Increased Incidence of juvenile onset systemic lupus erythematosus among children with asthma
Chang-Ching Wei, MD,a,b Cheng-Li Lin, MS,c,d Te-Chun Shen, MD,b,e Chi-Jung Chung, PhD, f,g and Tsai-Chung Li, PhDd
aDepartment of Pediatrics, China Medical University Hospital, Taichung, Taiwan; bCollege of Medicine, China Medical University, Taichung, Taiwan;
cManagement Office for Health Data, China Medical University Hospital, Taichung, Taiwan;
dDepartment of Public Health, China Medical University, Taichung, Taiwan;
eDivision of Pulmonary and Critical Care Medicine, Department of Internal Medicine, China Medical University Hospital, Taichung, Taiwan
fDepartment of Medical Research, China Medical University Hospital, Taichung, Taiwan;
gDepartment of Health Risk Management, College of Public Health, China Medical University, Taichung, Taiwan
Address correspondence to: Chi-Jung Chung, PhD; Department of Health Risk Management, College of Public Health, China Medical University; #91 Hsueh-Shih Road, Taichung City 404, Taiwan; Tel: 2205-3366 ext. 6505; Fax: +886-4-2207-0429; E-mail: cjchung@mail.cmu.edu.tw.
Short title: systemic lupus erythematosus in asthma
Key words: asthma, juvenile onset systemic lupus erythematosus Abbreviations used
Systemic lupus erythematous: SLE
Juvenile systemic lupus erythematous: JSLE T helper1 cells: Th1
T helper2 cells: Th2
Funding Source: The study was supported in part by the grants(DMR-101-061 and DMR-100-076) from China Medical University Hospital, Taiwan Department of Health Clinical Trial and Research Center and for Excellence (DOH102-TD-B-111-004), Taiwan Department of Health Cancer Research Center for Excellence
(DOH102-TD-C-111-005), Bureau of Health Promotion, Department of Health, R.O.C. (Taiwan) (DOH99-HP-1205), and International Research-Intensive Centers of Excellence in Taiwan (I-RiCE) (NSC101-2911-I-002-303).
Financial Disclosure: The authors have indicated that they have no financial relationships relevant to this article to disclose.
Conflict of Interest: None. Contributor’s Statement:
Chang-Ching Wei: Dr. Wei conceptualized and designed the study, drafted the initial manuscript, and approved the final manuscript as submitted.
Cheng-Li Lin, Chia-Hung Kao, Yen-Hsiu Liao, Jeng-Dau Tsai: carried out the initial analyses, reviewed and revised the manuscript, and approved the
final manuscript as submitted.
Yen-Jung Chang: coordinated and supervised data collection, critically reviewed the manuscript, and approved the final manuscript as submitted.
Abstract
Increased Incidence of juvenile onset systemic lupus erythematosus among children with asthma
Wei CC, Lin CL, Shen TC,Chung CJ, andLi TC Pediatr Allergy Immunol
Background:Children with systemic lupus erythematosus (SLE) especially have a more aggressive course and a worse outcome. Previous studies have revealed a possible link between SLE and allergy, but the nature of the relationship between these disorders remain unclear. This population-based cohort study is to investigate the incidence and risk of juvenile onset SLE (JSLE) among children with asthma. Methods: From 2000 to 2003, 120,939 children with newly diagnosed asthma and 483,756 randomly selected non-asthma controls were enrolled. A multivariate Cox proportional hazard regression model was used to measure the incidence rate and risk of JSLE in asthma compared to non-asthma cohorts.
Results: The overall incidence of JSLE was 2.52-fold greater in the asthma cohort than in the non-asthma cohort (3.49 vs. 1.53 per 100,000 person-years, 95% CI = 1.59-3.99). The increased JSLE risk was strongest in male gender (HR 3.02, 95% CI = 1.21-7.52) and children aged 6-10 years (HR 3.50, 95% CI = 1.75-7.02). The
medical visits, from 1.22 (95% CI =0.67-1.41) for those ≤ 2 visits/year to 5.88 (95% CI =3.43-10.1) for those > 2 visits/year (trend test p<0.001). The trends of increased JSLE risk declined over time.
Conclusion: In conclusion, the increased incidence of JSLE was observed among children with asthma. In the future, the mechanism of asthma on JSLE development should be better elucidated to establish innovative disease intervention programs.
Introduction
Systemic lupus erythematosus (SLE) is a severe multi-systemic autoimmune disease, characterized by autoantibody overproduction, accumulation of immune complexes, and subsequent organ damage (1, 2). Up to 20% of patients with SLE have an onset during childhood (3, 4). Children with SLE have more serious organ involvement, a more aggressive course and a worse outcome (3, 4). The annual incidence of juvenile onset SLE (JSLE) is less than one per 100,000 children per year. The low incidence makes it hard to perform a study with a substantial number of patients (5). To date, SLE has neither a specific treatment for disease healing nor a diagnostic tool for disease prevention. Long-term immunosuppressive therapy is still the mainstay of treatment, with multiple adverse side effects observed. Although great efforts have put towards better understanding and developing treatment strategies for SLE over the past 60 years, the etiology of SLE has not clearly established (1, 2).
Previous studies have described the association between SLE and allergic diseases but such results have been somewhat controversial(6-10). Recently, studies revealed elevated serum IgE in SLE patients and that this was associated with disease activity (11-16).Allergic disorders, such as asthma, are considered T-helper type 2 (Th2) related immune response with IgE mediated inflammation at sites of persistent or repetitive exposure to allergens (17). In contrast, SLE is considered T-helper type 1
(Th1) related immune response with the expansion of autoreactive T and B cells and autoantibody production and immune complex deposition at sites of involved system (1, 2). Whether the Th2 related allergic diseases contribute to the development of Th1 related autoimmune disease remains unknown. Asthma is on the rise worldwide in past decades, with increasing interest on their long-term health consequences. In addition, human epidemiologic studies that explore the cause and effect relationship between asthma and JSLE in children are lacking. Therefore, we performed a population-based cohort study to examine the hypothesis of childhood onset asthma may contribute to the development of SLE.
Methods Data Sources
The implementation of Taiwan’s National Health Insurance (NHI), as a single-payer, social insurance plan, supported coverage of almost all residents of Taiwan with minimal copayment fees required (18, 19). The Bureau of NHI (BNHI) has contracted with 99% of hospitals with total coverage of the population as high as 99%. NHI also provides datasets for corresponding research on issues related to cost, quality of health services, medical practice patterns, accessibility to health care programs and treatment outcomes at national or local levels
(http://www.nhi.gov.tw/english/index.aspx) (18, 19). This study used a dataset consisting of a randomly selected sample of half of all insured children in Taiwan. This study was exempt from the Institutional Review Board because the NHIRD database contains de-identified person identifiers and is publicly available through the proper application process. The International Classification of Disease, Ninth
Revision (ICD-9), was used to define diagnostic disease codes, which had been reported with high accuracy and validity for study using NHIRD database (20, 21). Study design and subjects
A total of 120,939 child patients (<18 year old) newly diagnosed with asthma (ICD-9 code 493 and 494) from 2000 and 2003 were identified as the asthma cohort. To improve diagnostic accuracy, only those having at least 3 consecutive corresponding
diagnoses of asthma could be designated as having asthma. The diagnosis date defined as the index date used to initiate follow-up for calculation of person-years. Child patients with asthma and SLE (ICD-9 code 710.0) recorded before the index dates were excluded. For each child with asthma, we randomly selected 4 non- asthma children with frequency matched by sex, age (in interval of 5 year), urbanization of residence area, parental occupation, and baseline year. The SLE events diagnosis was confirmed by the International Classification of Diseased, 9th Revision of the Clinical Modification (ICD-9-CM), and the Registry for Catastrophic Illness Patient Database (RCIPD), which includes selected major injuries or illnesses and is published by the Department of Health, Executive Yuan. To register as SLE, the diagnosis must be made by board-certified specialist and the application is further reviewed and approved by the Bureau of NHI, which ensures the accuracy and reliability of the diagnosis. Each subject was monitored from the index date until a new diagnosis of SLE was made, the end of 2010 or censored because of death or withdrawal from the insurance program.
Statistical analysis
The sociodemographic variables in this study were sex, age, urbanization, and parent’s occupation. The urbanization level was categorized by the population density of the residential area into 4 levels, with level 1 as the most urbanized and level 4 as
the least urbanized. Data analysis first compared the demographic characteristics between the asthma cohort and the non-asthma cohort, which were examined by using Chi-square test. We evaluated the overall incidence and incidence stratified by age and sex for both cohorts. Univariate and multiple Cox proportional hazards regression model were used to estimate the specific hazard ratio (HR) and 95% confidence intervals (CI) of SLE for the asthma cohort compared to the non-asthma cohort. The Cox model was also used to estimate the HR of SLE associated with the annual average of medical visits by asthma, compared to the non-asthmatic cohort. We further assessed the role of asthma duration using time-dependent covariates (≤3, 4-6, and >6 years since asthma diagnosis) on SLE risk. The study used the SAS software (version 9.2 for windows; SAS Institute Inc., Cary, NC) for all data analyses.
Results
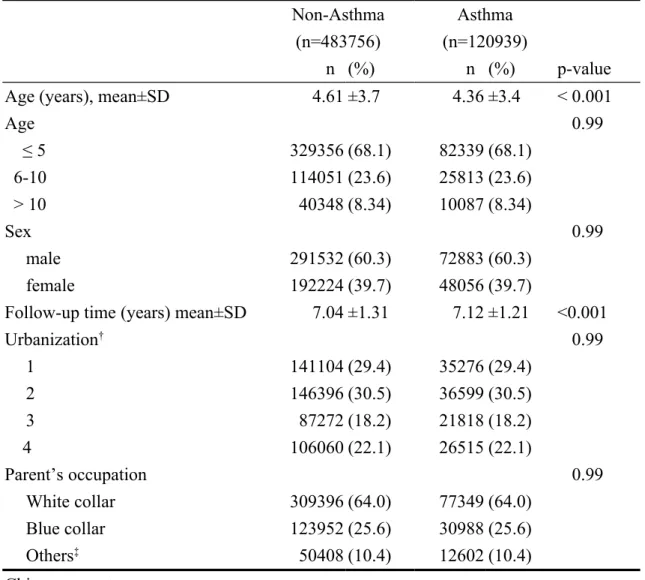
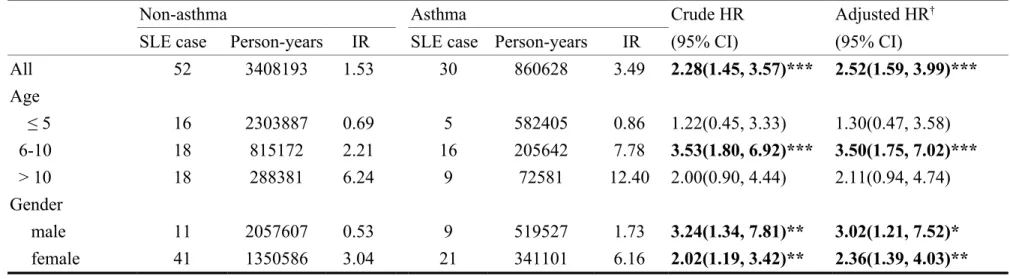
Demographic characteristics are shown in Table 1. The mean age was 4.36±3.44 years for the asthma cohort and 4.61±3.68 years for non-asthma cohort. More than half of children with asthma were age ≤ 5 years and 60.3% was boys. Patients with asthma were more likely to reside in urban areas (59.9%). Most of their parent’s occupations were white collar related (64.0%). The overall incidence of SLE was higher in the asthma cohort than in non-asthma cohort (3.49 vs. 1.53 per 100,000 person-years) (Table 2). The Cox proportional hazard regression model revealed that children with asthma were 2.52 times more likely to develop SLE (95% CI=1.59-3.99) after controlling for age. The incidence of SLE involve a greater relative risk estimate for those aged 6-10 years (adjusted HR=3.50, 95% CI=1.75-7.02). The risk of SLE was significantly increased for children with asthma regardless of gender (adjusted HR=3.02, 95% CI=1.21-7.52 for boys; and adjusted HR=2.36, 95%
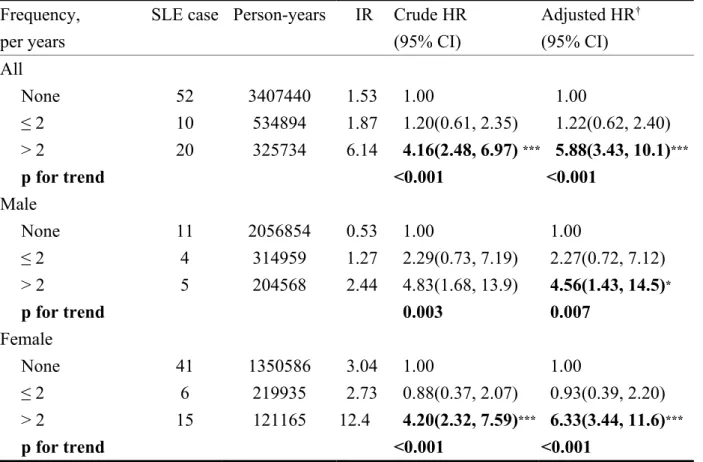
CI=1.39-4.03 for girls). The association between the average number of medical visits because of asthma and SLE development was measured using annual average
frequency (Table 3). Overall the risk of SLE increased from 1.22 in children with ≤2 annual medical visits for asthma to 5.88 in children with >2 visits (trend test
p<0.0001). The risk for both genders was shown to have similar results. During the first 3 years after asthma diagnosis, the SLE incidence rate was greater in the
asthmatic children than in the non-asthma cohort (3.59 vs. 1.11 per 100,000 person-years), with an adjusted HR of 3.55 (95% CI = 1.66-7.60) (Table 4). A similar result was also shown in those with 4-6 years of follow-up for their asthma.
Discussion
By far, this is the first population-based cohort study to investigate the incidence of JSLE in childhood with asthma compared to a non-asthma control group. The results show a significantly increased incidence rate of JSLE in childhood among those with asthma regardless of gender. During the first 6 years after asthma diagnosis, the clinic physicians should pay close attention to the expression of SLE-related biomarkers. JSLE is rare disease with an estimated annual incidence of 0.36-0.9 per 100,000 children per year, with a significantly higher incidence among non-Caucasian children, especially Asians, and African Americans (5, 22). The current study revealed an incidence rate of 1.5 per 100,000 person per year in non-asthma Han Chinese children, which is similar to previous study population. Interestingly, a significantly increased incidence rate of 3.49 per 100,000 persons per year was observed in asthmatic children. In addition, the incidence rate further increased in children with asthma older than 8 (11.95 per 100,000 person per year) and those with female gender (6.16 per 100,000 person per year). More medical visits for asthma with increased risk for incidence rate of JSLE indicated the risk of JSLE increasing with more serious or uncontrolled airway inflammation of asthma.
contributors to development or severity of disease are evident but their roles are poorly understood to date. Only a few studies with limited numbers of patients investigating the association between allergic diseases and SLE have produced inconsistent results (6-9). Most of these studies were cross sectional in nature, mainly focusing on adult patients (6-9). The causality between allergic disease and SLE in children has not been clearly established. The strength of this study is that it is the first cohort study to investigate the precise quantification of the incidence of JSLE in children with asthma. Our results, contrary to the Th1/Th2 paradigm (23-25), revealed increased incidence rate and risk of JSLE in asthma children cohort and the risk further increased with severity of asthma symptoms. Second, the current study is a population-based study which minimizes the selection bias in other case-control studies. Third, the diagnosis of asthma by physicians other than using questionnaires which minimizes the selection bias and recall bias. SLE was defined using ICD-9-CM, and RCIPD, which ensures better diagnosis validity. Fourth, we adjusted the possible confounding factors, including age, gender, urbanization of residence area, parental occupation, and baseline year. Ethic influence was not considered to be adjusted because most of the population in Taiwan is ethnic Chinese.
Elevated total serum IgE has also been described in SLE patients (11-16). Some studies suggest that elevated IgE correlates with disease activity and nephritis (11, 12,
14).A recent study, in a mouse model of SLE and in human SLE subjects, reported that the activation of the basophil by autoreactive IgE-containing immune complexes serves to amplify the production of autoantibodies and contributes to the pathogenesis of disease (15, 16) The above research advances support our finding of Th2
environment in contributing to the development of SLE.
There are several limitations to this study that are worth noting. Period of follow-up was about 10 years, which may not be enough to see the long-term relationship between asthma and SLE. However, the prominent positive effect of asthma on JSLE was still observed. Even though the current study is the largest population to investigate the incidence of JSLE in an asthma cohort, JSLE is a very rare disease and subsequent risk analysis of more frequently occurring presentations of JSLE, such as lupus nephritis, thrombocytopenia, encephalopathy and so on cannot be performed due to insufficient patient numbers. In addition, a number of possible confounding variables, including body mass index and family history of allergic diseases, which are associated with allergic diseases were not included in our database. Another limitation is the lack of data for the study group on genetic and behavioral factors which might affect the risks JSLE and allergic diseases.
In conclusion, this population-based cohort study revealed a significantly increased incidence rate of JSLE in children with asthma regardless of gender. In the future,
more research should look to explore the role of Th2 mediated allergic disease contributing to the development of Th1 mediated autoimmune disease, and provide a potential strategy of diagnosis and therapeutic promise in disease intervention.
ACKNOWLEDGEMENTS
The study was supported in part by the study projects (DMR-101-061 and DMR-100-076) in our hospital; Taiwan Department of Health Clinical Trial and Research Center and for Excellence (DOH102-TD-B-111-004), Taiwan Department of Health Cancer Research Center for Excellence (DOH102-TD-C-111-005); Bureau of Health
Promotion, Department of Health, R.O.C. (Taiwan) (DOH99-HP-1205); and
International Research-Intensive Centers of Excellence in Taiwan (I-RiCE) (NSC101-2911-I-002-303).
REFERENCES
1. Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med 2008: 358: 929-39.
2. Lipsky PE. Systemic lupus erythematosus: an autoimmune disease of B cell hyperactivity. Nat immunol 2001: 2: 764-6.
3. Brunner HI, Gladman DD, Ibanez D, Urowitz MD, Silverman ED. Difference in disease features between childhood-onset and adult-onset systemic lupus
erythematosus. Arthritis Rheum2008: 58: 556-62.
4. Tucker LB, Uribe AG, Fernandez M, et al. Adolescent onset of lupus results in more aggressive disease and worse outcomes: results of a nested matched case-control study within LUMINA, a multiethnic US cohort (LUMINA LVII). Lupus 2008: 17: 314-22.
5. Gardner-Medwin JM, Dolezalova P, Cummins C, Southwood TR. Incidence of Henoch-Schonlein purpura, Kawasaki disease, and rare vasculitides in children of different ethnic origins. Lancet2002: 360: 1197-202.
6. Sequeira JF, Cesic D, Keser G, et al. Allergic disorders in systemic lupus erythematosus. Lupus 1993: 2: 187-91.
7. Sekigawa I, Yoshiike T, Iida N, Hashimoto H, Ogawa H. Allergic disorders in systemic lupus erythematosus: prevalence and family history. Lupus 2002: 11: 426-9.
8. Cooper GS, Dooley MA, Treadwell EL, St Clair EW, Gilkeson GS. Risk factors for development of systemic lupus erythematosus: allergies, infections, and family history. J Clin Epidemiol2002: 55: 982-9.
9. Sekigawa I, Yoshiike T, Iida N, Hashimoto H, Ogawa H. Allergic diseases in systemic lupus erythematosus: prevalence and immunological considerations.
Clin Exp Rheumatol2003: 21: 117-21.
10. Wozniacka A, Sysa-Jedrzejowska A, Robak E, Samochocki Z, Zak-Prelich M. Allergic diseases, drug adverse reactions and total immunoglobulin E levels in lupus erythematosus patients. Mediators inflamm 2003: 12: 95-9.
11. Laurent J, Lagrue G, Sobel A. Increased serum IgE levels in patients with lupus nephritis. Am J Nephrol 1986: 6: 413-4.
12. Elkayam O, Tamir R, Pick AI, Wysenbeek A. Serum Ige Concentrations, Disease-Activity, and Atopic Disorders in Systemic Lupus-Erythematosus.
Allergy 1995: 50: 94-96.
13. Sekigawa I, Tokano Y, Yoshiike T, Iida N, Hashimoto H, Ogawa H. Relationship between serum IgE and autoantibody levels in SLE patients. Clin Exp Rheumatol
14. Rebhun J, Quismorio F, Dubois E, Heiner DC. Systemic Lupus-Erythematosus Activity and Ige. Ann Allergy 1983: 50: 34-36.
15. Charles N, Hardwick D, Daugas E, Illei GG, Rivera J. Basophils and the T helper 2 environment can promote the development of lupus nephritis. Nat Med 2010: 16: 701-7.
16. Charles N, Rivera J. Basophils and Autoreactive IgE in the Pathogenesis of Systemic Lupus Erythematosus. Cur Allergy Asthma Rep 2011: 11: 378-87. 17. Woodruff PG, Modrek B, Choy DF, et al. T-helper type 2-driven inflammation
defines major subphenotypes of asthma. Am J Respir Crit care Med 2009: 180: 388-95.
18. Davis K, Huang AT. Learning from Taiwan: experience with universal health insurance. Ann Intern Med 2008: 148: 313-4.
19. Cheng TM. Taiwan’s National Health Insurance system: high value for the dollar. In Okma KGHaC, L. ed. Six Countries, Six Reform Models: The Health Reform Experience of Israel, the Netherlands, New Zealand, Singapore, Switzerland and Taiwan. 2009: 71-204.
20. Cheng CL, Kao YHY, Lin SJ, Lee CH, Lai ML. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan.
Pharmacoepidem Dr S 2011: 20: 236-42.
21. Wei CC, Yu IW, Lin HW, Tsai AC. Occurrence of infection among children with nephrotic syndrome during hospitalizations. Nephrology2012: 17: 681-8.
22. Levy DM, Peschken CA, Tucker LB, et al. Influence of ethnicity on childhood-onset systemic lupus erythematosus: results from a multiethnic multicenter Canadian cohort. Arthritis care Res 2013: 65: 152-60.
23. Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med2002: 347: 911-20.
24. Okada H, Kuhn C, Feillet H, Bach JF. The 'hygiene hypothesis' for autoimmune and allergic diseases: an update. Clin Exp Immunol 2010: 160: 1-9.
25. Romagnani S. The increased prevalence of allergy and the hygiene hypothesis: missing immune deviation, reduced immune suppression, or both? Immunology 2004: 112: 352-63.
Table 1. Comparisons of demographic characteristics between children with asthma and non-asthma controls
Non-Asthma Asthma
(n=483756) (n=120939)
n (%) n (%) p-value
Age (years), mean±SD 4.61 ±3.7 4.36 ±3.4 < 0.001
Age < 0.99 ≤ 5 329356 (68.1) 82339 (68.1) 6-10 114051 (23.6) 25813 (23.6) > 10 40348 (8.34) 10087 (8.34) Sex < 0.99 male 291532 (60.3) 72883 (60.3) female 192224 (39.7) 48056 (39.7)
Follow-up time (years) mean±SD 7.04 ±1.31 7.12 ±1.21 <0.001
Urbanization† < 0.99 1 141104 (29.4) 35276 (29.4) 2 146396 (30.5) 36599 (30.5) 3 87272 (18.2) 21818 (18.2) 4 106060 (22.1) 26515 (22.1) Parent’s occupation < 0.99 White collar 309396 (64.0) 77349 (64.0) Blue collar 123952 (25.6) 30988 (25.6) Others‡ 50408 (10.4) 12602 (10.4) Chi-square test,
‡Other occupations included primarily retired, unemployed, or low income populations. †: The urbanization level was categorized by the population density of the residential area into 4 levels, with level 1 as the most urbanized and level 4 as the least urbanized.
Table 2. The incidence rate and risk of systemic lupus erythematosus (SLE) in children with asthma and non-asthma controls, stratified by age and gender
Non-asthma Asthma Crude HR Adjusted HR†
SLE case Person-years IR SLE case Person-years IR (95% CI) (95% CI)
All 52 3408193 1.53 30 860628 3.49 2.28(1.45, 3.57)*** 2.52(1.59, 3.99)*** Age ≤ 5 16 2303887 0.69 5 582405 0.86 1.22(0.45, 3.33) 1.30(0.47, 3.58) 6-10 18 815172 2.21 16 205642 7.78 3.53(1.80, 6.92)*** 3.50(1.75, 7.02)*** > 10 18 288381 6.24 9 72581 12.40 2.00(0.90, 4.44) 2.11(0.94, 4.74) Gender male 11 2057607 0.53 9 519527 1.73 3.24(1.34, 7.81)** 3.02(1.21, 7.52)* female 41 1350586 3.04 21 341101 6.16 2.02(1.19, 3.42)** 2.36(1.39, 4.03)** IR, incidence rate, per 100,000 person-years
HR, hazard ratio † adjusted for age
Table 3. The incidence rate and risk of systemic lupus erythematosus (SLE) in children with asthma, stratified by frequency of annual medical visits and gender
Frequency, per years
SLE case Person-years IR Crude HR (95% CI) Adjusted HR† (95% CI) All None 52 3407440 1.53 01.00 01.00 ≤ 2 10 534894 1.87 01.20(0.61, 2.35) 01.22(0.62, 2.40) > 2 20 325734 6.14 04.16(2.48, 6.97) ***05.88(3.43, 10.1)*** p for trend <0.001 <0.001 Male None 11 2056854 0.53 01.00 01.00 ≤ 2 4 314959 1.27 02.29(0.73, 7.19) 02.27(0.72, 7.12) > 2 5 204568 2.44 04.83(1.68, 13.9) 04.56(1.43, 14.5)* p for trend 00.003 00.007 Female None 41 1350586 3.04 01.00 01.00 ≤ 2 6 219935 2.73 00.88(0.37, 2.07) 00.93(0.39, 2.20) > 2 15 121165 12.4 04.20(2.32, 7.59)***06.33(3.44, 11.6)*** p for trend <0.001 <0.001
IR, incidence rate, per 100,000 person-years HR, hazard ratio
† adjusted for age
Table 4. The incidence rate and risk of systemic lupus erythematosus (SLE) in children with asthma, stratified by follow-up years
Non- Asthma Asthma
Follow time SLE case Person-years IR SLE case Person-years IR cHR (95% CI) aHR† (95% CI) ≤3 years 16 1443281 1.11 13 362527 3.59 3.24(1.56, 6.73)** 3.55(1.66, 7.60)** 4-6 years 15 1378414 1.09 8 348257 2.30 2.11(0.90, 4.98) 2.46(1.03, 5.86)* >6 years 21 585746 3.59 9 149844 6.01 1.67(0.77, 3.65) 1.82(0.83, 4.01) IR, incidence rate, per 100,000 person-years
HR, hazard ratio † adjusted for age * p<0.05, ** p<0.01