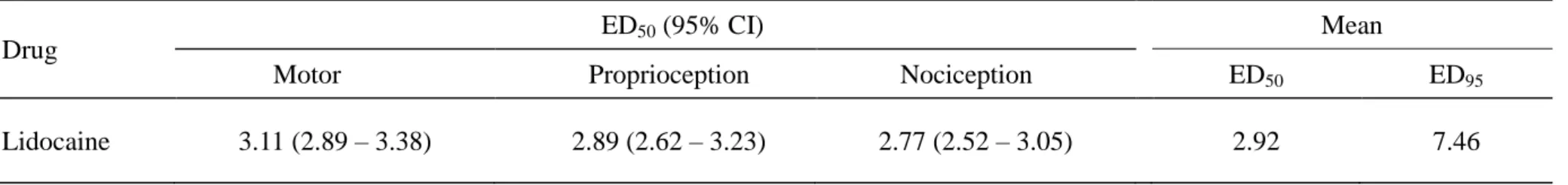
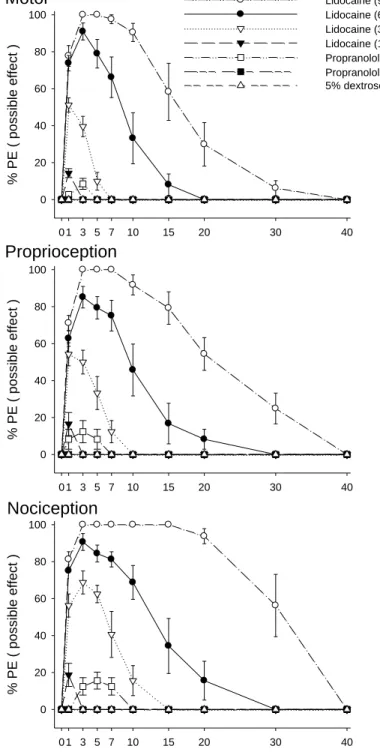
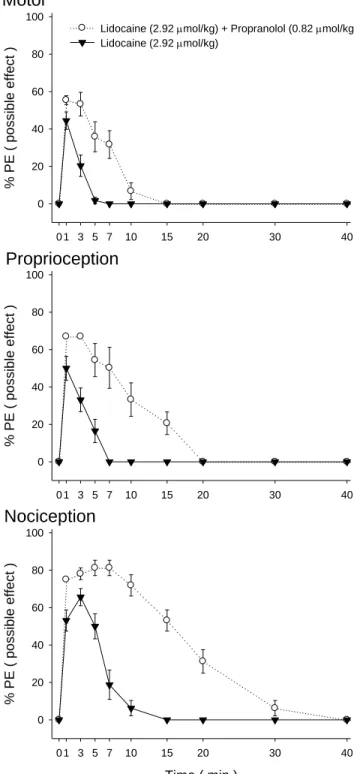
The spinal block effects of propranolol and lidocaine in motor function,
proprioception, and nociception has been demonstrated in Figure 1. The ED50s of
lidocaine in motor, proprioception, and nociception are shown in Table 1. At the dose of 1.63 μmol/kg (Fig. 1), propranolol showed 8.5, 12.4, and 15.6% of blockades (% MPE) in motor function, proprioception, and nociception with duration of action of
about 2.50.9, 2.81.1, and 5.91.8 min, respectively. Intrathecal injection of 5%
dextrose or propranolol at the dose of 0.82 μmol/kg elicited no spinal blockades in
μmol/kg produced complete blockade (100% MPE) of motor function, proprioception, and nociception (Fig. 1).
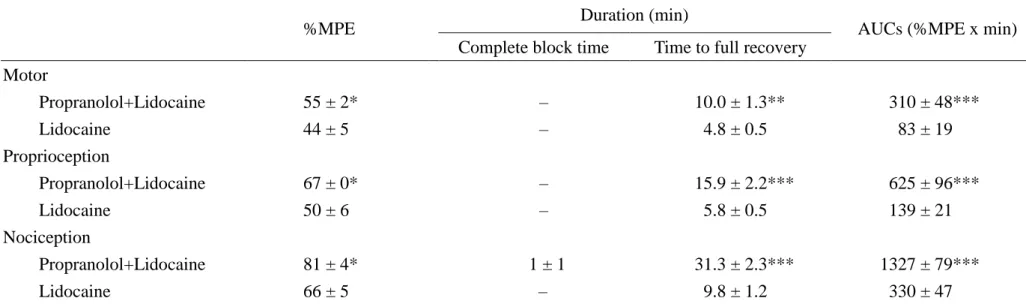
We have known that propranolol at the dose of 0.82 µmol/kg showed no spinal
anesthesia (Fig. 1). Co-administration of lidocaine at ED50 (2.92 μmol/kg) and
propranolol (0.82 µmol/kg) demonstrated greater motor, proprioceptive, and
nociceptive blockade (55% MPE, 67% MPE, and 81% MPE; P < 0.05) than that of
the same dose of lidocaine alone (44% MPE, 50% MPE, and 66% MPE) (Fig. 2 and
Table 2). The time to full recovery and AUCs of co-administration of lidocaine (2.92 μmol/kg) and propranolol (0.82 µmol/kg) also displayed greater motor, proprioceptive, and nociceptive blockade than that of the same dose of propranolol alone (Fig. 2 and
Table 2).
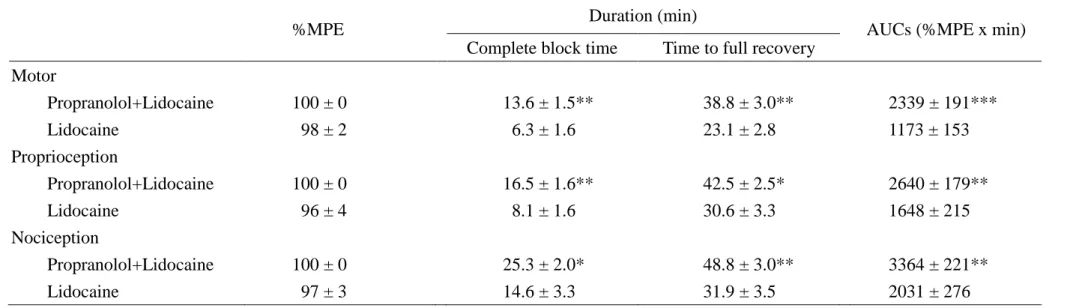
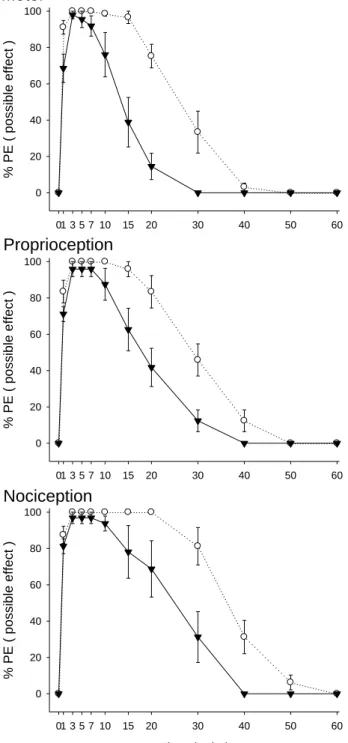
Lidocaine at the dose of 7.46 μmol/kg (ED95) co-injected with propranolol (0.82
µmol/kg) caused similar motor, proprioceptive, and nociceptive blockade (100% MPE,
100% MPE, and 100% MPE) to that of the same dose of lidocaine alone (98% MPE,
96% MPE, and 97% MPE) (Fig. 3 and Table 3). The time to full recovery and AUCs
of lidocaine at the dose of ED95 with propranolol (0.82 µmol/kg) were greater (P<0.05)
than those of lidocaine (ED95) in Figure 3 and Table 3. All rats recovered completely
after intrathecal drug injections.
anesthesia in rats. Propranolol dramatically improves the spinal blocking effect and
duration by lidocaine.
Local anesthetics are agents that elicit neural blockade via a direct blocking effect
on the voltage-gated Na+ channels of the nervous tissues [2, 8]. Because propranolol
has been known to have a blocking effect of veratridine-stimulated Na+ influx in rat
cerebrocortical synaptosomes [7], theoretically, it may have a local anesthetic effect.
In this study, we did find that the beta-blocker propranolol displayed a spinal (local)
anesthetic effect. Similarly to propranolol spinal anesthetic effect in rats, propranolol
administration into the sciatic nerve area produced the local anesthetic effect and
neuromuscular blocking activity [18] in mice.
The coadministration of lidocaine with propranolol resulting in pharmacokinetic
interactions that may be significant for the determination of the correct dose of
lidocaine in clinical practice [24]. Furthermore, both the reduced concentrations and
the protein-binding of lidocaine in mandible after the coadministration with
propranolol may result in decreased depth and duration of local anesthesia [24]. In
addition, Tesseromatis et al. reported that propranolol can displace lidocaine from
liver proteins and therefore the co-administration of these two drugs may increase the
free fraction of lidocaine excreted by the liver [28]. However, we showed that
lidocaine.
Tetrodotoxin is also a local anesthetic agent in that it does not cause seizures,
arrhythmias or local neurotoxicity [23]. Interestingly, it has been demonstrated that
co-injection with the high concentrations of adrenergic antagonists (e.g. propranolol)
markedly prolong the duration of block of tetrodotoxin in rat sciatic nerve blockade,
by an effect that does not appear to be adrenergic receptor-specific [16]. This report is
agreement in our data showed that adding propranolol at 0.82 µmol/kg to lidocaine, a
common local anesthetic, produced a supra-additive effect in spinal anesthesia (Figs.
2 and 3).
It is well known that the adrenergic system is a prime controller of blood pressure.
In in vitro binding assays, propranolol shows high affinity for β1- and β2-adrenoceptors [9, 25]. Though we have made no study about the known cardiovascular effects, Berg et al. demonstrated that centrally active propranolol (β1+2+[3], 44 μmol/kg) in rats had little effect on blood pressure, heart rate, cardiac
output, and total peripheral vascular resistance [1]. In this study, we only evaluated
the doses of propranolol between 1.63 and 0.82 µmol/kg. Besides, spinal anesthesia is
a relatively simple method, which supplies competent surgical conditions by
administrating a small amount of local anesthetics [15]. In addition to the spinal
lidocaine and propranolol elicited greater spinal blockades than that of the same dose
of lidocaine alone.
In conclusion, our results showed that propranolol and lidocaine produced spinal
anesthetic effects in rats. Co-injection with propranolol markedly potentiated the
Acknowledgements
The authors gratefully acknowledge the financial support provided for this study
Table 1. The 50% effective doses (ED50s) of lidocaine with 95% confidence interval (95% CI) on spinal blockades of motor, proprioception,
and nociception in rats Drug
ED50 (95% CI) Mean
Motor Proprioception Nociception ED50 ED95
Lidocaine 3.11 (2.89 – 3.38) 2.89 (2.62 – 3.23) 2.77 (2.52 – 3.05) 2.92 7.46
Table 2. The %MPE, duration, and AUCs of lidocaine at ED50 (2.92 µmol/kg) or co-administration of lidocaine (2.92 µmol/kg) and
propranolol (0.82 µmol/kg) in rats
%MPE Duration (min) AUCs (%MPE x min)
Complete block time Time to full recovery Motor Propranolol+Lidocaine 55 ± 2* – 10.0 ± 1.3** 310 ± 48*** Lidocaine 44 ± 5 – 4.8 ± 0.5 83 ± 19 Proprioception Propranolol+Lidocaine 67 ± 0* – 15.9 ± 2.2*** 625 ± 96*** Lidocaine 50 ± 6 – 5.8 ± 0.5 139 ± 21 Nociception Propranolol+Lidocaine 81 ± 4* 1 ± 1 31.3 ± 2.3*** 1327 ± 79*** Lidocaine 66 ± 5 – 9.8 ± 1.2 330 ± 47
Percent of maximal possible effect (%MPE), duration of drug action, and area under curves (AUCs) of motor, proprioceptive, and nociceptive blockades (mean SEM) for lidocaine alone or co-administration of lidocaine and propranolol (n = 8 in each group). Symbols (*,**,***) indicate P < 0.05, P < 0.01, P < 0.001, respectively, when co-administration of propranolol and lidocaine compared to lidocaine alone.
Table 3. The %MPE, duration, and AUCs of lidocaine at ED95 (7.46 µmol/kg) or co-administration of lidocaine (7.46 µmol/kg) and
propranolol (0.82 µmol/kg) in rats
%MPE Duration (min) AUCs (%MPE x min)
Complete block time Time to full recovery Motor Propranolol+Lidocaine 100 ± 0 13.6 ± 1.5** 38.8 ± 3.0** 2339 ± 191*** Lidocaine 98 ± 2 6.3 ± 1.6 23.1 ± 2.8 1173 ± 153 Proprioception Propranolol+Lidocaine 100 ± 0 16.5 ± 1.6** 42.5 ± 2.5* 2640 ± 179** Lidocaine 96 ± 4 8.1 ± 1.6 30.6 ± 3.3 1648 ± 215 Nociception Propranolol+Lidocaine 100 ± 0 25.3 ± 2.0* 48.8 ± 3.0** 3364 ± 221** Lidocaine 97 ± 3 14.6 ± 3.3 31.9 ± 3.5 2031 ± 276
Percent of maximal possible effect (%MPE), duration of drug action, and area under curves (AUCs) of motor, proprioceptive, and nociceptive blockades (mean SEM) for lidocaine alone or co-administration of lidocaine and propranolol (n = 8 in each group). Of note, all of the rats in the co-administration of propranolol and lidocaine group show complete blockade (100% MPE) of any function tested. Symbols (*,**,***) indicate P < 0.05, P < 0.01, P < 0.001, respectively, when co-administration of propranolol and lidocaine compared to lidocaine alone.
Legends to figures
Fig. 1. Time courses of spinal blockades of motor, proprioception, and nociception
(% PE) by lidocaine and propranolol in rats (n=8 at each testing point). The 5%
dextrose (vehicle) group is as the control. Data are mean ± SEM.
Fig. 2. The time course of lidocaine at 2.92 µmol/kg (ED50) or coadministration of
lidocaine at 2.92 µmol/kg and propranolol at 0.82 µmol/kg on spinal anesthesia in
rats. Values are expressed as mean ± SEM. For each group of the time course study,
n=8 rats.
Fig. 3. The time course of lidocaine at 7.46 µmol/kg (ED95) or coadministration of
lidocaine at 7.46 µmol/kg and propranolol at 0.82 µmol/kg on spinal anesthesia in
rats. Data are mean ± SEM. Each testing point of the time course study contained
References
[1] T. Berg, B.W. Piercey, J. Jensen, Role of beta1-3-adrenoceptors in blood
pressure control at rest and during tyramine-induced norepinephrine release in
spontaneously hypertensive rats, Hypertension 55 (2010) 1224-1230.
[2] A. Borgeat, J. Aguirre, Update on local anesthetics, Curr. Opin. Anaesthesiol.
23 (2010) 466-471.
[3] L.M. Buckmiller, Propranolol treatment for infantile hemangiomas, Curr.
Opin. Otolaryngol. Head Neck Surg. 17 (2009) 458-459.
[4] Y.W. Chen, Y.C. Chen, C.N. Lin, C.C. Chu, M.T. Lin, J.J. Wang, C.H. Kao,
The spinal anaesthetic effect of dextromethorphan, dextrorphan, and
3-methoxymorphinan, Eur. J. Pharmacol. 569 (2007) 188-193.
[5] Y.W. Chen, C.C. Chu, Y.C. Chen, J.J. Wang, C.H. Hung, The dose-dependent
study of verapamil and diltiazem on spinal anesthesia in the rat, Neurosci. Lett.
482 (2010) 76-80.
[6] Y.W. Chen, C.C. Chu, Y.C. Chen, J.J. Wang, C.H. Hung, Isobolographic
analysis of caramiphen and lidocaine on spinal anesthesia in rats, Neurosci.
Lett. 469 (2010) 174-178.
[7] G. Chidlow, J. Melena, N.N. Osborne, Betaxolol, a beta(1)-adrenoceptor
interaction with Na(+) channels: comparison with other beta-adrenoceptor
antagonists, Br. J. Pharmacol. 130 (2000) 759-766.
[8] H.A. Fozzard, P.J. Lee, G.M. Lipkind, Mechanism of local anesthetic drug
action on voltage-gated sodium channels, Curr. Pharm. Des. 11 (2005)
2671-2686.
[9] P.F. Fraundorfer, R.H. Fertel, D.D. Miller, D.R. Feller, Biochemical and
pharmacological characterization of high-affinity trimetoquinol analogs on
guinea pig and human beta adrenergic receptor subtypes: evidence for partial
agonism, J. Pharmacol. Exp. Ther. 270 (1994) 665-674.
[10] W.H. Frishman, W. Shapiro, S. Charlap, Labetalol compared with propranolol
in patients with both angina pectoris and systemic hypertension: a
double-blind study, J. Clin. Pharmacol. 29 (1989) 504-511.
[11] P. Gerner, T. Nakamura, C.F. Quan, D.C. Anthony, G.K. Wang, Spinal
tonicaine: potency and differential blockade of sensory and motor functions,
Anesthesiology 92 (2000) 1350-1360.
[12] L.J. Heaton, D.W. McNeil, P. Milgrom, Propranolol and D-cycloserine as
adjunctive medications in reducing dental fear in sedation practice, SAAD
Dig. 26 (2010) 27-35.
cardiomyopathy: effects of propranolol and verapamil on diastolic stiffness,
Eur. Heart J. 4 Suppl F (1983) 47-56.
[14] C.H. Hung, K.S. Liu, D.Z. Shao, K.I. Cheng, Y.C. Chen, Y.W. Chen, The
systemic toxicity of equipotent proxymetacaine, oxybuprocaine, and
bupivacaine during continuous intravenous infusion in rats, Anesth. Analg.
110 (2010) 238-242.
[15] C.H. Hung, J.J. Wang, Y.C. Chen, C.C. Chu, Y.W. Chen, Intrathecal
oxybuprocaine and proxymetacaine produced potent and long-lasting spinal
anesthesia in rats, Neurosci. Lett. 454 (2009) 249-253.
[16] D.S. Kohane, N.T. Lu, G.A. Crosa, Y. Kuang, C.B. Berde, High concentrations
of adrenergic antagonists prolong sciatic nerve blockade by tetrodotoxin, Acta.
Anaesthesiol. Scand. 45 (2001) 899-905.
[17] C. Leaute-Labreze, E. Dumas de la Roque, T. Hubiche, F. Boralevi, J.B.
Thambo, A. Taieb, Propranolol for severe hemangiomas of infancy, N. Engl. J.
Med. 358 (2008) 2649-2651.
[18] K. Leszczynska, S.T. Kau, A sciatic nerve blockade method to differentiate
drug-induced local anesthesia from neuromuscular blockade in mice, J.
Pharmacol. Toxicol. Methods. 27 (1992) 85-93.
inhibited sodium currents and produced spinal anesthesia,
Neuropharmacology 58 (2010) 1147-1152.
[20] K. Linde, K. Rossnagel, Propranolol for migraine prophylaxis, Cochrane
Database Syst. Rev. (2004) CD003225.
[21] J.C. Matthews, J.K. Baker, Effects of propranolol and a number of its
analogues on sodium channels, Biochem. Pharmacol. 31 (1982) 1681-1685.
[22] S. Maturo, C. Hartnick, Initial experience using propranolol as the sole
treatment for infantile airway hemangiomas, Int. J. Pediatr. Otorhinolaryngol.
74 (2010) 323-325.
[23] S. Sakura, A.W. Bollen, R. Ciriales, K. Drasner, Local anesthetic
neurotoxicity does not result from blockade of voltage-gated sodium channels,
Anesth. Analg. 81 (1995) 338-346.
[24] T. Saranteas, C. Mourouzis, F. Koumoura, C. Tesseromatis, Effects of
propranolol or paracetamol on lidocaine concentrations in serum and tissues, J.
Oral Maxillofac. Surg. 61 (2003) 604-607.
[25] A. Schotte, P.F. Janssen, W. Gommeren, W.H. Luyten, P. Van Gompel, A.S.
Lesage, K. De Loore, J.E. Leysen, Risperidone compared with new and
reference antipsychotic drugs: in vitro and in vivo receptor binding,
[26] E.C. Siegfried, W.J. Keenan, S. Al-Jureidini, More on propranolol for
hemangiomas of infancy, N. Engl. J. Med. 359 (2008) 2846; author reply
2846-2847.
[27] I.E. Tchivileva, P.F. Lim, S.B. Smith, G.D. Slade, L. Diatchenko, S.A.
McLean, W. Maixner, Effect of catechol-O-methyltransferase polymorphism
on response to propranolol therapy in chronic musculoskeletal pain: a
randomized, double-blind, placebo-controlled, crossover pilot study,
Pharmacogenet. Genomics 20 (2010) 239-248.
[28] C. Tesseromatis, A. Kotsiou, M. Tsagataki, E. Tigka, J. Vovou, A. Alevizou, C.
Perisanidis, T. Saranteas, D. Karakitsos, A. Karabinis, G. Kostopanagiotou, In
vitro binding of lidocaine to liver tissue under the influence of propranolol:
another mechanism of interaction?, Eur. J. Drug Metab. Pharmacokinet. 32
(2007) 213-217.
[29] A.P. Zimmermann, S. Wiegand, J.A. Werner, B. Eivazi, Propranolol therapy
for infantile haemangiomas: review of the literature, Int. J. Pediatr.
Fig. 1.
0 1 3 5 7 10 15 20 30 40 % PE ( p o ssib le e ffe ct ) 0 20 40 60 80 Lidocaine (3.08 mol/kg) Lidocaine (1.54 mol/kg) Propranolol (1.63 mol/kg) Propranolol (0.82 mol/kg) 5% dextrose 0 1 3 5 7 10 15 20 30 40 % PE ( p o ssib le e ffe ct ) 0 20 40 60 80 100 Proprioception Time ( min ) 0 1 3 5 7 10 15 20 30 40 % PE ( p o ssib le e ffe ct ) 0 20 40 60 80 100 NociceptionFig. 2.
0 1 3 5 7 10 15 20 30 40 % P E ( po ssib le e ffec t ) 0 20 40 60 80Lidocaine (2.92 mol/kg) + Propranolol (0.82 mol/kg) Lidocaine (2.92 mol/kg) 0 1 3 5 7 10 15 20 30 40 % P E ( po ssib le e ffec t ) 0 20 40 60 80 100 Proprioception Time ( min ) 0 1 3 5 7 10 15 20 30 40 % P E ( po ssib le e ffec t ) 0 20 40 60 80 100 Nociception