57
Journal of Organometaliic Chemistry, 371 (1989) 57-69 Else&r Sequoia S.A., Lausanne - Printed in The Netherlands
JOM 09971
Synthesis, spectra and crystal structure
of (CO),(NO)Cr( $-C,H,)CH,(
$-C,H,)Fe[vinyl-(
q5-C,H,)]
Yu-Pin Wang *, Tso-Shen Lin, Rong-Sen Shyu, Jin-Mei Hwu, Department of Chemistry, Tunghai University, Taichung, Taiwan (R. 0. C.)
Yu Wang, and Ming-Chu Cheng
National Taiwan University, Taipei, Taiwan (R. O.C.) (Received October 21st, 1988)
Abstract
Friedel-Crafts acetylation of [ q5-(ferrocenylmethyl)cyclopentadienyl]dicarbonyl- nitrosylchromium (hereafter called cynichrodenylferrocenyhnethane) (3) has af- forded (1’-acetylferrocenyl)~cynichrodenylmethane (5) in 63% yield. Reduction of 5 with sodium borohydride gives 1-[l’-(cynichrodenylmethyl)ferrocenyl]ethanol (6). Dehydration of 6 produces [ $-(l’-vinylferrocenyl)methyl]cyclopentadienyl]di- carbonylnitrosylchromium (7), a promising organometallic monomer, in 80% yield. The structure of 7 has been solved by an .X-ray diffraction study: space group Pbca, a 10.966(2), b 12.595(l), c 26.137(3) A, and 2 = 8. The vinyl group and the exocyclic carbon are in the 1,3’-configuration with an average rotation of 7 o of the corresponding Cp(Fe) rings from the eclipsed configuration.
Introduction
The synthesis of novel organometallic polymers and studies of their properties [l-6] have accelerated in recent years. Reports on the polymerization and copo- lymerization of organometallic carbonyl monomers are rare in contrast to their metallocene analogs. Furthermore, only a few organochromium monomers have been synthesized and polymerized. These include $-styrenetricarbonylchromium, ~6-(benzylacrylate)tricarbonylchromium [7], and q6-(2-phenylethylacrylate)tricar- bonylchromium [ 81.
* Author to whom correspondence should be addressed. 0022-328X/89/%03.50 0 1989 Elsevier Sequoia S.A.
0
0 Cfco/
1
‘co
NO ferroccne cynichroclcne (1) (2)Since the compound cynichrodenyl ferrocenyl methane (3) containing both cynichrodenyl and ferrocenyl groups has been prepared by reduction of cynichro- deny1 ferrocenyl ketone (4) with lithium-aluminum hydride-aluminum chloride [9,10], it is of interest to prepare some vinyl-substituted derivatives of 3 as mono- mers for the synthesis of new organometallic polymers. Further, while the chemistry of dicarbonylcyclopentadienylmtrosyl complexes of chromium has become the subject of considerable study, the crystal structure and 13C NMR of these com- plexes have not been examined thoroughly [ll-151. Herein, we reported the pre- paration and spectra of 5-7 and crystal structure of (C0)2(NO)($-CsHq)CH2(~5-
C,H,)Fe[vinyl-(775-C,H,)1 (7.
Experimental
All operations were carried out under a nitrogen atmosphere by means of Schlenk techniques. Trace oxygen in the nitrogen was removed by BASF catalyst and the deoxygenated nitrogen was dried with molecular sieve 3A and P,O,. Hexane, pentane, benzene, toluene and dichloromethane were dried over calcium hydride and freshly distilled under argon from calcium hydride. Diethyl ether was dried over sodium and redistilled under argon from sodium-benzophenone. All other solvents were used as commercially obtained.
Column chromatography was carried out under nitrogen using Merck Rieselgel 60. The silica gel was heated with a heat gun while mixing in a rotary evaporator attached to a vacuum pump for 2 h to remove water and oxygen. The silica gel was kept under nitrogen before use. Cynichrodenylferrocenylmethane (3) was prepared according to the literature procedure [lo].
‘H and r3C (300 and 400 MHz) NMR spectra were obtained on a Bruker AM-300-WB or AM-400 spectrometer. ‘H and 13C were referenced to tetramethyl-
silane. Infrared spectra were recorded on a Perk&Elmer 682 spectrophotometer. Microanalyses were carried out in Microanalytic Laboratory at National Taiwan University.
Preparation of (I ‘-acetylferrocenyI)cinychrodenylmethane (5)
Acetyl chloride (0.5 ml, 6.8 mmol) was stirred with aluminum chloride (1.8 g, 13.5 mmol) in 60 ml methylene chloride for 2 h at room temperature to give a yellow solution of the corresponding acylium ion. The solution was filtered from excess AlCl,. Subsequently, the filtrate was added dropwise to a solution of cyni- chrodenylferrocenylmethane (3) (2.7 g, 6.7 mmol) in 50 ml methylene chloride at 0 * C. After the addition was completed, the reaction mixture was allowed to stir at
59
room temperature for 12 h. The reaction was then cooled to
0"
C
and slowlyhydrolyzed with 50 ml of ice followed by 2 drops of concentrated hydrochloric acid. The aqueous and organic layers were separated, and the aqueous layer was extracted twice with methylene chloride. The combined organic portion was washed once with water, once with a sodium bicarbonate solution, once again with water, and dried with anhydrous magnesium sulfate. The solution was filtered, concentrated to 50 ml under vacuum, silica gel (20 g) was added, and the solvent removed under vacuum. The residue was added to a dry-packed column (1.8 cm X 10 cm) of silica gel. Elution of the column with hexane/ether (10/l) gave an orange band which upon removal of solvent under vacuum gave 5 1.7 g (63%) m.p. 83-84OC. IR (CH,Cl,) 202O(vs), 194O(vs), 169O(vs), 1664(m,sh); mass spectrum, m/e 443 (W). Analysis: Found: C, 54.54; H, 4.05; N, 3.21. C,H$rFeNO, calcd.: C, 54.20; H, 3.87; N, 3.16%.
Preparation of {[(I -a-hydroxyethyl)f errocenyl]methylcyclopentadienyl}dicarbonyl-
nitrosykhromium (I -[l ‘-(cynichrodenylmethyl)-ferrocenyl]ethanol) (6)
(1’-Acetylferrocenyl)cynichrodenylmethane (5) (1.8 g, 4.06 mmol) was dissolved in 100 ml of ethanol, sodium borohydride (0.7 g, 1.84 mmol) was added, and the mixture was refluxed for 30 min. To this solution was added 20 ml of 6N sodium hydroxide, and the reaction mixture continued to reflux for 15 min. The solution was concentrated by removing the solvent under aspirator vacuum. The residue was extracted with ether and then dried with anhydrous magnesium sulfate. The solution was filtered, concentrated to 50 ml under aspirator vacuum. Silica gel (2 g) was added and the solvent removed under vacuum. The residue was added to a dry-packed column (1.8 cm X 9 cm) of silica gel. Elution of the column with hexane/ether (6/l) gave an orange band which upon removal of the solvent gave 6 1.71 g (95%). Melting point 52-53OC. IR (CH&l,): 3200-3600 (bs), 2032(vs), 196O(vs), 1715(vs); mass spectrum, m/e 445(M+).
Analysis. Found: C, 54.04; H, 4.12; N, 2.99. C,H,,CrFeNO, calcd.: C, 53.93; H, 4.30; N, 3.15%.
Preparation of [q5-(1 ‘-vinylferrocenyl)methylcycZopentadienyl]dicarbonylnitrosylchro-
mium (7)
1-[l’-(Cynichrodenylmethyl)ferrocenyl]ethanol (6) (1 g, 2.24 mmol), anhydrous cupric sulfate (3 g, 18.73 mmol), and 5 mg of hydroquinone were dissolved in 100 ml of toluene. The mixture was refluxed for 45 min and then cooled to room temperature. After the filtration the solvent was removed under vacuum. The residue was extracted with ether and dried with anhydrous magnesium sulfate. The solution was filtered, concentrated to 50 ml under vacuum. The residue was added to a dry-packed column (1.8 cm X 9 cm) of silica gel. Elution of the column with hexane gave an orange band which upon removal of the solvent gave 7 0.81 g (84%). An analytical sample was obtained by recrystallization from hexane/ether as red crystals: m-p. 56-57” C. An X-ray sample was obtained by solvent evaporation method from hexane/ether at 0 o C. IR (CH,Cl 2) 203O(vs), 194O(vs), 169O(vs); mass spectrum, m/e 427 (AI+).
Analysis. Found: C, 56.71; H, 3.96; N, 3.37. CZOH,,CrFeNO, calcd.: C, 56.23; H, 4.01; N, 3.28%.
60
X-ray diffraction analysis of 7
The X-ray diffraction intensity data were collected on a CAD4 diffractometer in the 8/28 scan fashion with a graphite monochromated MO-K, radiation. The other experimental detail is given in Table 1.
The structure was determined by heavy atom method. The full matrix least squares refinements were based on F. The atomic scattering factors, fO, were taken from ref. Uk, the anomalous dispersion corrections were included based on ref. 16b. The secondary extinction corrections were applied. All the data processing were done on a PDP-11 and VAX 11/785 using NRCC programs [17].
Table 1
Summary of crystal data and intensity collection Empirical formula
Color
crystal size (mm3) Space group Unit cell dimensions
Volume Formula units/cell Formula weight Density (talc.) Absorption coefficient WOO) Diffractometer used Radiation Temperature Monochromator 28 range Scan type Scan speed Scan range Background measurement Standard reflections Index ranges Reflections collected Unique reflections Final residuals Goodness-of-fit Largest and mean A/o Data to parameter ratio Largest difference peak Largest difference hole No. of variables S g u (extinction coefficient) * E;p"b," =F,,(l+ g/3F:)-“’ C,,,H,,NO,CrFe Orange 0.3 X0.5 x0.6 mm orthorhombic, P&J a 10.966(2) A b 12.595(l) A c 26.137(3) A 1032.73 A3 8 427 AMU 1.57 g/cm3 14.1 cm-’ 1744 e- CAD4 MO-K, (h 0.7107 A) 27OC Graphite crystal 2t050° e/2 e Variable; 20/20 to 20/3 deg/min 2(0.8 +0.35 tan 8)
Stationary counts with l/4 of total scan time at each side of the scan
-5 0 4 i
-5 0 -4 )
for every 2 hour 0 -8 -3 0~h~13,0~k~15,091I31 3641 3163 (2074 Z =- 2,5a (I)) R 3.518, R, 2.6% 2.319 0.166,0.03 8.78/l 0.27 e-/A3 - 0.27 e-/A3 236 2.32 9.0x10-5
61 Cp(CrJ Cp’iFe) cpum Cp’(Fcl &Fe) (3) Cptcr) Cp’(Fel (6) ii CHyC+AICi,- C--H3 II *. Cp”(Fe1 o (5) cp (0) Cp’(FeJ
c<
1 ‘co“\
e
/ b NO ccc \ Cp? Fe) HA (71Results and discussion
Preforming the Perrier-type complex [14] of acetyl chloride and aluminum chloride in the absence of 3 and then subsequently allowing this complex to react with 3 led to the acetylated derivative 5 in 63% yield. Reduction of ketone 5 with sodium borohydride in 95% ethanol produced the corresponding secondary alcohol 6 in high yield. Dehydration of 6 in refhucing toluene using anhydrous cupric sulfate gave 7 in 80% yield based on 5.
All compounds 5-7 exhibit two carbonyl stretching bands, the symmetric mode occurring at 2020-2032 cm-’ and the asymmetric mode at 1940-1960 cm-‘. A nitrosyl stretching band is also observed at 1690-1715 cm-‘. The normal absorp- tion of the acetyl group of 5 is somewhat obscured by the NO stretching band and exhibited a shoulder on the v(N0) at 1664 cm-‘.
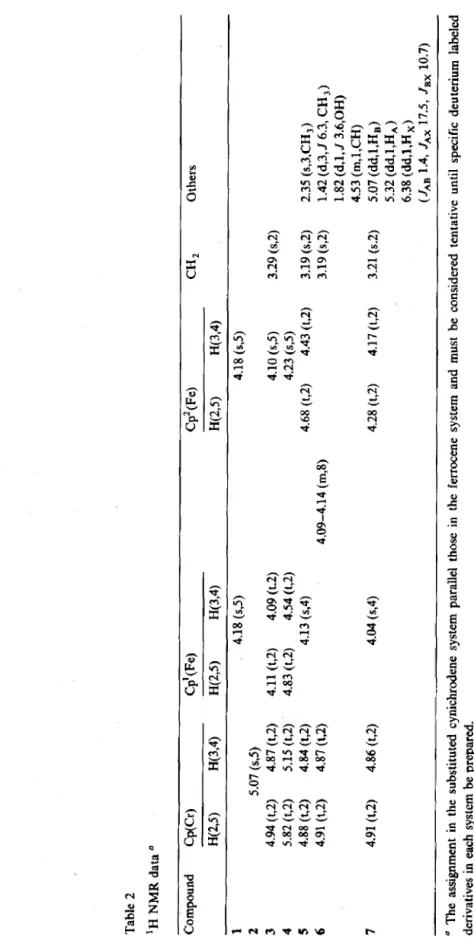
The lH NMR spectrum of 5 exhibits a singlet cyclopentadienyl resonance at 6 4.13 corresponding to the protons of Cp(Fe), a pair of apparent triplets at S 4.43 and 4.68 for the protons of Cp2(Fe), another pair of apparent triplets at S 4.84 and 4.88 corresponding to the protons of Cp(Cr), and a 2H singlet at 6 3.19 for methylene protons (Table 2). As expected, Cp’(Fe) experienced a stronger carbonyl deshielding effect than the remote Cp(Cr) and Cp’(Fe) rings.
The alcohol 6, in which the hydroxyl substituted carbon is chiral, and thus expected to exhibit a A,BB’ pattern for the protons of Cp’(Fe). It would consist of two closely spaced quartets of relative intensity 1H downfield and a triplet of relative intensity 2H upfield. The two downfield quartets can be assigned to the H(2,5) protons since the protons nearer to the alcohol would be expected to show the greater diastereotopic effect, whereas a proton at either the 3- and 4-position
would show little or no diastereotopic effect. However, since the chemical shifts of Cp(Fe) and Cp’(Fe) protons overlap, a multiplet of relative intensity of 8H at S 4.09-4.16 was observed. Definite assignments could not be made.
Table 2 ‘H NMR data a Compound WCr) H(2S) H(3,4) CP’(W H(2S) H(3,4) Cp2(W H(2S) H(3,4) CH, Others 1 4.18 (s,5) 2 5.07 (SJ) 3 4.94 (t,z) 4.87 (&2) 4.11 (t,2) 4.09 (t.2) 4 5.82 (t,2) 5.15 (t,Z) 4.83 (t,2) 4.54 (t,2) 5 4.88 (t,l) 4.84 (t,2) 4.13 (s,4) 6 4.91 (t,2) 4.87 (t,Z) 4.09-4.14 (m,8) 7 4.91 (t,2) 4.86 (t,2) 4.04 (s,4) LI The assignment in the substituted cynichrodene system parallel those in the ferrocene system and must be considered tentative until specific deuterium labeled derivatives in each system be prepared. 4.18 (0) 4.10 (s,5) 3.29 (s,2) 4.23 (s,5) 4.68 (t,Z) 4.43 (&2) 3.19 (s,2) 2.35 (s,3,CH,) 3.19 (s,2) 1.42 (dJ,J 6.3, CH,) 1.82 (d,l, J 3.6,OH) 4.53 (m,l,CH) 4.28 (t,2) 4.17 (t,2) 3.21 (s.2) 5.07 (dd,l,H,) 5.32 (dd,l,H,) 6.38 (dd,l,H,) (J*n 1.4, JAX 17.5, Jax 10.7) __ - -- __ - - .- - ..- - -- - - - - __
Table 3 13C[H] NMR’ Compound Cp(Cr) C&Fe) Cp’(Fe) CH, Cr-C=O >C=O Others C(l) q2,5) (C(3,4) C(1) c(2,5) C(3,4) C(l) ~(2~5) C(3,4) 1 67.88 (C(l-5)) 67.88 (C(l-5)) 2 ,90.31 (cql-5)) 237.10 3 113.41 90.09 88.66 88.17 67.73 68.47 68.68 (C(l-5)) 28.59 237.62 4 103.03 93.89 91.06 78.39 70.43 72.25 70.24 (C(l-5) - 234.76 192.52 5 112.66 90.06 88.63 87.39 69.71 70.2 79.83 70.2 73.01 27.49 237.16 201.55 27.42 (CH, ) 6 113.20 89.98 88.68 86.38 68.06 68.8 94.86 66.71 68.8 28.33 237.32 - 23.84 (CH,) 66.74 68.9 65.50 (CH) 7 113.56 90.00 88.63 86.20 69.01 69.63 83.85 67.29 69.36 27.88 237.46 - 111.45 (C’H,) 134.06 (CH) ’ Chemical shifts reported in ppm with respect to internal MeJi.
- -z - P
.s
_ -2 z -8 0 -8 -X65
A study of the ‘H NMR spectrum of 7 demonstrated that a vinyl group exerts an electron-withdrawing effect. Downfield shifts of the Cp2(Fe) protons relatively to the corresponding protons of 3 are observed. A typical ‘H NMR pattern of the vinylic system is also observed, namely an ABX pattern at 6 5.07, 5.32 and 6.38 for HB, HA and HX with coupling constants of JAB 1.4 Hz, Ja 17.5 HZ, and JBx 10.7 Hz. This assignment is made on the basis that HA and H* are not chemically equivalent. HA (6 5.32) is deshielded as compared with HB, because of its relative proximity of the ring. HX (6 6.38) is strongly deshielded by the ring, and couples with HA and H*. The HB proton couples with HX and HA.
The assignments of 13C NMR spectra for compounds 5-7 (Table 3) are based on: standard 13C NMR correlations [18]; 2D-HetCOR; DEPT technique and compared with other metallo-aromatic system [19,20]. In the case of 7, four relatively less intense signals at S 237.46, 111.56, 86.20 and 83.85 corresponding to terminal carbonyl carbon, C(1) of Cp(Cr), C(1) of Cp’(Fe) and C(1) of Cp*(Fe), all show no short range coupling. The line assignments for C(2-5) of Cp(Cr), Cp’(Fe) and Cp2(Fe) were more difficult to make. Based on 2D-HetCOR (Fig. 1) chemical shifts at 6 88.63 and 90.00 were assigned to C(3,4) and C(2,5) of Cp(Cr) ring and chemicals shifts at 6 69.01 and 69.63 were assigned to C(2,5) and C(3,4) of Cp’(Fe), and at S 67.29 and 69.36 were assigned to C(2,5) and C(3,4) of Cp2(Fe), respec- tively. Chemical shifts at 6 27.88, 111.45, 134.06 were assigned to CH,, CH=CH, and CH=CH Z respectively.
The mass spectra of 5-7 all exhibit a parent peak and the expected chromium and iron isotopic pattern. Fragment peaks at (M - CO)+, (M - 2CO)+, and (M - 2C0 - NO)+ are also always observed.
Cpfcrl
66 Table 4
Selected bond distances (A) and angles (deg) of 7 Bond distances Fe-C(11) Fe-C(13) Fe-C(H) Fe-C(32) Fe-C(34) Cr-C(21) Cr-C(23) cr-C(25) C(ll)-C(15) c(13)-W4) c(21)-c(22) C(22)-C(23) C(24)-C(25) C(31)-C(35) c(33)-c(34) Cr-N(1) Cr-C(3) C(2)-o(2) c-C(11) C(33)-C(36) Bond angles C(ll)-C(12)-C(13) C(13)-c(14)-C(15) C(12)-C(ll)-C(15) C(22)-C(21)-C(25) C(23)-C(24)-C(25) C(32)-C(31)-C(35) c(32)-C(33)-C(34) C(31)-C(35)-C(34) N(l)-Cr-C(3) C(ll)-c-C(21) Cr-C(2)-O(2) c-C(ll)-C(12) C(32)-C(33)-C(36) C(33)-C(36)-C(37) C-C(21)-C(25) ten.-Cr-C(2) 2.037(4) 2.036(5) 2.040(5) 2.036(4) 2.029(5) 2.212(5) 2.185(4) 2.194(4) 1.415(7) 1.391(U) 1.403(6) 1.411(7) 1.409(7) 1.390(7) 1.412(7) 1.704(4) 1.832(5) 1.134(6) 1.488(6) l&7(7) 108.4(5) 108.4(5) 106.q4) 107.9(4) 108.0(4) 108.6(4) 107.2(4) 108.5(4) 94.72(20) 114.1(4) 176.6(4) 127.8(4) 126.5(4) 126.5(4) 124.8(4) 120.1(2) Fe-C(12) Fe-C(14) Fe-C(3 1) Fe-C(33) Fe-C(35) Cr-C(22) Cr-C(24) C(ll)-C(12) C(12)-C(13) C(14)-C(15) C(21)-C(25) C(23)-C(24) C(31)-C(32) C(32)-c(33) C(34)-C(35) G-C(2) N(l)-O(l) C(3)-O(3) c-C(21) C(36)-C(37) C(12)-C(13)-C(14) C(ll)-C(15)-C(14) C(21)-C(22)-C(23) C(22)-C(23)-C(24) C(21)-C(25)-C(24) C(31)-c(32)-C(33) C(33)-C(34)-C(35) N(l)-Cr-C(2) C(2)-Cr-C(3) Cr-N(l)-O(1) Cr-C(3)-O(3) c-C(H)-C(15) C(34)-C(33)-C(36) c-C(21)-c(22) cm.-Cr-N(1) ten.-Cr-C(3) 2.032(5) 2.03q5j 2.033(4) 2.041(4) 2.032(5) 2.203(5) 2.188(5) 1.42q7) l.O48(9) 1.406(9) 1.417(6) 1.402(7) 1.421(7) 1.436(6) 1.420(g) 1.851(5) 1.183(5) 1.146(6) 1.507(6) 1.296(S) 108.2(5) 108.6(5) 108.0(4) 108.3(4) 107.8(4) 107.5(4) 108.3(4) 92.97(20) 94.67(22) 177.9(3) 176.4(5) 125.7(4) 126.3(4) 127.2(4) 127.3(2) 119.3(2)
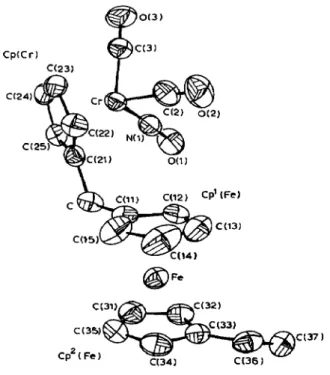
The molecular structure of 7 is shown in fig. 2. Selected bond distances and angles are given in Table 4. The atomic coordinates of the non-hydrogen atoms are listed in Table 5.
Compound 7 adopts a transoid conformation at methylene carbon and the cynichrodenyl moiety resides at exo site of ferrocenyl fragment. The dihedral angle between the Cp(Cr) and @(Fe) planes is 102.2” which deviates from the corre- sponding angle C(ll)-C-C(21) by 11.9 O. The coordination geometry about the Cr center is approximately a distorted tetrahedron with two carbonyl groups, the Cp group and nitrosyl group as the four coordination sites. The nitrosyl group of the cynichrodenyl moiety is located at the site toward the exocyclic carbon of the corresponding Cp(Cr) with a twist angle 43.4O. The twist angle is defined as the
67
Table 5
Atomic parameters x, y, z and By of 7,e.s.d.s refer to the last digit printed
X Y 2 Fe Cr
&1,
o(1) C(2) O(2) c(3) O(3) Cw) c(12) c(13) c(l4) C(15) C(21) c(22) ~(23) ~(241 c(251 c(31) c(32) c(33) c(34) c(35) c(36) c(37) 0.25530(6) 0.10693(5) 0.23038(6) 0.26451(5) 0.5400(4) 0.0723(4) 0.2137(4) 0.1901(3) 0.2012(4) 0.1414(3) 0.1169(5) 0.1853(4) 0.0433(3) 0.1377(3) 0.3621(5) 0.1898(4) O&33(4) 0.1461(3) 0.4213(4) 0.1117(4) 0.3715(5) 0.2157(4) 0.2593(6) 0.2181(5) 0.2387(6) 0.1181(6) 0.3381(5) 0.052q5) 0.6449(4) 0.0842(4) 0.638q4) 0.0928(4) 0.7586(5) 0.0970(4) 0.8393(4) 0.0908(4) 0.7696(4) 0.0837(3) 0.2847(4) 0.0505(4) 0.2237(4) 0.1499(4) 0.1107(4) 0.1387(4) 0.1053(5) 0.0333(4) 0.2128(5) -0.0203(4) 0.0181(4) 0.2212(4) 0.0038(5) 0.3063(4) 0.157609(21) 0.91759(3) 0.14286(16) 0.86359(14) 0.82527(11) 0.95353(19) 0.97362(14) 0.94131(19) 0.95444(15) 0.12275(15) 0.12723(19) 0.10110(22) 0.08027(18) 0.0928q18) 0.10607(17) 0.05262(18) 0.03349(16) 0.07499(20) 0.12015(16) 0.22941(17) 0.23153(H) 0.20432(16) 0.18609(19) 0.20196(19) 0.19667(17) 0.22394(19) 3.49(3) 3.26(3) 3.79(21) 4.22(20) 6.41(22) 3.96(24) 6.45(21) 4.15(24) 6.89(22) 3.45(22) 5.1(3) 7.1(4) 7.1(4) 5.0(3) 3.33(22) 3.96(23) 4.41(24) 4.16(24) 3.59(21) 4.3(3) 3.69(22) 3.62(22) 4.6(3) 4.8(3) 4.3(3) 5.3(3)torsional angle between the nitrogen atom, the chromium atom, the Cp ring center and the ring carbon atom bearing exocychc carbon.
In the cynichrodene moiety, the observed average bond lengths of Cr-C(ring) 2.196 A, compare favorably with the 2.188(5) A average found in ($- C,H,)Cr(CO),NO [ll] and with the 2.20(l) A average value in [(q5-C,H,)Cr(CO),], [21] and in ($-C,H5)Cr(NO)$1 [22]. The Cr-N length 1.704(4) A, falls in the range of reported values, 1.687(7) A in ($-C,,H,)Cr(CO),NO [ll] to 1.72(l) A in ($-C,H,)Cr(N0)2(NCO) [23]. The Cr-C(carbony1) distance: l-851(5) A (Cr-C(2)); l-832(5) (C~-C(3)) agree well with the 1.864(6) found in ($-C13Hs)Cr(CO)zN0 and 1.86 A found in [(q5-C,H5)Cr(CO)3]2 [21]. The N=O length of 1.183(5) (N(l)-O(1)) is longer than the C%O distances of 1.134(6) (C(2)-O(2)), 1.146(6) (C(3)-O(3)), in keeping with the greater antibonding population in the nitrosyl ligand. The Cr-N-O angle of 177.9(3) A (Cr-N(l)-O(1)) is consistent with the NO+ formalism typical of linear M-NO linkage, while the Cr-C-O angle of 176.6(4) (Cr-C(2)-O(2)), 176.4(5) (Cr-C(3)-O(3)) indicate the usual mode of bonding in the terminal metal carbonyl complexes. The Cr-centroid (Cp(Cr)) distance is 1.841(5), agrees with the 1.844 A in ($-C5H5)Cr-(C0)2N0 and 1.884 A in (n5-C,3H9)Cr(CO)2N0 [ll]. The average C-C distance in the ring (Cp(Cr)) is 1.408 A. The mean angle in the ring is 108 O.
The two cyclopentadienyl rings of ferrocenyl moiety exhibit an average twist angle of 7 O. The twist angle is defined by Palenik [24] as the torsional angle between
68
a ring carbon atom, the two ring centers and the corresponding carbon on the opposite ring. It is apparent that compound 7 is close to the eclipsed configuration, which is in good agreement with other ferrocenyl compounds [25,26]. There is 1.2” offset from parallelism between the two Cp rings of ferrocene moiety and those are separated by 3.29 A. The mean bond distances of ferrocenyl moiety in compound 7 are very similar to those in related molecules [25]. The average Fe-C(ring) is 2.04 Ai, the average C-C distances in the rings are C-C(Cp’(Fe)) 1.41 A, C-C(Cp2(Fe)) 1.42 A, The exocyclic C-C bond measures l-488(6) (C-C(ll)), 1.507(6) (C-C(21)) and the mean angle in the rings is 108O.
The exocyclic carbon (C) is bent away from both metals, Cr and Fe, with 19 angles of - 3.0 o and - 1.6 o respectively. The 8 angle is defined as the angle between the exocyclic C-C bond and Cp ring with positive angle toward metal. The 8 angle between bond C(33)-C(36) and the ring plane of Cp’(Fe) is -0.2O. The vinyl group and the exocyclic carbon are in the 1,3’-configuration and the vinyl plane (C(33)-C(36)-C(37)) turns away from the ring plane of Cp2(Fe) by 21.0”. This rotation is the result of intramolecular steric interference between atoms H(C(32)) and H(C(37)). This is supported by the enlargement of bond angle (C(33)-C(36)-C(37)) to 126.5 O.
Supplementary material available. List of anisotropic temperature factors of
non-hydrogen atoms and the coordinates with isotropic temperature factors of hydrogen atoms as well as list of structure amplitudes (21 pages) are deposited. Ordering information can be obtained from the authors.
Acknowledgements
The author is grateful to the National Science Council for grants in support of this research program.
References
1 C.U. Pittman, Jr., G.V. Marlin and T.D. Rounsefell, Macromol. 6 (1973) 1.
2 D.O. Cowan, J. Park, C.U. Pittman, Jr., Y. Sasaki, T.K. Mukherjee and N.A. Diamond, J. Am. Chem. Sot., 94 (1972) 5110.
3 C.E. Carraher, Jr., J.E. Sheats and C.U. Pittman, Jr. @Is.), Grganometalhc Polymers, Academic Press, New York, 1978.
4 J.E. Shea@ C.E. Carraher, Jr., and CU. Pittman, Jr. (Eds.), Metal-Containing Polymeric Systems, Plenum Press, New York, 1985.
5 M. Zeklin, K.J. Wynne, and H-R Allcock (Pds.), Inorganic and Organometallic Polymers, ACS Symposium Series 360, American Chemical Society, Washington, DC, 1988.
6 CU. Pittman, Jr., and M.D. Rausch, Pure & Appl. Chem., 58 (1986) 617. 7 CU. Pittman, Jr., R.L. Voges and J. Elder, Macromol. 4 (1971) 302. 8 C.U. Pittman, Jr., and G.V. Marlin, J. Polymer Sci. A-l, 11 (1973) 2753.
9 C.U. Pittman, Jr., T.D. Rounsefell, E.A. Lewis, J.E. Sheats, B.H. Edwards, M.D. Rausch and E.A. Mintz, Macromol., 11 (1978) 560.
10 Yu-Pin Wang, R-S. Shyu, J.-M. Hwu and Y.-H. Li, Proc, Natl. Sci. Qxmc. RGC (A), 11 (1987) 110. 11 J.L. Atwood, R Shaker, J.T. Malito, M. Herberhold, W. Kremnitz, W.P.E. Bemhagen and H.G. Ah,
J. Organomet. Chem., 165 (1979) 65.
12 T.J. Greenhough, B.W.S. Kolthammer, P. Legidins and J. Trotter, Inorg. Chem., 18 (1979) 3548. 13 D.W. Macomber and M.E. Rausch, Grganometallics, 2 (1983) 1523.
14 M.D. Rausch, E.A. Mintz and D.W. Macomber, J. Grg. Chem., 45 (1980) 689. 15 M.D. Rausch, D.J. Kowalski and E.A. Mintz, J. Grganomet. Chem., 342 (1988) 201.
69 16 International Tables for X-ray Crystahography, Kynoch, Birmingham, England, 1974, (a) Vol IV. (b)
Vol. III. (Present distributor D. Reidel, Dordrecht).
17 E.J. Gabe, Y. Lepage, P.S. White and F.L. Lee, Acta Cryst. A, 43 (1987) C294. 18 J.B. Stother, Ed. Carbon-13-NMR Spectroscopy, Academic Press, New York, 1972. 19 B.E. Mann, Adv. Organomet. Chem., 12 (1974) 135.
20 G.H. Wilkuns, D.D. Traficante and D. Seyferth, J. Organomet. Chem., 60 (1973) C53. 21 R.D. Adams, D.E. Collins and F.A. Cotton, J. Am. Chem. Sot., 96 (1974) 749. 22 O.L. Carter, A.T. McphaiI and G.A. Sim, J. Chem. Sot. A, (1966) 1095. 23 M.A. Bush and G.A. Sim. J. Chem. Sot. A, (1970) 605.
24 G.J. Palenik, Inorg. Chem., 9 (1970) 2424.
25 A.P. Krukonis, J. Silverman, N.F. Yannoni, Acta Cryst. B, 28 (1972) 987. 26 J. Trotter and A. MacDonald, Acta Cryst., 21 (1966) 359.
![Table 3 13C[H] NMR’ Compound Cp(Cr) C&Fe) Cp’(Fe) CH, Cr-C=O >C=O Others C(l) q2,5) (C(3,4) C(1) c(2,5) C(3,4) C(l) ~(2~5) C(3,4) 1 67.88 (C(l-5)) 67.88 (C(l-5)) 2 ,90.31 (cql-5)) 237.10 3 113.41 90.09 88.66 88.17 67.73 68.47 68.68 (C(l-5))](https://thumb-ap.123doks.com/thumbv2/9libinfo/8874329.249600/7.772.289.520.55.983/table-nmr-compound-fe-cp-fe-ch-cr.webp)