Evaluation Manufacturing Technique and the Mechanical Properties of Polylactide Acid Net / Chitosan Composite Membrane
Ching-Wen Lou1, Ming-Gene Tu2,3, Chao-Tsang Lu4, Po-Ching Lu5 and Jia-Horng Lin 5, 6,7*
1Institute of Biomedical Engineering and Material Science, Central Taiwan University of Science and Technology, Taichung 40601, Taiwan, R.O.C.
2School of Dentistry, China Medical University, Taichung, 40402, Taiwan. 3Department of Dentistry, China Medical University Hospital, Taichung, 40402, Taiwan.
4Graduate Institue of Biotechnology, Central Taiwan University of Science and Technology, Taichung 40601, Taiwan, R.O.C.
*5Laboratory of Fiber Application and Manufacturing, Department of Fiber and Composite Materials, Feng Chia University, Taichung City 40724, Taiwan, R.O.C. *6School of Chinese Medicine, China Medical University, Taichung 40402, Taiwan, R.O.C.
*7Department of Fashion Design, Asia University, Taichung 41354, Taiwan, R.O.C.
Feng Chia University, No. 100, Wenhwa Rd., Seatwen, Taichung, Taiwan 40724, R.O.C. Tel.: +886-4-24517250 ext.3405; Fax: +886-4-24510871; E-mail:
jhlin@fcu.edu.tw.
Abstract
Guided Tissue Regeneration is an important treatment for periodontal diseases, as it helps periodontal tissues to regenerate. This study aims to evaluate the influence of different procedures for gelation on the mechanical properties of low melting-point polylactic acid (LPLA) net/ chitosan composite membranes. After immersion in a chitosan solution, LPLA nets undergo different steps of gelation and freeze-drying, forming the porous LPLA/Chitosan composite membranes. The tensile strength, swelling, degradation and water content of the resulting membranes are then evaluated. According to the experimental results, different sequences for gelation influence the tensile strength and swelling ratio, but do not significantly influence the water content ratio and contact angle.
Keywords: Guided Tissue Regeneration (GTR), gelation, low melting-point polylactic acid (LPLA), chitosan.
Introduction
Guided Tissue Regeneration (GTR), invented by Nyman in 1982, is commonly used to treat periodontal diseases. It uses a membrane to obstruct the fast-growing gingival tissue, creating room for the growth and filtration of the periodontal tissue cells to form new tissues, including alveolar bone, periodontal ligament, and cementum [1-4]. Therefore, membranes must have a good strength in order to block the growth of the tissues and be able to withstand sutures. It takes six months for periodontal tissue to grow; therefore, the membranes require a low degradation level. They should also have a certain porosity and pore size, enabling the exchange of body fluid which helps cell growth. Kuo et al. (2009) produced membranes with β-TCP and chitosan, and found that after the 60-day biodegradation, the membranes still maintained 97 % of their weight. In an experiment on animals, the membranes effectively blocked the connective tissue from growing; in addition, new bone growth appeared [5].
Polylactic acid (PLA) is a biopolymer, which results from the polymerization of lactic acid monomers. PLA has good mechanical properties, biodegradation, biocompatibility, and is non-toxic; thus, is commonly used in tissue engineering. PLA has a complete degradation time of a year, and often further synthesizes with other glycolic acid (GA) monomers to make PLGA, the degradation of which
varies, providing a greater variety for their applications. This study uses PLA fibers, which are made by melt-spinning PLA chips. The PLA fibers have high crystallinity, molecular orientation, and heat-resistance, and their degradation time is the same as that for PLA.
Chitosan is made by the deacetylation of chitin with alkali treatment. It comes from the shells of shrimps and crabs, both of which are abundant in the ocean. It can be dissolved in an environment of pH< 6, and the greater the deacetylation, the higher the solubility [6]. Chitosan is anti-bacterial and has good biocompantity, biological activity, and biodegradation [7]. It is degraded more slowly in vivo, and the degradation becomes slower with an increase in deacetylation [8]. The byproduct after degradation is non-toxic, and therefore, chitosan is commonly used in the biomedical field for wound dressings [9, 10], drug release [11-13], and as an antibacterial [14-15]. Chitosan also has good hemostasis as blood that encounters chitosan coagulates. After the red blood cells adhere to each other, NH3+ resulting from the chitosan’s being dissolved in an acid solution gives the chitosan positive ions, and thus attracts the negative molecules from the surface of the red blood cells. The red blood cells subsequently coagulate and cause hemostasis. Fan et al. (2006) combined 30 wt% or 10 wt% of carboxymethyl-chitosan, sodium alginate, and fibers, and found good compatibility between the first two materials [17]. This
study creates LPLA/chitosan membranes by immersing the Low melting-point polylactic acid (LPLA) nets in a chitosan solution. The physical properties of the resulting membranes are explored, determining their applications in GTR.
Materials and Methods Material
Chitosan (VA&G Bioscience Inc, Taiwan, ROC) has a deacetylation of 80 %. Low melting-point polylactic acid (LPLA)fibers (Far Eastern New Century Corporation, Taiwan, ROC) have a melting point of 130 °C, a fineness of 20 um, a length of 50 mm, a monofilament’s strength of 3.2 g/D, and an elongation of 52 %. Sodium hydroxide and acetic acid are purchased from Kojima Chemicals Co., Ltd, Japan.
Preparation of the LPLA Net
The LPLA fibers have skin-core structure. When the fibers are thermally treated, their surfaces melt, creating bonding points between them. The fibers undergo an opening, mixing, and carding process, and then were lain creating for 4 layers. The 4-layer net is removed, placed in a stainless steel mold measuring 15×15 cm2, and thermally treated in an oven at 150 °C for 30 minutes. The
resulting nets are cut into 40×40 mm2 pieceswith thicknesses of 210 μm, forming the LPLA nets.
Preparation of LPLA/Chitosan Composite Membranes
Chitosan is first dissolved in 2 % acetic acid, forming a 5 wt% chitosan solution. LPLA nets are then soaked in the chitosan solution for 10 minutes, until they are fully saturated. The LPLA/chitosan net is placed in a Petri dish and frozen at -20 °C for 24 hours, forming the LPLA/chitosan membranes. The membranes are then prepared with three different procedures: First, one batch is soaked in a 1M sodium hydroxide (NaOH) solution for 3 minutes for gelation, washed with deionized water, frozen for another 24 hours, and then freeze-dried for 24 hours, forming the LPLA/chitosan composite membranes that are ante—freeze-drying gelled (AG). The second batch is first frozen and freeze-dried, both for periods of 24 hours, and then immersed in a 1 M NaOH solution for gelation, forming the LPLA/chitosan composite membranes that are post—freeze-drying gelled (PG). The third batch is frozen for 24 hours, freeze-dried for 24 hours, washed with deionized water three times, and dried at room temperature for 48 hours, forming the LPLA/chitosan composite membrane that is non-gelled (NG).
Tests
Differential Scanning Calorimetry (DSC) Analysis
5 mg of LPLA fibers are meausred with a DSC Q200 (TA instrument, U.S.) at a temperature range of 40-200 °C with a heating speed of 40 °C /min.
Fiber Direction
The LPLA nets that are thermally and alkali treated are placed in a scanner on top of a piece of black paper, and scanned, 1200 dots per inch. The fiber orientation ratio of the scanned images is analyzed by the software developed by our laboratory.
Air Permeability
The LPLA nets that are thermally and alkali treated are prepared as specified in ASTM D737-04. The nets measuring 25×25 cm2 are evaluated with an air permeability tester (Textest FX3300, Switzerland) under a pressure of 125 Pa, and the number of samples is 12 (n=12).
Tensile Strength
AG, PG, and NG are cut into 10×20 mm2 and immersed in phosphate buffer solution (PBS) for 24 hours. An Instron 5566 (US) is then employed to test the tensile strength of the three composite membranes with settings: a 1 mm/min tensile speed, and
a distance between clamps is 10mm.
Degradation Test
AG, PG, and NG are weighed and the immersed in a 40 ml PBS, which is placed in the water bath at 37 °C for 1, 2, and 3 months, the PBS is changed every three days. The samples are thermally treated at 50 °C for 24 hours before they are weighed. The degradation of the various samples is calculated according to the equation below.
Degradation = (W0-Wd)/W0 × 100 %... (1)
where Wd is the sample weight after being immersed in PBS and then dried in an oven and W0 is the sample weight before it is immersed in deionized water.
Swelling Ratio and Water Content
AG, PG, and NG are thermally treated at 50 °C for 24 hours, and then weighed as W0. Then, samples are immersed in 10 mL deinoized water, and removed every other minute, wiped off the excess water, and weighed as W1. The increase in weight indicates samples’ swelling ratio and water content.
Swelling Ratio = (W1-W0)/W0 × 100 % ………(2) Water Content = (W1-W0)/W1× 100 %...(3)
where W1 is the weight of water-logged sample and W0 is the sample weight before immersion in the deionized water.
Water Contact Angle
The instrument (OCA-15 SCA20) is used to evaluate the hydrophilicity AG, PG, and NG. A 20μl of deionized water is dripped on their surface to observe the contact angle of water drop and the surface of the samples, determining the hydrophilicity of the composite membranes.
Statistical analysis
Differences in tensile strength and swelling ratio were analysed using Student’s T-test with two-tailed distribution tails and two-sample equal variance. P-values less than 0.05 were considered significant
Results and Discussion DSC Analysis
Temperature (°C)
exo
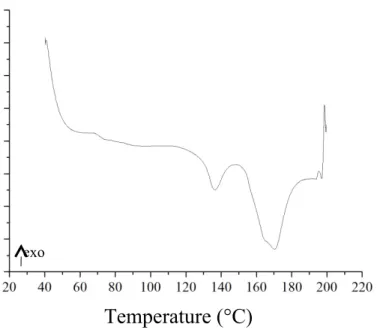
Figure 1. DSC curves of the LPLA fibers.
Figure 1 shows that with a skin-core structure, the LPLA fibers have two different melting points of 135 °C and 170 °C for the skin and the core, respectively. The skin melts as a result of heating and forms the thermal bonding points between fibers in the LPLA fiber webs.
Fiber Direction in the LPLA Net
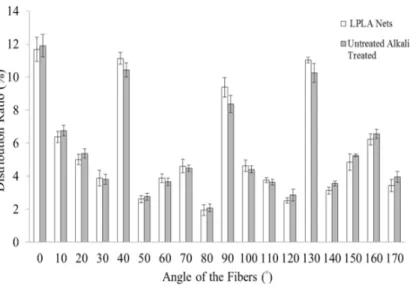
Figure 2 reveals the distribution ratio of fibers at various angles in the LPLA net with and without alkali treatment. Fibers at 0°, 40°, 90°, and 130° have the greatest proportion, indicating that their distribution in the net is even, which gives the net the same strength when pulled in all directions.
Figure 2. The distribution ratio of various angles of fibers in the LPLA nets with and without alkali treatment.
Air Permeability of the LPLA Net
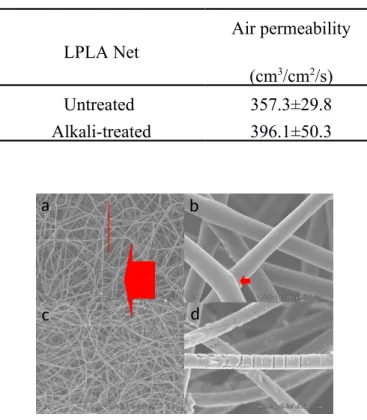
Table 1 reveals the air permeability of the LPLA nets as related to alkali treatment. The air permeability of the treated LPLA net is slightly greater than that of the untreated one, as the alkali treatment damages the thermal bonding points between the fibers, changing the pore size, and thus resulting in an increase in the air permeability. Figure 3 (a) and (b) compares the surface of the LPLA nets with and without alkali treatment, respectively, and the untreated LPLA nets have thermal bonding points. Figure 3 (c) shows that the alkali-treated LPLA nets have significantly fewer thermal bonding points, and there are also breakages in the fibers. The required sample size is 15 cm ×15 cm as specified in the test standard
a b
c d
of the air permeability test. However, the maximum size of LPLA/chitosan composite membranes is 5 cm × 5 cm; therefore, the membranes are not qualified to be tested for air permeability in order to determine the influence of the chitosan immersion on the air permeability, pore size, and amount of pores.
Table 1. Air permeability of the 4-layer LPLA nets with and without being heated at 150 °C for 30 minutes. LPLA Net Air permeability (cm3/cm2/s) Untreated 357.3±29.8 Alkali-treated 396.1±50.3
Figure 3. SEM images of the LPLA net without alkali treatment a) ×50, and b) ×1.0 k, and with treatment c) ×50, and d) ×1.0 k.
Thermal bonding points are incidated
by the red arrows.
SEM Observation of the LPLA/Chitosan Composite Membranes
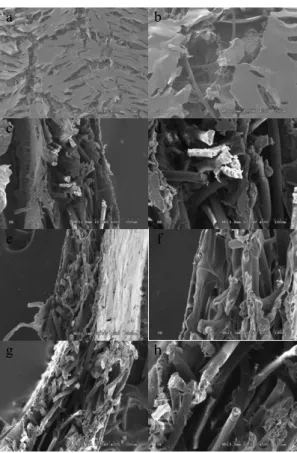
Figure 4 shows the SEM images of AG, PG, and NG. Figure 4 (a) shows that there are still many pores on the surface of AG. Figure 4 (b) illustrates the fibers beneath the pores, and there are no fibers exposed according to morphology observation. All of the LPLA fibers in Figure 4 are coated with a layer of chitosan, proving that during manufacturing, the nets were completely soaked by a chitosan solution. From the cross-sectional images, it can be seen that the chitosan also connects the pores between the fibers inside the internal net, thereby enhancing the overall mechanical properties of the composite membranes.
The thermal bonding points are composed of the low melting-point PLA in the skin of the LPLA fibers; thus, the molecular weight of the PLA in the skin is smaller than that in the core. This makes the LPLA fibers more vulnerable to the alkali, decreasing the thermal bonding points between the fibers. Figure 4 (d) shows the obvious etching on the surface of the fibers that resulted from the alkali treatment, causing the surface to become rough. The breakage of the fibers reduces the continuity of the LPLA net and enlarges its pore size, resulting in an increase in the air permeability. Alkali treatment damages the fibers, but does not loosen the whole structure, making its concentration and treatment time only minorly destructive to the overall structure of the LPLA nets.
a b
c d
e f
g h
Figure 4 shows that after the alkali treatment, the angles of the fibers in the LPLA net are not significantly changed, still arranged in almost the same directions. This is due to the alkali treatment, which removes the oil that adhered to the fibers during the spinning process, and thus giving rise to etching of the fibers; it does not, however, influence the fiber direction.
Alkali treatment etches the LPLA fibers, resulting in a decrease in their diameters that makes them subject to easy breaking; thus, the fibers are easily broken.
Figure 4. SEM images of AG a) ×50, b) ×200; cross-section of AG c) ×200, d) ×500; cross-section of PG e) ×200, f) ×500; and cross-section of NG g) ×200, (h) ×500.
Tensile Strength
LPLA nets are immersed in the chitosan solution to reinforce the tensile strength of the resulting membranes. Membranes that were not immersed in PBS for 24 hours serve as the control group. Figure 5 compares the tensile strengths of AG, PG, and NG, showing that immersion in PBS does not make a difference.
Figure 5 shows that the composite membranes exhibit a lower tensile strength when wet than when dry. Two explanations are: When composite membranes are wet, water invades the pores, and the strength of the wet chitosan membrane, as well as that of the fibers themselves, is low.
Though alkali treatment damages the LPLA nets, it does not influence the tensile strength of the resulting membranes. Being made by freeze-drying, the composite membranes are full of pores, which discontinues the transmission of stress. The pores have a greater influence on the tensile strength of the structure, which is demonstrated by the insignificant variation in the tensile strength in Figure 4. This result is also in line with the finding of Sundararajan, which reveals that chitosan membrane without pores have 30 times the tensile strength of porous chitosan membranes [17].
Figure 5 shows the tensile strengths of AG, PG, and NG. The tensile strength of the chitosan membrane is lessened by PBS and thus cannot be measured. There
is no significant variation in the tensile strengths of AG, PG, and NG; therefore, the different sequences for gelation have no influence. In sum, the tensile strength of the composite membranes in this study is desirable, being superior to that of the sodium alginate cross-linked with calcium chlorine (35 KPa) in a study by Ueyama et al. [18]. Both our LPLA nets and composite membranes have a greater tensile strength, regardless of the different sequences of gelation.
The tensile strengths of the LPLA/chitosan composite membranes, the LPLA nets and the pure chitosan membranes are 1.3-1.6 MPa, 0.3 MPa, and 0.4-0.5 MPa, respectively. The tensile strength of the composite membrane is greater than the sum of that of both the LPLA net and chitosan membrane, indicating the addition of the net acted as a reinforcing agent. When the composite membranes are stretched, the force is conveyed to the nets by their connections with the chitosan, and then distributed via the thermal bonding points between the fibers, resulting in a better tensile strength than the sum of that of pure chitosan membrane and that of LPLA net.
**
**
**
a
b
Figure 5. Tensile strength of AG, PG, and NG as related to (with or without) immersion in PBS for 24 hours. Significance levels indicated by asterisks: **p > 0.001; compared to LPLA Net
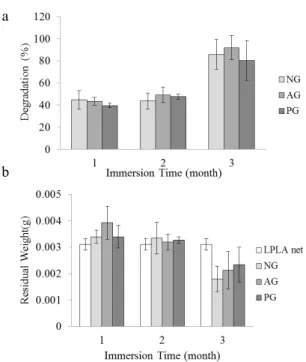
Figure 6. The degradation (a) residual weight (b) of the NG, AG, and PG as related to various immersion times in PBS.
Degradation
The time required for bones to recover from injuries of different degrees varies from 3 to 6 months; hence, the membranes used for GTR must have a slower degradation. Premature degradation allows gingival tissue to grow between the periodontal tissues, obstructing and eventually ceasing the growth of the alveolar bone. According to Figure 6 (a), degradation of NG in the first month is 45 %, which is lower than that of AG and PG; however, it is greater than that of AG and PG in the second month.
Figure 6 (b) shows that after immersion for 1 month, AG, PG, and NG have residual weights which are close to the average weight of a 1 × 1 cm2 LPLA net. This indicates the chitosan is completely dissolved in one month. After one month, the amount of chitosan in AG and PG is greater than that in NG, and after two months, the chitosan in AG, PG, and NG all dissolves in the PBS. The residual weight of membranes is the weight of the LPLA nets exclusively. In addition, PG and AG both undergo alkali treatment, which allows the chitosan and NaOH to interact and then form the membrane; during this period, the thermal bonding points are damaged by the alkali, resulting in a decrease in the tensile strength of the LPLA nets. Therefore, in the degradation test, the fibers fall apart as a result of
the strength deficiency in the LPLA nets. The nets’ structure is damaged and their overall weight decreases, in turn increasing their degradation. After immersion in the PBS for three months, degradation of all composite membranes reaches 70-80 % as the strength of the fibers when wet is lower than that when dry. Moreover, the alkali treatment weakens the strength of the thermal bonding points, fracturing the LPLA nets and greatly increasing the degradation.
The crosslinking with glutaraldehyde is commonly used to reduce the degradation ratio of chitosan, which is attained as a result of the bonding of the aldehyde group of glutaraldehyde and the amine group of chitosan. Another method to decrease the degradation ratio of chitosan is the use of plasma treatment or chemical grafting for a surface modification of the LPLA fibers, thereby chemically bonding the LPLA fiber and chitosan and thus mitigating the degradation of chitosan.
b a
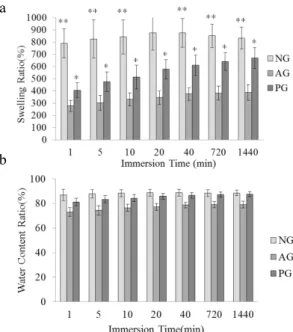
Figure 7. The swelling ratio(a) water content ratio (b)of the NG, PG, and AG as related to various immersion times in PBS. Significance levels indicated by asterisks: **p > 0.001; *p > 005; compared to AG.
Swelling Ratio
Figure 7 (a) reveals the swelling ratio of NG, AG, and PG as related to various immersion times in PBS. In the initial first minute, the swelling ratio of all samples reaches the maximum range; although it later increases with an increase in immersion time, its range is not as great as that observed in the first minute. Though Figure 7 (a) shows significant variations in swelling ratios of the composite membranes with and without gelation, the overall water content ratio of AG, PG, and NG is similar, as seen in Figure 7 (b), and the variation is not significant.
The composite membranes are made by freeze-drying, they are porous, causing the swelling ratio to reach its maximum. The value later increased due to the amount of PBS that the LPLA fibers and net structure absorbed, not significantly, however, increasing the swelling ratio. The swelling ratio of the NG is the greatest, followed by that of AG and PG. During the gelation between the chitosan and NaOH solution, the chitosan has a more compact and stable form which does not easily swell; thus, the swelling ratio is low. Figure 7 (a) shows the statistical analysis for the swelling ratio of the NG, AG, and PG, and their variation is significant at P < 0.05, indicating that there is a significant difference in the swelling ratio between any two of the NG, AG, and PG.
Water Contact Angle
A material’s hydrophilicity influences its adhesion with periodontal cells. Gingiva tissue is subject to having voids when attached to a low hydrophilic material, allowing bacteria to decay the alveolar bone through the voids or fast-growing connective tissues to grow through the voids, and eventually obstructing the growth of the alveolar bones. As composite membranes are made by freeze-drying, there are pores on the surface, giving them great hydrophilicity. Therefore, in the water contanct angle test, water is fast absorbed by the composite membrane before the angle between it and the surface can be measured. The contact angles of
NG, AG, and PG are determined to be 0°, indicating that all the composite membranes have good hydrophilicity.
Conclusion
This study successfully produces LPLA/chitosan composite membranes with various sequences of gelation—NG, AG, and PG. They are evaluated with different tests to determine their clinical application for periodontal diseases. LPLA nets have a even distribution of fibers. The air permeability of alkali-treated nets is 9.5 % greater than that of untreated nets. Alkali treatment does not significantly influence the tensile strength of composite membranes. The swelling ratio of NG is 51 % and 145 %, respectively, greater than that of AG and PG. However, the water content ratio of the NG is only 3.5 % and 15 %, respectively, greater than that of AG and PG. When comparing to the swelling ratio results, it can be seen that NG, AG, and PG have almost the same water contents. According to the results of the water contact angle test, the composite membranes are porous, resulting in a 0° water contact angle for NG, AG, and PG which indicates the good hydrophilicity of all composite membranes.
References
[1] R. C. Page and J. D. Beck,Int. Dent. J., 47(2), 61 ,1997.
[2] I. Aukhil, D. M. Simpson and T. V. Schaberg, J. Periodontal. Res., 18(6), 643, 1983.
[3] S. J. Card, R. G. Caffesse, B. A. Smith and C. E. Nasjleti, Int. J. Periodont. Rest.,
9(1), 59, 1989.
[4] Caffesse R.G., Nasjieti C.E., Morrison E.C. and Sanchez R., J. Periodontol., 65(6), 583, 1994.
[5] S. M. Kuo, S. J. Chang, G. C. C. Niu, C. W. Lan, W. T. Cheng and C. Z. Yang, J
Appl. Polym. Sci., 112(5), 3127, 2009.
[6] S. Aiba, N. Minoura, K. Taguchi and Y. Fujiwara, Biomaterials, 8(6), 481, 1987. [7] J. Rhoades and S. Roller, Appl. Environ. Microb., 66(1), 80, 2000.
[8] K. Y. Lee, W. S. Ha and W. H. Park, Biomaterials, 16(16), 1211, 1995.
[9] T. Wang, X. K. Zhu, X. T. Xue and D. Y. Wu, Carbohyd. Polym., 88(1), 75, 2012. [10] W. C. Lin, C. C. Lien, H. J. Yeh, C. M. Yu and S. H. Hsu, Carbohyd. Polym.,
94(1), 603, 2013..
[11] S. Das, A. Chaudhury and K. Y. Ng, Int. J. Pharmaceut., 406(1–2), 11, 2011. [12] L. Li, L. L. Wang, Y. Shao, R. Ni, T. T. Zhang and S. R. Mao, Int. J. Pharmaceut.,
450(1–2), 197, 2013.
[14] S. Elsaka and A. Elnaghy, J. Biomed. Res., 26(4), 288, 2012. [15] Y. S. Liu and H. I. Kim, Carbohyd. Polym., 89(1), 111, 2012.
[16] L. H. Fan, Y. M. Du, B. Z. Zhang, J. H. Yang, J. P. Zhou and J. F. Kennedy,
Carbohyd. Polym., 65(4), 447, 2006.
[17] V. M. Sundararajan and W. T. M. Howard, Biomaterials, 20(12), 1133, 1999. [18] U. Yoshiya, I. Kunio, M. Takamitsu, K. Takahiro, N. Hitoshi, S. Kazuomi and R.