Review
Article
Non-canonical signaling mode of the
epidermal growth factor receptor family
Heng-Huan Lee1, Ying-Nai Wang1,2, Mien-ChieHung1,2,3,4,
1Department of Molecular and Cellular Oncology, The University of Texas MD Anderson
Cancer Center, Houston
77030, TX, USA; 2Center for Molecular Medicine and Graduate Institute of Cancer Biology,
China Medical Univer- sity, Taichung 404, Taiwan; 3The University of Texas Graduate School of
Biomedical Sciences at Houston, Houston
77030, TX, USA; 4Department of Biotechnology, Asia University,
Taichung 413, Taiwan
Received August 17, 2015; Accepted August 18, 2015; Epub September 15, 2015; Published October 1, 2015
Abstract: Epidermal growth factor receptor (EGFR) and its family members are key players in both physiological and pathological settings for which they are well recognized as models for investigating the functions and regulations of other membrane receptor tyrosine kinases (RTKs) and serve as therapeutic targets critical to clinical need and fundamental research. The canonical view of the pivotal functions in the EGFR family has been well documented as being an initiator of signaling amplification cascades from the plasma membrane to different subcellular compart- ments via receptor endocytic trafficking, intermolecular interaction, and kinase-substrate reaction in a temporal- spatial manner. However, several lines of evidence have identified non-canonical roles of the EGFR family, acting as a transcriptional factor and a chromatin regulator in the nucleus to regulate gene expression, DNA replication, and DNA damage repair. Moreover, the EGFR family can even exert its impact outside the host cell through exosomal vesicle secretion. The emerging concept of the non-canonical roles of the EGFR family reveals an astonishing and elaborate scheme on the molecular functions of membrane RTKs, offering new insights into the receptor biology as well as the development of comprehensive therapeutic strategies in the future.
Keywords: EGFR family, nuclear function, nuclear translocation, vesicle membrane-associated pathways, exo- somal secretion
Introducti on
To date, non-canonical localization of cell sur- face RTKs in the nucleus, named membrane receptors in the nucleus (MRIN) [1], has been reported in 11 out of 20 RTK classes, including subfamilies of EGFR and insulin, PDGF, VEGF, FGF, HGF, Trk, ROR, Mer, Eph, and Ryk recep- tors [2-4]. It has been more than two decades since the discovery of EGFR family members in the nucleus, where they exist as intact or trun- cated forms to transduce signals and exert a number of important functions [5-8]. The traf-ficking mechanisms for nuclear transport of EGFR and ErbB-2 have
been gradually elucidat- ed [9-13]. In addition to their subcellular traf- ficking from the cell surface, extracellular secretion of EGFR and ErbB-2 from cells in the form of exosomes has also been reported
[14-21]. In this review, we highlight the functions, clinical relevance, and potential pathways of the members of the EGFR family, which is one
of the best-documented RTK subfamilies in the MRIN field, trafficked from the cell surface to a variety of intracellular organelles and to the exosomes in the extracellular environment.
Nuclear functions of EGFR
The functions of nuclear EGFR have been exten- sively studied and are mainly related to tran- scriptional regulation, kinase signaling trans-duction, and protein-protein interaction, which affect a variety of physiological and pathologi- cal functions, such as cell proliferation,
tumor progression, DNA
replication/synthesis, DNA damage repair, and resistance to certain anti-cancer therapies.
Nuclear EGFR as a transcriptional regulator
The first RTK identified to have direct role in transcriptional regulation was nuclear ErbB-2 [22, 23], and subsequently, similar activities
were demonstrated for EGFR and ErbB-4 [24,
25]. Thus far, nuclear EGFR is the best docu- mented. Nuclear EGFR possesses an intrinsic transactivation activity at the carboxyl terminus that regulates target gene promoters, such as cyclin D1 [24], iNOS [26], Aurora-A [27], cyclo- oxygenase-2 (cox-2) [28], c-Myc [29], B-Myb [30], thymidylate synthase [31], breast cancer- resistant protein (BCRP) [32], and STAT1 [33], all of which are related to either tumorigenesis, chromosome instability, drug-resistance, or inflammation. In 2001, EGF-activated nuclear EGFR was first reported as a transcriptional co-activator that binds indirectly to an AT-rich response sequence (ATRS) within the cyclin D1 promoter to activate its gene expression [24]. Huo et al. further identified RNA helicase A (RHA) as EGFR’s DNA-binding partner [34] and showed that RHA functions as a mediator for EGFR’s transactivation activity by binding to the ATRS. Moreover, the authors further demon-strated a positive correlation between the nuclear expression of EGFR, RHA, and cyclin D1 in human breast cancer samples [34]. Additionally, the mucin MUC1 has also been found to associate with EGFR in the nucleus and increase EGFR-mediated cyclin D1 gene expression [35]. Two related studies reported that the Epstein-Barr virus oncoprotein latent membrane protein 1 upregulates the cyclin D1 promoter activity by associating with nuclear EGFR through transcriptional factors, such as transcriptional intermediary factor 2 (a mem- ber of the p160 nuclear receptor co-activators) and STAT3, in nasopharyngeal carcinoma [36,
37]. On the other hand, the tumor suppressor promyelocytic leukemia protein (PMLIV) was shown to interact with nuclear EGFR and repress the transcriptional activity of nuclear EGFR-targeted cyclin D1 gene promoter by inhibiting the levels of acetylation in the histone promoter [38]. Similar to nuclear EGFR studies in regulating cyclin D1 gene promoter,
research- ers demonstrated a bidirectional crosstalk between progesterone receptor and ErbB-2 sig-naling in breast tumor growth in which proges- tin triggers nuclear translocation of ErbB-2 to activate cyclin D1 expression through the recruitment of STAT3, progesterone receptor, and ErbB-2 to the gene promoter [39].
In addition to cyclin D1, activated nuclear EGFR transactivates certain target genes, such as iNOS, Aurora-A, c-Myc, and B-Myb, through
association with transcriptional factors that harbor DNA binding capability, including STAT proteins and E2F1 [26, 27, 29, 30]. Given that EGFR does not contain a DNA binding domain, its association with transcriptional factors appears to be essential for transactivation of each target gene. However, it is not yet clear whether any DNA-binding partners are involved in nuclear EGFR-mediated transactivation of thymidylate synthase and BCRP [31, 32]. In glioblastoma cells, Lo and colleagues per- formed microarray analysis to identify 19 nuclear EGFR target genes and found that nuclear EGFR and EGFRvIII (a constitutively activated EGFR type III variant) associated with STAT3 to activate cox-2 gene expression, which contributes to glioblastoma tumorigenesis [28]. In addition to EGFR and EGFRvIII, nuclear
ErbB-2 also transactivates cox-ErbB-2 gene expression by binding to a specific DNA element, the ErbB-2- associated sequence, in breast cancer cells [23]. Other transcriptional factors participated remain to be discovered. STAT1 gene expres- sion is also upregulated by nuclear EGFR, EGFRvIII, and ErbB-2 via their association with STAT3, providing a link between oncogenic RTK pathways and inflammatory processes through STAT1 signaling [33]. In 2013, Bogler and col-leagues performed a ChIP-Seq in conjunction with bioinformatics analysis to identify genome- wide targets of EGFRvIII and demonstrated that nuclear EGFRvIII associates with c-Myc on E-box-containing promoters, driving oncogenic functions in glioblastoma cells [40]. Inte- restingly, analysis of the human protein-DNA interactome via an unbiased approach identi- fied EGFR as a DNA-binding protein [41], further supporting the nuclear function of EGFR in tran- scriptional regulation. It should be mentioned that both ErbB-2 and ErbB-4 are also associat- ed with transcriptional regulation [22, 23, 25]. ErbB-2 also associates with nuclear actin [42,
43] and plays a role in transcriptional activation of ribosomal RNA by
forming a complex with RNA polymerase-I and β-actin, which in turn enhances translation and cell growth [44].
Nuclear EGFR as a protein kinase
The tyrosine kinase activity of the EGFR family members, except for ErbB-3, plays an impor- tant role in their functions although previous studies have also reported additional functions that are kinase independent [45-49]. In the
nucleus, EGFR also exerts its protein kinase function as exemplified by EGFR-mediated phosphorylation of chromatin-bound prolifera- tive cell nuclear antigen (PCNA) to increase its protein stability and maintain its functions, such as DNA replication and damage repair [50]. In 2013, Weiss and colleagues reported that EGFRvIII serves as a substrate for EGFR and the EGFR-catalyzed phosphorylation of EGFRvIII associates with EGFR to activate STAT3 in the nucleus, which leads to malignant progression of glioblastoma [51, 52]. Various types of post-translational modifications of his-tones are known to play fundamental roles in chromatin dynamics and functions [53]; how- ever, little is known as to how these important functions are regulated by the upstream stimu- li. Chou et al. reported that nuclear EGFR phos- phorylates histone H4 at residue tyrosine-72,
which recruits histone
methyltransferases to enhance its methylation at K20, an event that is critical in regulating DNA synthesis and DNA double-strand break repair [54]. Their findings open a new avenue toward the investigation of interrelationship between RTKs and chromatin structure.
ATM is a serine/threonine protein kinase that modulates DNA damage response, particularly DNA double-strand breaks, by controlling IR-induced foci formation, cell cycle arrest, and apoptosis [55, 56]. However, how upstream stimuli regulate ATM activation has not been well explored. More recently, Lee et al. reported a novel mechanism underlying ATM regulation and radiotherapy resistance in which EGFR associates with and phosphorylates ATM at tyrosine 370 (Y370) at the site of DNA double- strand breaks after IR stimulation. Inhibition of EGFR kinase activity blocked EGFR/ATM asso-ciation, impaired ATM-mediated downstream functions, such as foci formation and DNA repair ability, and enhanced radio-sensitivity. The
authors suggested that EGFR-mediated ATM Y370 phosphorylation has the potential to serve as a biomarker to stratify patients for either radiotherapy alone or in combination with EGFR inhibition [57]. Interestingly, EGFR signaling is functionally involved in DNA replica-tion licensing, which is an important step for initiating cell proliferation in human cancers, by enhancing the phosphorylation of minichromo- some maintenance 7 (MCM7), a licensing fac- tor critical for DNA replication, through a nonre- ceptor Lyn tyrosine kinase phosphorylation
[58]. Further efforts are required to determine whether nuclear EGFR also contributes to the MCM7-mediated DNA replication licensing.
Nuclear EGFR as a protein interactor
A series of reports indicated that certain DNA damage pathways, such as those activated by ultraviolet, IR, or cisplatin treatment, trigger nuclear translocation of EGFR and subsequent protein-protein interaction of nuclear EGFR with DNA-dependent protein kinase (DNAPK), a central enzyme required for the non-homolo- gous end-joining DNA repair of double-strand breaks that drives drug- and radio-resistance [59-62]. Moreover, IR-induced phosphorylation of EGFR at threonine 654 modulates nuclear shuttling of EGFR and confers radio-resistance [63]. A mechanism was proposed for nuclear EGFR/DNAPK complex-mediated elevation of c-Myc mRNA, leading to cell survival and radio- resistance in which upon IR, nuclear EGFR inac- tivates a newly-identified nuclear EGFR- associated protein, polynucleotide phosphory-lase (PNPase), which harbors exoribonuclease activity in controlling c-Myc mRNA degradation, through DNAPK-induced serine phosphoryla-tion on PNPase [64]. A physical association between nuclear EGFR and fused toes homo- log, which is an oncogene known to be respon- sible for the radio-resistance in cervical cancer cells, was recently reported [65]. Silencing of fused toes homolog reduced the phosphoryla- tion of EGFR and DNAPK along with increased DNA double-strand breaks, suggesting it may contribute to radio-sensitization. In addition to nuclear EGFR, radiation treatment also ele- vates the expression level of nuclear ErbB-2, which nuclear import can be blocked by trastu- zumab, a monoclonal antibody against ErbB-2 [66]. It remains unclear but worthwhile to deter- mine whether ErbB-2 also associates with DNAPK to regulate DNA damage response.
Clinical relevance of nuclear EGFR
Nuclear EGFR as a marker for prognosis and therapeutics
The correlation between nuclear EGFR and poor patient outcome has been shown in differ- ent types of malignancies, including breast cancer [67, 68], ovarian cancer [69], non-small cell lung cancer (NSCLC) [70], gallbladder carci- noma [71], and oropharyngeal [72] and esoph- ageal [73] squamous cell carcinomas. In
hor-mone-refractory prostate cancer, nuclear EGFRvIII expression is associated with poor overall survival [74]. In head and neck squa- mous cell carcinoma, nuclear EGFR expression is negatively correlated with p16 serving as a surrogate marker for human papillomavirus infection [75]. This inverse relationship may explain the resistance mechanism to cisplatin and radiation in p16-negative tumors, which express higher level of nuclear EGFR than that in p16-positive tumors, and may provide a ther- apeutic benefit for targeting nuclear EGFR in p16-negative patients [75]. In addition, the loss of TIP30, a HIV-1 Tat interacting protein that is known to suppress metastasis of lung cancer, delays EGFR endocytic degradation followed by increased EGFR cytoplasmic and nuclear sig-naling pathways [76], suggesting a potential therapeutic strategy using EGFR-targeted ther- apies for patients with low TIP30-expressing lung adenocarcinoma. In this regard, it is worth- while to mention that Woloschak and col- leagues developed a novel cancer therapy by using EGFR-targeted Fe O @TiO2
nanoparti-tumors, namely Sym004, which is a mixture of two antibodies that target non-overlapping epi- topes on EGFR and effectively degrades EGFR and delays xenograft tumor growth, may pro- vide a promising opportunity to overcome acquired resistance to cetuximab [82]. In addi- tion, Sym004-directed degradation of EGFR may also overcome cetuximab resistance through the inhibition of EGFR nuclear translo- cation [82]. With regard to EGFR-TKIs resis- tance, studies have demonstrated that nuclear EGFR confers acquired resistance to EGFR-TKI gefitinib. Specifically, the authors demonstrat- ed that nuclear EGFR phosphorylated by Akt binds to the promoter of BCRP, which is an ATP-binding cassette efflux drug transporter that is capable of transporting chemotherapeutic agents out of cells, and subsequently upregu-lates BCRP expression in gefitinib-resistant cells [32, 83]. Treatment with an Akt inhibitor to diminish EGFR nuclear function rendered resis- tant cells more sensitive to gefitinib, further supporting a therapeutic role of Akt interfer- ence in gefitinib re-sensitization [32]. Likewise,
3 4
cles to induce significantly more double-
strand-ed DNA breaks, which is dependent on suc- cessful EGFR nuclear accumulation [77, 78].
Nuclear EGFR in therapeutic resistance
Nuclear EGFR is responsible for resistance to various cancer therapeutics, including above-mentioned DNA damage events involving DNAPK interaction (radiation and cisplatin) [60,
61] and EGFR-targeted therapies (cetuximab and EGFR-TKIs) [32, 79]. A series of studies by Wheeler and colleagues indicated a positive cooperation between nuclear EGFR and Src family kinases (SFKs) activity in acquired resis- tance to cetuximab therapy. They first demon- strated that cetuximab-resistant NSCLC cells are
associated with increased nuclear EGFR expression, which can be blocked by dasatinib, an inhibitor of SFKs, leading to re-sensitization to cetuximab [79]. A follow-up report identified Yes and Lyn as the SFK members that contrib- ute to cetuximab resistance via phosphoryla- tion of EGFR at tyrosine 1101, which impairs its nuclear translocation [80]. Most recently, a potential benefit by blocking both nuclear EGFR translocation with SFK inhibitors and EGFR sig- naling pathway with cetuximab for triple-nega- tive breast cancer (TNBC) was reported [81]. It is worthwhile to mention that a potential EGFR targeted therapy for cetuximab resistant
treatment with cell-penetrating anti-PCNA
pep-tides to inhibit EGFR-mediated phosphorylation of PCNA more effectively suppressed tumor growth in EGFR-TKIs resistant TNBC compared with the parental cells, suggesting that this peptide-based strategy has potential for the drug development against EGFR-TKIs resistant TNBC [84]. Another group found that nuclear EGFR and ErbB-2 bind to and transactivate the gene promoter of thymidylate synthase, which is frequently overexpressed in fluoropyrimidine- resistant cancer cells; meanwhile, treatment with lapatinib, a dual TKI for EGFR and ErbB-2, blocked the nuclear translocation of EGFR and ErbB-2 and consequently led to fluoropyrimi- dine-sensitization by downregulating thymi- dylate synthase [31]. The evidence to date indi- cates that nuclear EGFR strongly associates with clinical relevance, and thus raises an inter- esting question whether nuclear EGFR may serve as a biomarker to predict resistance to the current EGFR target therapies as well as a potential therapeutic target.
Subcellular trafficking mechanisms of nuclear
EGF R
EGFR targeting to the nucleus
Dynamin-dependent receptor endocytosis via clathrin-coated pits is required for nuclear entry
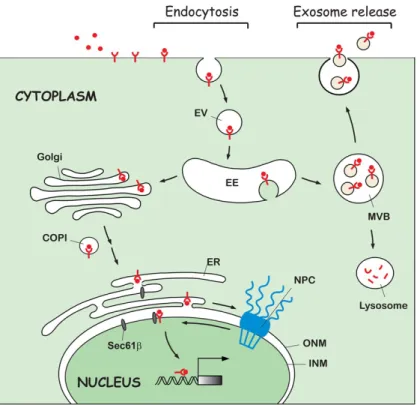
Figure 1. A diagram of non-canonical EGFR trafficking to the intracellular compartments and extracellular space via exosomes. Following endocytosis induced by ligand stimulation, the endocytic vesicles (EV) carrying ligand- bound EGFR fuse with the early endosomes (EE) and are subsequently traf- ficked to several intracellular organelles, such as the Golgi apparatus, ER, and nucleus. COPI vesicle-mediated Golgi-to-ER retrograde transport is in- volved in active EGFR nuclear trafficking. The integral EGFR inserted into the ER membrane is targeted to the INM by crossing the NPC via INTERNET. The INM-embedded EGFR is then released from the lipid bilayer to the nu- cleoplasm by the association with Sec61β translocon located in the INM. In addition to the nuclear import of cell surface EGFR, the internalized EGFR can also be transported into the extracellular environment via exosomal se- cretion after fusion of the MVBs with the cell surface membrane. The scale of the diagram does not reflect the relative size of the molecules or subcel- lular structures. EV, endocytic vesicle; EE, early endosomes; MVB, multive- sicular body; COPI, coat protein complex I; NPC, nuclear pore complex; ER, endoplasmic reticulum; ONM, outer nuclear membrane; INM, inner nuclear membrane.
juxtamembrane domain of EGFR that is conserved among the EGFR family mem- bers [89]. Moreover, NLS-bearing molecules form a complex with importin-β for binding to the nucleoporins of nuclear pore complexes (NPCs) to facilitate nuclear trafficking of EGFR and
ErbB-2 [85, 86]. Notably, a recent study mapped the nuclear delivery of EGFR by a high- resolution imaging using X-ray fluorescence microscopy [77,
78]. Over the past few
years, a more
comprehensive path-way was demonstrated for EGFR nuclear transport th- rough membrane-bound traf-ficking from the cell surface to the Golgi apparatus, the ER, and the nucleus [9-11]. We summarize the details of this pathway (Figure 1) below.
Nuclear transport of EGFR via microtubule and Golgi-to- ER retrograde trafcking
Microtubule cytoskeleton me- diates EGF-induced Golgi translocation via dynamin- associated vesicle, and con-sequently the nuclear entry of EGFR by syntaxin 6-depen- dent fusion with the Golgi membrane, which is respon- sible for the nuclear functions of EGFR, including cell prolif- eration and drug-resistance
of EGFR and ErbB-2 [85, 86]. In addition, the association of EGFR and ErbB-2 with early endosomal markers in the nucleus further sup- ports the notion that the endosomal sorting machinery after endocytosis is involved in nuclear translocation of endosome-embedded EGFR and ErbB-2 [85, 86]. The putative nuclear localization signal (NLS) has been identified in all of the EGFR family members, including EGFRvIII [23, 26, 28, 87, 88]. Unlike the tradi- tional mono- and bi-partite NLSs, Hsu et al. identified a tri-partite NLS of EGFR that con- tains three clusters of basic amino acids at the
[90]. Interestingly, the phosphoinositide lipid kinase PIKfyve, known to regulate endosome- to-trans-Golgi trafficking, was found to promote heparin-binding EGF-like growth factor (HB-EGF)-induced EGFR nuclear transport and the associated function in cell cycle progres- sion [91], further supporting the important role of Golgi apparatus during the EGFR nuclear trafficking. Moreover, Wang et al. reported the first example of Golgi-to-ER retrograde pathway utilized by cell surface RTK in which EGFR reaches the nucleus through a membrane-associated route from the Golgi apparatus to
the ER membrane [92]. The authors further identified an association between coat protein complex I, which is known to deliver cargo pro- teins involving in Golgi-to-ER vesicular trans- port, and EGFR, that is necessary for EGF- induced EGFR nuclear translocation [92]. The questions of whether other EGFR family mem- bers and cell surface RTKs also travel along the microtubule to the nucleus and whether Golgi- to-ER retrograde trafficking is involved remain to be addressed.
Membrane-bound trafcking of EGFR from the
ER to the inner nuclear membrane
EGFR is found in the nuclear matrix and inner nuclear membrane (INM) [93, 94], but the traf- ficking mechanism of subnuclear translocation from the cell surface remains largely unex- plored. Cell surface EGFR stimulated by EGF is targeted to the INM as demonstrated by multi- ple experimental methods, such as immuno- electron microscopy, confocal immunofluores- cence, sucrose gradient purification, and biochemical subcellular fractionation [95]. Importin-β facilitates and regulates EGFR trans- port to the INM by passing the outer nuclear membrane (ONM) that is contiguous and func-tionally related to the ER membrane, suggest- ing a central role of importin-β in EGFR traffick- ing from the ER/ONM to the INM as well as the nucleus [95]. Together, this proposed mecha- nism via the NPCs, termed INTERNET, which stands for the integral trafficking from the ER to the nuclear envelope transport, outlines the route by which the full-length RTKs embedded in the endosomal membrane travel all the way from the cell surface to the nucleus through the NPCs [7, 95]. It should be mentioned that the entire process of EGFR trafficking from the cell surface to the INM is membrane associated, e.g., vesicle-embedded (Figure 1). This vesicle membrane-associated pathway (V-MAP)
mech-anism provides a logical route for cell surface receptors to translocate to different subcellular compartments inside a cell, including the nucleus. It is not clear whether this V-MAP can be reversed to transport nuclear materials back to the cell surface. If so, reversed V-MAP may utilize the exosome formation process, which could explain how the exosomes carry nuclear materials (Please see the later sec-tion). Of note, a recent study presented another solid evidence of EGFR nuclear trafficking at a
single molecule level by a novel high-resolution three-dimensional tracking method [96]. All these findings together indicate that EGFR trav- els from the cell surface to the nucleus in a spa- tiotemporal manner (see Supplementary Movie
6 [96]).
Subnuclear trafcking of EGFR from the INM
to the
nucleoplasm
Following the INTERNET pathway from ER to the INM of the nuclear envelope, how EGFR trans- ports from the INM to the nucleus is still not clear. An endocytosis-like mechanism that occurs in the nuclear envelope has been pro- posed to facilitate internalization of INM- embedded EGFR into the nucleoplasm, where EGFR remains associated with the vesicle membrane [95]. This model was supported by the newly identified location and function of Sec61β, one of the subunits of Sec61 translo- con complex traditionally thought to be associ- ated solely with the ER membrane [97]. Wang et al. reported that Sec61β associates with EGFR in the INM and is required for nuclear translocation of INM-embedded EGFR [95]. Since Sec61β is known to export cargo proteins from ER to cytosol [98], the newly discovered INM location and function of Sec61β support the proposed model above. An earlier model suggested that the ER-localized Sec61β assists EGFR retrotranslocation from the ER mem- brane into the cytosol and that cytosolic EGFR associates with importin-β, resulting in its nuclear translocation of EGFR [99]. While this model is attractive based on prior knowledge on Sec61β, it does not explain the undetect- able level of EGFR in the cytosol [99] and how EGFR could escape from ER-embedded mem- brane-association to become as a free cytosol protein. Further systematic investigation is required to unveil the detailed mechanisms of EGFR nuclear translocation.
Comparison of nuclear trafcking
mechanisms
between EGFR and others
ErbB-2 is reported to utilize the same INTERNET trafficking mechanism as EGFR, followed by Sec61β association in the INM, for transloca- tion from the cell surface to the nucleus [100]. In addition to nuclear localization, ErbB-2 is also detected in the nucleolus, where it associ- ates with RNA polymerase-I to enhance ribo- somal RNA gene transcription [44]. Currently,
we do not known how ErbB-2 is targeted to the nucleolus or whether EGFR family receptors other than ErbB-2 are localized in the nucleo- lus. Another pathway named INFS (integrative nuclear FGFR-1 signaling) [101], has been shown to facilitate the nuclear transport of FGFR-1 in which FGFR-1 is extracted from the cytoplasmic membranes into the cytosol and subsequently enters the nucleus by interacting with importin-β indirectly because FGFR-1 has atypical transmembrane domain without an NLS that typically associates with importin-β [102, 103]. Thus, at least two routes of nuclear entry have been identified for cell surface receptors which further advances our knowl-edge of nuclear trafficking mechanisms for vari- ous endosome-embedded RTKs [100].
EGFR in the exosomes
Membrane-enclosed nanoparticles with a diameter ranging from 30 to 100 nm, also known as exosomes, are derived from multive- sicular bodies (MVBs). After fusion of the MVBs with the cell surface membrane, exosomes are secreted from the cell into the extracellular environment [104, 105]. Exosome secretion was first observed in transferrin receptor traf- ficking by electron microscopy during reticulo-cyte maturation three decades ago [106, 107], and later thought to be a way of dumping mis- folded or unessential proteins. More recently, however, various groups have reported biologi- cally important functions of exosomes. For instance, exosomes are present in many body fluids, such as blood, urine, ascites, saliva, and milk, and released from both normal and path- ological conditions including cancer [108, 109]. Exosomes also act as mediators in intercellular signal communication by delivering a variety of molecules, including proteins, lipids, mRNAs, microRNAs, and DNAs, from donor cells to recipient cells via membrane fusion [18-20,
110-113]. The packaged exosomes prevent the contents from degradation and allow for pro- longed transport to local or distant sites, and correlate to cancer progression, metastasis, tumor microenvironment support, angiogene-sis, and immune suppression [114-116]. In addition to the non-canonical subcellular trafficking described above, EGFR secreted into exosomes can exist as membrane-associated full-length or C-terminal remnants, both of which are enhanced by EGF treatment in human
keratinocyte [14] (Figure 1). The intact and C-terminal exosomal forms of EGFR are also detected in pancreatic cancer cells whereas extracellular domain of EGFR exists as a solu- ble form in conditional culture media [14, 15,
117, 118]. We will highlight the functional roles of exosomal form of EGFR family members in cell-cell signal transduction, immune therapy, and drug resistance in cancers.
Exosomal EGFR as a biomarker for cancer diagnosis
EGFR localized in the exosomes has been stud- ied in different malignancies, such as pancre- atic [15, 119], lung [120, 121], bladder [122], and colorectal cancers [123, 124]. For exam- ple, membrane-bound isoforms of EGFR within the exosomes are found in five human pancre- atic cancer cell lines through the secretome analysis coupled with mass spectrometry [15]. Lung cancer patients have higher exosomal EGFR levels in the plasma than normal individu- als [120]. In addition to the blood circulation where the exosomes accumulate, EGFR and its interacting proteins are also highly enriched in the exosomes derived from human lung cancer pleural effusions [121]. These findings suggest the feasibility of utilizing exosomal EGFR as bio- markers. Interestingly, however, EGFR is not only present in the exosomes isolated from pooled sera of 12 patients with high-grade glio- mas but also detected in human AB serum from normal individuals [125]. Thus, more stud- ies are required to examine the differential roles of exosomal EGFR in various cancer types.
Exosomal EGFR in intercellular
communication
Tumor-derived exosomes carrying EGFR is secreted into the extracellular space that in turn can be taken up by endothelial cells, which leads to tumor angiogenesis [126]. This tumor exosomes-mediated intercellular transfer of EGFR triggers an angiogenic switch via activa- tion of MAPK and Akt pathways, accompanied
by the upregulation of VEGF expression and its autocrine signaling, in endothelial cells [126]. Another study reported that human tumor virus modulates tumor microenvironment upon the uptake of exosomes containing EGFR by epithe- lial, endothelial, and fibroblast cells. For instance, the Epstein-Barr virus latent mem- brane protein 1 increases the release of EGFR- containing exosomes from nasopharyngeal
car-cinoma, which results in the activation of ERK
and Akt pathways in neighboring cells [127].
Exosomal EGFR in immune therapy
Exosome research was first reported in immune response following the discovery of antigen- presenting exosomal vesicles secreted from B-lymphocytes [128]. Exosomes have been well recognized to participate in cell-cell com- munication particularly between immune and cancer cells [129, 130]. A large number of EGFR carried by the lung cancer-derived exo- somes can modulate the immune systems by increasing tumor-specific regulatory T (Treg) cells, which suppresses cytotoxic CD8+ T cells, leading to immunosuppression in the tumor microenvironment [131]. The release of exo- somes containing distinct microRNAs, which can be transferred to T helper 1 (Th1) cells to inhibit Th1 cell proliferation and cytokine secre- tion functions in Treg cell-mediated immuno- suppression [132]. Since Treg cells are known to inhibit tumor-specific T cell immunity and negatively impact patient survival [133, 134], more studies relating to exosomes, oncogenic signals, and Treg cells are warranted.
EGFR variant in the exosomes
The constitutively active EGFR type III variant, EGFRvIII, as a unique brain tumor antigen was first identified in the exosomes released by glio- ma cells [135]. The tumor-released exosomes are transferred intercellularly to deliver EGFRvIII to neighboring cells lacking EGFRvIII to promote their malignant transformed phenotype [135]. Analysis of the exosomes isolated from sera of glioblastoma patients (7 out of 25) but not of normal individuals (30) detected the EGFRvIII mRNA; thus, the serum exosomes-specific EGFRvIII transcript may be used as a biomarker in a non-invasive diagnostic approach to detect
glioblastoma [19]. Moreover, a systematically proteomic and immunologic analysis of murine brain tumor exosomes revealed a high content of EGFRvIII [125]. Most of the brain tumor exo- somes isolated from sera of pooled patient but not normal individuals contain EGFRvIII [125]. Interestingly, transforming growth factor beta (TGF-β), a putative immunosuppressive cyto- kine, was also present in the murine brain tumor exosomes and human patient sera, sup- porting the notion of immune modulatory prop- erties of tumor exosomes [125].
Exosomal ErbB-2 in therapeutic resistance
In addition to the exosomal forms of EGFR and its variants, ErbB-2 has also been shown to exist in exosome which was first observed in the ascites fluid of patients with breast and ovarian cancer [16]. Furthermore, ErbB-2-overexpressing cancers such as breast, ovari- an, and gastric cancer were reported to harbor exosomes containing full-length ErbB-2 [17, 136]. Notably, exosomal ErbB-2 has been shown to confer therapeutic resistance to the humanized monoclonal antibody, trastuzumab, which is mainly used to treat breast cancers with ErbB-2 amplification [137]. Trastuzumab interacts with exosomal ErbB-2 on the surface membrane of exosomes that are either secret- ed from ErbB-2-positive breast cancer cells or found in the sera of breast cancer patient, resulting in continued tumor cell proliferation. This suggests that exosomal ErbB-2 can pro- mote tumor aggressiveness by limiting the availability of therapeutic agents [137].
Ligands of EGFR in the exosomes
In line with the studies of exosomal EGFR family members, ligands of EGFR, including amphireg- ulin, TGF-α, and HB-EGF, are also present as full-length forms in the tumor-derived exo- somes [138]. Specifically, uptake of exosomal amphiregulin to recipient breast cancer cells contributes to a 4-fold increased invasiveness over two other exosomal EGFR ligands [138]. Furthermore, exosomes purified from colon cancer cells with mutant KRAS are highly enriched with amphiregulin and enhances recipient cell invasion, compared with those from isogenically matched wild-type KRAS cells [138]. Recently, Singh and Coffey reported their unpublished observation that ubiquitylation of amphiregulin on its three Lysyl residues in the cytoplasmic tail is responsible for the expres- sion level of exosomal amphiregulin [21], which provides a potential mechanism
underlying delivery of amphiregulin into exosomes.
Future perspective of exosomal EGFR
A highly sensitive and rapid analytical tech- nique was designed for profile circulating exo- somes directly from blood samples of glioblas- toma patients with a microfluidic chip, which allows real-time monitoring of glioblastoma therapy [139]. The use of protein typing of
circu-lating exosomes, labeled with target-specific magnetic nanoparticles and detected by a min- iaturized nuclear magnetic resonance system, have consistently revealed elevated expression of several protein markers, including EGFR and EGFRvIII mutant [139]. Together, exosomes are important molecules in the progression and diagnosis of various diseases ranging from can- cer and diabetes to liver and neurodegenera-tive diseases [140-142]. It would be worthwhile to determine that whether other EGFR family members carried by exosomes are also associ- ated with diseases besides cancer.
Conclusio n
The presence of cell surface RTKs in the nucle- us was observed over two decades ago and has been a mystery in the MRIN field due to lack of molecular mechanism to demonstrate how a membrane receptor embedded in the lipid bilayer can find its way into the nucleus. The biological significance behind this nucleus- oriented phenomenon was uncertain since it can be easily confounded by its traditional function in non-nuclear compartments, such as the plasma membrane and endocytic vesicles. These non-canonical roles of EGFR and its fam- ily members are gradually unraveled by researchers’ continuous efforts from different groups and laboratories. A main theme behind all these exciting observation is to identify and characterize a new location for a membrane receptor to transmit signals from inside the cell or even function in the extracellular space. Adding to the well-established knowledge of membrane RTK signaling, the finding of non- canonical roles of EGFR provides a novel view- point and research direction to the field. Since it is well known that a membrane RTK like EGFR can transduce signal cascades through interac- tion with different proteins in a temporal-spa- tial manner, a nucleus-localized EGFR is
also shown associate with a variety of nuclear pro- teins to regulate diverse functions, including histone modification, gene transcription, and DNA replication and repair. On the other hand, the detection of membrane receptors and their cognate ligands on secretory exosomes sug- gests another novel feature of EGFR to regulate cells in a paracrine manner, in addition to its conventional role as an autocrine signaling transducer. Together, both nuclear and exo- somal EGFR studies opens a new avenue to
explore unknown functions and molecular insight of membrane RTKs, especially with accumulating evidence indicating that they can be critical to disease treatment, including ther-apeutic efficacy, tumor recurrence, metastasis, etc. A systematic investigation of the molecular mechanism of EGFR trafficking to various desti- nations will advance our knowledge regarding the unique functions of EGFR in different sub-cellular compartments and shed light on both the receptor biology and clinical application of anti-EGFR target therapies.
Acknowledgeme nts
This review was supported by the following grants: National Institutes of Health (CA109311, CA099031, and CCSG CA16672); National Breast Cancer Foundation, Inc.; Breast Cancer Research Foundation; Patel Memorial Breast Cancer Endowment Fund; The University of Texas MD Anderson-China Medical University and Hospital Sister Institution Fund; Ministry of Science and Technology, International Research-intensive Centers of Excellence in Taiwan (I-RiCE; MOST 104-2911-I-002-302); Ministry of Health and Welfare, China Medical University Hospital Cancer Research Center of Excellence (MOHW104-TDU-B-212-124-002); and Center for Biological Pathways.
Disclosure of conflict of interest
None .
Address correspondence to: Dr. Mien-Chie Hung, Department of Molecular and Cellular Oncology, The University of Texas MD Anderson Cancer Center, Houston 77030, TX, USA. Tel: 713-792-3668; Fax:
713-794-3270; E-mail: mhu n g @md a nd e r s o n. or g
Reference s
[1] Wang SC and Hung MC. Nuclear translocation of the epidermal growth factor receptor family membrane
tyrosine kinase receptors. Clin Can- cer Res 2009; 15: 6484-6489.
[2] Du Y, Hsu JL, Wang YN and Hung MC. Nuclear functions of receptor tyrosine kinases. In: Wheeler DL, Yarden Y, editors. Receptor tyro- sine kinase: structure, functions and role in human disease. New York, NY: Springer; 2014. [3] Huo L, Hsu JL and Hung MC. Receptor
tyrosine kinases in the nucleus: nuclear functions and therapeutic implications in cancers. In: Kumar R, editor. Nuclear signaling pathways and tar- geting transcription in cancer. New York, NY: Springer; 2014.
[4] Stephenson SA, Mertens-Walker I and Hering- ton A. Signaling of Receptor Tyrosine Kinases in the Nucleus. In: Najman S, editor. Current Frontiers and Perspectives in Cell Biology. In Tech; 2012.
[5] Brand TM, Iida M, Luthar N, Starr MM, Huppert EJ and Wheeler DL. Nuclear EGFR as a molecu- lar target in cancer. Radiother Oncol 2013;
108: 370-377.
[6] Carpenter G and Liao HJ. Receptor tyrosine ki- nases in the nucleus. Cold Spring Harb Per- spect Biol 2013; 5: a008979.
[7] Wang YN, Yamaguchi H, Hsu JM and Hung MC.
Nuclear trafficking of the epidermal growth fac- tor receptor family membrane proteins. Onco- gene 2010; 29: 3997-4006.
[8] Han W and Lo HW. Landscape of EGFR signal- ing network in human cancers: biology and therapeutic response in relation to receptor subcellular locations. Cancer Lett 2012; 318:
124-134.
[9] Wang YN, Hsu JL and Hung MC. Nuclear func- tions and trafficking of receptor tyrosine kinas- es. In: Yarden Y, Tarcic G, editors. Vesicle traf-ficking in cancer. New York, NY: Springer;
2013. pp. 159-176.
[10] Wang YN and Hung MC. Nuclear functions and subcellular trafficking mechanisms of the epi- dermal growth factor receptor family. Cell Bios- ci 2012; 2: 13.
[11] Brand TM, Iida M, Li C and Wheeler DL. The nuclear epidermal growth factor receptor sig- naling network and its role in cancer. Discov Med 2011; 12: 419-432.
[12] Bertelsen V and Stang E. The Mysterious Ways of ErbB2/HER2 Trafficking. Membranes (Ba- sel) 2014; 4: 424-446.
[13] Carpenter G and Liao HJ. Trafficking of recep- tor tyrosine kinases to the nucleus. Exp Cell Res 2009; 315: 1556-1566.
[14] Sanderson MP, Keller S, Alonso A, Riedle S, Dempsey PJ and Altevogt P. Generation of nov- el, secreted epidermal growth factor receptor (EGFR/ErbB1) isoforms via
metalloprotease- dependent ectodomain shedding and exo- some secretion. J Cell Biochem 2008; 103:
1783-1797.
[15] Adamczyk KA, Klein-Scory S, Tehrani MM, Warnken U, Schmiegel W, Schnolzer M and Schwarte-Waldhoff I. Characterization of solu- ble and exosomal forms of the EGFR released from pancreatic cancer cells. Life Sci 2011;
89: 304-312.
[16] Andre F, Schartz NE, Movassagh M, Flament C, Pautier P, Morice P, Pomel C, Lhomme C, Es- cudier B, Le Chevalier T, Tursz T, Amigorena S, Raposo G, Angevin E and Zitvogel L. Malignant effusions and immunogenic tumour-derived exosomes. Lancet 2002; 360: 295-305.
[17] Koga K, Matsumoto K, Akiyoshi T, Kubo M, Ya- manaka N, Tasaki A, Nakashima H, Nakamura M, Kuroki S, Tanaka M and Katano M. Purifica- tion, characterization and biological signifi-cance of tumor-derived exosomes. Anticancer Res 2005; 25: 3703-3707. [18] Valadi H, Ekstrom K, Bossios A,
Sjostrand M, Lee JJ and Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007; 9: 654-659.
[19] Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT Jr, Carter BS, Krichevsky AM and Breakefield XO. Glio- blastoma microvesicles transport RNA and proteins that promote tumour growth and pro- vide diagnostic biomarkers. Nat Cell Biol 2008;
10: 1470-1476.
[20] Cha DJ, Franklin JL, Dou Y, Liu Q, Higginbotham JN, Demory Beckler M, Weaver AM, Vickers K, Prasad N, Levy S, Zhang B, Coffey RJ and Pat- ton JG. KRAS-dependent sorting of miRNA to exosomes. Elife 2015; 4: e07197. [21] Singh B and Coffey RJ. Trafficking of
epidermal growth factor receptor ligands in polarized epi- thelial cells. Annu Rev Physiol 2014; 76: 275-300.
[22] Xie Y and Hung MC. Nuclear localization of p185neu tyrosine kinase and its association with transcriptional transactivation. Biochem Biophys Res Commun 1994; 203: 1589-1598.
[23] Wang SC, Lien HC, Xia W, Chen IF, Lo HW, Wang Z, Ali-Seyed M, Lee DF, Bartholomeusz G, Ou-Yang F, Giri DK and Hung MC. Binding at and transactivation of the COX-2 promoter by nuclear tyrosine kinase receptor ErbB-2. Can- cer Cell 2004; 6: 251-261.
[24] Lin SY, Makino K, Xia W, Matin A, Wen Y, Kwong KY, Bourguignon L and Hung MC. Nuclear lo- calization of EGF receptor and its potential new role as a transcription factor. Nat Cell Biol 2001; 3: 802-808.
[25] Ni CY, Murphy MP, Golde TE and Carpenter G. gamma -Secretase cleavage and nuclear local- ization of ErbB-4 receptor tyrosine kinase. Sci-ence 2001; 294: 2179-2181.
[26] Lo HW, Hsu SC, Ali-Seyed M, Gunduz M, Xia W, Wei Y, Bartholomeusz G, Shih JY and Hung MC. Nuclear interaction of
EGFR and STAT3 in the activation of the iNOS/NO pathway. Cancer Cell 2005; 7: 575-589.
[27] Hung LY, Tseng JT, Lee YC, Xia W, Wang YN, Wu ML, Chuang YH, Lai CH and Chang WC. Nucle- ar epidermal growth factor receptor (EGFR) in-teracts with signal transducer and activator of transcription 5 (STAT5) in activating Aurora-A gene expression. Nucleic Acids Res 2008; 36:
[28] Lo HW, Cao X, Zhu H and Ali-Osman F. Cyclo- oxygenase-2 is a novel transcriptional target of the nuclear EGFR-STAT3 and EGFRvIII-STAT3 signaling axes. Mol Cancer Res 2010; 8:
232-245 .
[29] Jaganathan S, Yue P, Paladino DC, Bogdanovic J, Huo Q and Turkson J. A functional nuclear epidermal growth factor receptor, SRC and Stat3 heteromeric complex in pancreatic can- cer cells. PLoS One 2011; 6: e19605.
[30] Hanada N, Lo HW, Day CP, Pan Y, Nakajima Y and Hung MC. Co-regulation of B-Myb expres- sion by E2F1 and EGF receptor. Mol Carcinog 2006; 45:
10-17.
[31] Kim HP, Yoon YK, Kim JW, Han SW, Hur HS, Park J, Lee JH, Oh DY, Im SA, Bang YJ and Kim TY. Lapatinib, a dual EGFR and HER2 tyrosine kinase inhibitor, downregulates thymidylate synthase by inhibiting the nuclear transloca- tion of EGFR and HER2. PLoS One 2009; 4: e5933.
[32] Huang WC, Chen YJ, Li LY, Wei YL, Hsu SC, Tsai SL, Chiu PC, Huang WP, Wang YN, Chen CH, Chang WC, Chang WC, Chen AJ, Tsai CH and Hung MC. Nuclear translocation of epidermal growth factor receptor by Akt-dependent phos- phorylation enhances breast cancer-resistant protein expression in gefitinib-resistant cells. J Biol Chem 2011; 286: 20558-20568. [33] Han W, Carpenter RL, Cao X and Lo
HW. STAT1 gene expression is enhanced by nuclear EGFR and HER2 via cooperation with STAT3. Mol Car-cinog 2013; 52: 959-969.
[34] Huo L, Wang YN, Xia W, Hsu SC, Lai CC, Li LY, Chang WC, Wang Y, Hsu MC, Yu YL, Huang TH, Ding Q, Chen CH, Tsai CH and Hung MC. RNA helicase A is a DNA-binding partner for EGFR-mediated transcriptional activation in the nu- cleus. Proc Natl Acad Sci U S A 2010; 107:
16125-16130.
[35] Bitler BG, Goverdhan A and Schroeder JA.
MUC1 regulates nuclear localization and func- tion of the epidermal growth factor receptor. J Cell Sci 2010; 123: 1716-1723.
[36] Shi Y, Tao Y, Jiang Y, Xu Y, Yan B, Chen X, Xiao L and Cao Y. Nuclear epidermal growth factor re- ceptor interacts with transcriptional intermedi-ary factor 2 to activate cyclin D1 gene expres- sion triggered by the oncoprotein latent membrane protein 1. Carcinogenesis 2012;
33: 1468-1478.
[37] Xu Y, Shi Y, Yuan Q, Liu X, Yan B, Chen L, Tao Y and Cao Y. Epstein-Barr Virus encoded LMP1 regulates cyclin D1 promoter activity by nucle- ar EGFR and STAT3 in CNE1 cells. J Exp Clin Cancer Res 2013; 32: 90.
[38] Kuo HY, Chen YC, Chang HY, Jeng JC, Lin EH, Pan CM, Chang YW, Wang ML, Chou YT, Shih HM and Wu CW. The PML isoform IV is a nega- tive regulator of nuclear EGFR’s transcriptional
activity in lung cancer. Carcinogenesis 2013;
34: 1708-1716.
[39] Beguelin W, Diaz Flaque MC, Proietti CJ, Cayrol F, Rivas MA, Tkach M, Rosemblit C, Tocci JM, Charreau EH, Schillaci R and Elizalde PV. Pro-gesterone receptor induces ErbB-2 nuclear translocation to promote breast cancer growth via a novel transcriptional effect: ErbB-2 func- tion as a coactivator of Stat3. Mol Cell Biol
2010; 30: 5456-5472.
[40] Gururaj AE, Gibson L, Panchabhai S, Bai M, Manyam G, Lu Y, Latha K, Rojas ML, Hwang Y, Liang S and Bogler O. Access to the nucleus and functional association with c-Myc is re- quired for the full oncogenic potential of Del-taEGFR/EGFRvIII. J Biol Chem 2013; 288:
3428-3438.
[41] Hu S, Xie Z, Onishi A, Yu X, Jiang L, Lin J, Rho HS, Woodard C, Wang H, Jeong JS, Long S, He X, Wade H, Blackshaw S, Qian J and Zhu H. Pro-filing the human protein-DNA interactome re- veals ERK2 as a transcriptional repressor of interferon signaling. Cell 2009; 139: 610-622. [42] Philimonenko VV, Zhao J, Iben S,
Dingova H, Kysela K, Kahle M, Zentgraf H, Hofmann WA, de Lanerolle P, Hozak P and Grummt I. Nuclear actin and myosin I are required for RNA poly- merase I transcription. Nat Cell Biol 2004; 6:
1165-1172.
[43] de Lanerolle P, Johnson T and Hofmann WA.
Actin and myosin I in the nucleus: what next? Nat Struct Mol Biol 2005; 12: 742-746.
[44] Li LY, Chen H, Hsieh YH, Wang YN, Chu HJ, Chen YH, Chen HY, Chien PJ, Ma HT, Tsai HC, Lai CC, Sher YP, Lien HC, Tsai CH and Hung MC. Nuclear ErbB2 enhances translation and cell growth by activating transcription of ribosomal RNA genes. Cancer Res 2011; 71: 4269-4279.
[45] Weihua Z, Tsan R, Huang WC, Wu Q, Chiu CH, Fidler IJ and Hung MC. Survival of cancer cells is maintained by EGFR independent of its ki- nase activity. Cancer Cell 2008; 13: 385-393.
[46] Ren J, Bollu LR, Su F, Gao G, Xu L, Huang WC, Hung MC and Weihua Z. EGFR-SGLT1 interac- tion does not respond to EGFR modulators, but inhibition of SGLT1 sensitizes prostate
cancer cells to EGFR tyrosine kinase inhibitors. Pros- tate 2013; 73: 1453-1461.
[47] Huang WC, Hsu SC, Huang SJ, Chen YJ, Hsiao YC, Zhang W, Fidler IJ and Hung MC. Exoge- nous expression of human SGLT1 exhibits ag- gregations in sodium dodecyl sulfate polyacryl-amide gel electrophoresis. Am J Transl Res
2013; 5: 441-449.
[48] Tan X, Thapa N, Sun Y and Anderson RA. A ki- nase-independent role for EGF receptor in au- tophagy initiation. Cell 2015; 160: 145-160.
[49] Rauch J, Volinsky N, Romano D and Kolch W.
The secret life of kinases: functions beyond ca- talysis. Cell Commun Signal 2011; 9: 23.
[50] Wang SC, Nakajima Y, Yu YL, Xia W, Chen CT, Yang CC, McIntush EW, Li LY, Hawke DH, Ko- bayashi R and Hung MC. Tyrosine phosphoryla- tion controls PCNA function through protein stability. Nat Cell Biol 2006; 8: 1359-1368.
[51] Fan QW, Cheng CK, Gustafson WC, Charron E, Zipper P, Wong RA, Chen J, Lau J, Knobbe- Thomsen C, Weller M, Jura N, Reifenberger G, Shokat KM and Weiss WA. EGFR phosphory- lates tumor-derived EGFRvIII driving STAT3/5 and progression in glioblastoma. Cancer Cell
2013; 24: 438-449.
[52] Zadeh G, Bhat KP and Aldape K. EGFR and EG- FRvIII in glioblastoma: partners in crime. Can- cer Cell 2013; 24: 403-404.
[53] Bannister AJ and Kouzarides T. Regulation of
chromatin by histone modifications. Cell Res
2011; 21: 381-395.
[54] Chou RH, Wang YN, Hsieh YH, Li LY, Xia W, Chang WC, Chang LC, Cheng CC, Lai CC, Hsu JL, Chang WJ, Chiang SY, Lee HJ, Liao HW, Ch- uang PH, Chen HY, Wang HL, Kuo SC, Chen CH, Yu YL and Hung MC. EGFR Modulates DNA Syn- thesis and Repair through Tyr Phosphorylation of Histone H4. Dev Cell 2014; 30: 224-237.
[55] Shiloh Y and Ziv Y. The ATM protein kinase: regulating the cellular response to genotoxic stress, and more. Nat Rev Mol Cell Biol 2013; 14: 197-210.
[56] Cremona CA and Behrens A. ATM signalling and cancer. Oncogene 2014; 33: 3351-3360.
[57] Lee HJ, Lan L, Peng G, Chang WC, Hsu MC, Wang YN, Cheng CC, Wei L, Nakajima S, Chang SS, Liao HW, Chen CH, Lavin M, Ang KK, Lin SY and Hung MC. Tyrosine 370 phosphorylation of ATM positively regulates DNA damage re- sponse. Cell Res 2015; 25: 225-236.
[58] Huang TH, Huo L, Wang YN, Xia W, Wei Y, Chang SS, Chang WC, Fang YF, Chen CT, Lang JY, Tu C, Wang Y, Hsu MC, Kuo HP, Ko HW, Shen J, Lee HH, Lee PC, Wu Y, Chen CH and Hung MC. Epidermal growth factor receptor potenti- ates MCM7-mediated DNA replication through tyrosine phosphorylation of Lyn kinase in
hu-man cancers. Cancer Cell 2013; 23: 796-810.
[59] Dittmann K, Mayer C, Fehrenbacher B, Schaller M, Raju U, Milas L, Chen DJ, Kehlbach R and Rodemann HP. Radiation-induced epidermal growth factor receptor nuclear import is linked to activation of DNA-dependent protein kinase. J Biol Chem 2005; 280: 31182-31189.
[60] Liccardi G, Hartley JA and Hochhauser D. EGFR nuclear translocation modulates DNA repair following cisplatin and ionizing radiation treat-ment. Cancer Res 2011; 71: 1103-1114.
[61] Hsu SC, Miller SA, Wang Y and Hung MC. Nu- clear EGFR is required for cisplatin resistance and DNA repair. Am J Transl Res 2009; 1:
[62] Dittmann KH, Mayer C, Ohneseit PA, Raju U, Andratschke NH, Milas L and Rodemann HP. Celecoxib induced tumor cell radiosensitiza- tion by inhibiting radiation induced nuclear EGFR transport and DNA-repair: a COX-2 inde- pendent mechanism. Int J Radiat Oncol Biol Phys 2008; 70: 203-212.
[63] Dittmann K, Mayer C, Fehrenbacher B, Schaller M, Kehlbach R and Rodemann HP. Nuclear EGFR shuttling induced by ionizing radiation is regulated by phosphorylation at residue Thr654. FEBS Lett 2010; 584: 3878-3884.
[64] Yu YL, Chou RH, Wu CH, Wang YN, Chang WJ, Tseng YJ, Chang WC, Lai CC, Lee HJ, Huo L, Chen CH and Hung MC. Nuclear EGFR sup- presses ribonuclease activity of polynucleotide phosphorylase through DNAPK-mediated phosphorylation at serine 776. J Biol Chem
2012; 287: 31015-31026.
[65] Muthusami S, Prabakaran DS, Yu JR and Park WY. FTS is responsible for radiation-induced nuclear phosphorylation of EGFR and repair of DNA damage in cervical cancer cells. J Cancer Res Clin Oncol 2015; 141: 203-210.
[66] Luo B, Yu S, Zhuang L, Xia S, Zhao Z and Rong L. Induction of ERBB2 nuclear transport after radiation in breast cancer cells. J Huazhong Univ Sci Technolog Med Sci 2009; 29:
350-353.
[67] Lo HW, Xia W, Wei Y, Ali-Seyed M, Huang SF and Hung MC. Novel prognostic value of nucle- ar epidermal growth factor receptor in breast cancer. Cancer Res 2005; 65: 338-348.
[68] Hadzisejdic I, Mustac E, Jonjic N, Petkovic M and Grahovac B. Nuclear EGFR in ductal inva- sive breast cancer: correlation with cyclin-D1 and prognosis. Mod Pathol 2010; 23:
392-403.
[69] Xia W, Wei Y, Du Y, Liu J, Chang B, Yu YL, Huo LF, Miller S and Hung MC. Nuclear expression of epidermal growth factor receptor is a novel prognostic value in patients with ovarian can- cer. Mol Carcinog 2009; 48: 610-617.
[70] Traynor AM, Weigel TL, Oettel KR, Yang DT, Zhang C, Kim K, Salgia R, Iida M, Brand TM, Hoang T, Campbell
TC, Hernan HR and Wheel- er DL. Nuclear EGFR protein expression pre-dicts poor survival in early stage non-small cell lung cancer. Lung Cancer 2013; 81: 138-141.
[71] Li CF, Fang FM, Wang JM, Tzeng CC, Tai HC, Wei YC, Li SH, Lee YT, Wang YH, Yu SC, Shiue YL, Chu PY, Wang WL, Chen LT and Huang HY. EGFR nuclear import in gallbladder carcinoma: nuclear phosphorylated EGFR upregulates iNOS expression and confers independent prognostic impact. Ann Surg Oncol 2012; 19: 443-454.
[72] Psyrri A, Yu Z, Weinberger PM, Sasaki C, Hafty
B, Camp R, Rimm D and Burtness BA.
Quanti-tative determination of nuclear and cytoplas- mic epidermal growth factor receptor expres- sion in oropharyngeal squamous cell cancer by using automated quantitative analysis. Clin Cancer Res 2005; 11: 5856-5862. [73] Hoshino M, Fukui H, Ono Y, Sekikawa
A, Ichika- wa K, Tomita S, Imai Y, Imura J, Hiraishi H and Fujimori T. Nuclear expression of phosphory- lated EGFR is associated with poor prognosis of patients with esophageal squamous cell carcinoma. Pathobiology 2007; 74: 15-21.
[74] Edwards J, Traynor P, Munro AF, Pirret CF, Dunne B and Bartlett JM. The role of HER1- HER4 and EGFRvIII in hormone-refractory prostate cancer. Clin Cancer Res 2006; 12:
123-130.
[75] Husain H, Psyrri A, Markovic A, Rampias T, Pec- tasides E, Wang H, Slebos R, Yarbrough WG, Burtness B and Chung CH. Nuclear epidermal growth factor receptor and p16 expression in head and neck squamous cell carcinoma. La-ryngoscope 2012; 122: 2762-2768. [76] Li A, Zhang C, Gao S, Chen F, Yang C,
Luo R and Xiao H. TIP30 loss enhances cytoplasmic and nuclear EGFR signaling and promotes lung ad-enocarcinogenesis in mice. Oncogene 2013;
32: 2273-2281, 2281e.1-12.
[77] Yuan Y, Chen S, Paunesku T, Gleber SC, Liu WC, Doty CB, Mak R, Deng J, Jin Q, Lai B, Bris- ter K, Flachenecker C, Jacobsen C, Vogt S and Woloschak GE. Epidermal growth factor recep- tor targeted nuclear delivery and high-resolu- tion whole cell X-ray imaging of Fe3O4@TiO2 nanoparticles in cancer cells. ACS Nano 2013;
7: 10502-10517.
[78] Paunesku T, Gutiontov S, Brown K and Wolos- chak GE. Radiosensitization and nanoparti- cles. Cancer Treat Res 2015; 166: 151-171. [79] Li C, Iida M, Dunn EF, Ghia AJ and Wheeler DL.
Nuclear EGFR contributes to acquired resis- tance to cetuximab. Oncogene 2009; 28:
3801-3813.
[80] Iida M, Brand TM, Campbell DA, Li C and Wheeler DL. Yes and Lyn play a
role in nuclear translocation of the epidermal growth factor receptor. Oncogene 2013; 32: 759-767.
[81] Brand TM, Iida M, Dunn EF, Luthar N, Kosto- poulos KT, Corrigan KL, Wleklinski MJ, Yang D, Wisinski KB, Salgia R and Wheeler DL. Nuclear epidermal growth factor receptor is a function- al molecular target in triple-negative breast cancer. Mol Cancer Ther 2014; 13:
1356-1368.
[82] Iida M, Brand TM, Starr MM, Li C, Huppert EJ, Luthar N, Pedersen MW, Horak ID, Kragh M and Wheeler DL. Sym004, a novel EGFR anti- body mixture, can overcome acquired resis-tance to cetuximab. Neoplasia 2013; 15:
1196-1206.
[83] Chen YJ, Huang WC, Wei YL, Hsu SC, Yuan P, Lin HY, Wistuba, II, Lee JJ, Yen CJ, Su WC, Chang KY, Chang WC, Chou TC, Chou CK, Tsai CH and Hung MC. Elevated BCRP/ABCG2 ex- pression confers acquired resistance to gefi-tinib in wild-type EGFR-expressing cells. PLoS One 2011; 6: e21428. [84] Yu YL, Chou RH, Liang JH, Chang WJ,
Su KJ, Tseng YJ, Huang WC, Wang SC and Hung MC. Targeting the EGFR/PCNA signaling suppress- es tumor growth of triple-negative breast can- cer cells with cell-penetrating PCNA peptides. PLoS One 2013; 8: e61362.
[85] Lo HW, Ali-Seyed M, Wu Y, Bartholomeusz G, Hsu SC and Hung MC. Nuclear-cytoplasmic transport of EGFR involves receptor endocyto- sis, importin beta1 and CRM1. J Cell Biochem
2006; 98: 1570-1583.
[86] Giri DK, Ali-Seyed M, Li LY, Lee DF, Ling P, Bar- tholomeusz G, Wang SC and Hung MC. Endo- somal transport of ErbB-2: mechanism for nu- clear entry of the cell surface receptor. Mol Cell Biol 2005; 25: 11005-11018. [87] Ofterdinger M, Schofer C,
Weipoltshammer K and Grunt TW. c-erbB-3: a nuclear protein in mammary epithelial cells. J Cell Biol 2002; 157: 929-939.
[88] Williams CC, Allison JG, Vidal GA, Burow ME, Beckman BS, Marrero L and Jones FE. The ERBB4/HER4 receptor tyrosine kinase regu- lates gene expression by functioning as a STA- T5A nuclear chaperone. J Cell Biol 2004; 167:
469-478.
[89] Hsu SC and Hung MC. Characterization of a novel tripartite nuclear localization sequence in the EGFR family. J Biol Chem 2007; 282: 10432-10440.
[90] Du Y, Shen J, Hsu JL, Han Z, Hsu MC, Yang CC, Kuo HP, Wang YN, Yamaguchi H, Miller SA and Hung MC. Syntaxin 6-mediated Golgi translo- cation plays an important role in nuclear func- tions of EGFR through microtubule-dependent trafficking. Oncogene 2014; 33: 756-770.
[91] Kim J, Jahng WJ, Di Vizio D, Lee JS, Jhaveri R, Rubin MA, Shisheva A and Freeman MR. The phosphoinositide kinase PIKfyve mediates epi- dermal growth factor receptor trafficking to the
nucleus. Cancer Res 2007; 67: 9229-9237.
[92] Wang YN, Wang H, Yamaguchi H, Lee HJ, Lee HH and Hung MC. COPI-mediated retrograde trafficking from the Golgi to the ER regulates EGFR nuclear transport. Biochem Biophys Res Commun 2010; 399: 498-504. [93] Wang ZH, Tian XX, Cheng Y, Yam GH,
Ng HK, Ding MX, Chew-Cheng SB and Chew EC. Asso- ciation of EGFR gene fragments with nuclear matrices in glioblastoma cell lines. Anticancer Res 1998; 18: 4329-4332.
[94] Klein C, Gensburger C, Freyermuth S, Nair BC, Labourdette G and Malviya AN. A 120 kDa nu- clear phospholipase Cgamma1 protein frag-ment is stimulated in vivo by EGF signal phosphorylating nuclear membrane EGFR. Biochemistry 2004; 43: 15873-15883.
[95] Wang YN, Yamaguchi H, Huo L, Du Y, Lee HJ, Lee HH, Wang H, Hsu JM and Hung MC. The translocon Sec61beta localized in the inner nuclear membrane transports membrane-em-bedded EGF receptor to the nucleus. J Biol Chem 2010; 285: 38720-38729. [96] Perillo EP, Liu YL, Huynh K, Liu C,
Chou CK, Hung MC, Yeh HC and Dunn AK. Deep and high-resolution three-dimensional tracking of single particles using nonlinear and multi-plexed illumination. Nat Commun 2015; 6:
7874.
[97] Park E and Rapoport TA. Mechanisms of Sec61/SecY-mediated protein translocation across membranes. Annu Rev Biophys 2012;
41: 21-40.
[98] Romisch K. Endoplasmic reticulum-associated degradation. Annu Rev Cell Dev Biol 2005; 21:
435-456.
[99] Liao HJ and Carpenter G. Role of the Sec61 translocon in EGF receptor trafficking to the nucleus and gene expression. Mol Biol Cell
2007; 18: 1064-1072.
[100] Wang YN, Lee HH, Lee HJ, Du Y, Yamaguchi H and Hung MC. Membrane-bound trafficking regulates nuclear transport of integral epider-mal growth factor receptor (EGFR) and ErbB-2. J Biol Chem 2012; 287: 16869-16879.
[101] Stachowiak MK, Maher PA and Stachowiak EK.
Integrative nuclear signaling in cell develop- ment--a role for FGF receptor-1. DNA Cell Biol 2007; 26: 811-826.
[102] Reilly JF and Maher PA. Importin beta-mediat- ed nuclear import of fibroblast growth factor receptor: role in cell proliferation. J Cell Biol
2001; 152: 1307-1312.
[103] Myers JM, Martins GG, Ostrowski J and Sta- chowiak MK. Nuclear trafficking of FGFR1: a role for the transmembrane domain. J Cell Bio-chem 2003; 88: 1273-1291.
[104] Mathivanan S, Ji H and Simpson RJ. Exosomes: extracellular organelles important in intercel- lular communication. J Proteomics 2010; 73: 1907-1920.
[105] Raposo G and Stoorvogel W. Extracellular vesi- cles: exosomes, microvesicles, and friends. J Cell Biol 2013; 200: 373-383.
[106] Pan BT, Teng K, Wu C, Adam M and Johnstone RM. Electron microscopic evidence for exter- nalization of the transferrin receptor in vesicu- lar form in sheep reticulocytes. J Cell Biol 1985; 101: 942-948.
[107] Johnstone RM, Adam M, Hammond JR, Orr L
and Turbide C. Vesicle formation during
reticu-locyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem 1987; 262:
9412-9420 .
[108] van der Pol E, Boing AN, Harrison P, Sturk A and Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev 2012; 64: 676-705. [109] Simons M and Raposo G.
Exosomes-vesicular carriers for intercellular communication. Curr Opin Cell Biol 2009; 21: 575-581.
[110] Balaj L, Lessard R, Dai L, Cho YJ, Pomeroy SL, Breakefield XO and Skog J. Tumour microvesi- cles contain retrotransposon elements and amplified oncogene sequences. Nat Commun
2011; 2: 180.
[111] Guescini M, Genedani S, Stocchi V and Agnati LF. Astrocytes and Glioblastoma cells release exosomes carrying mtDNA. J Neural Transm 2010; 117:
1-4.
[112] Record M, Carayon K, Poirot M and Silvente- Poirot S. Exosomes as new vesicular lipid transporters involved in cell-cell communica- tion and various pathophysiologies. Biochim Biophys Acta 2014; 1841: 108-120.
[113] Hannafon BN and Ding WQ. Intercellular Com- munication by Exosome-Derived microRNAs in Cancer. Int J Mol Sci 2013; 14: 14240-14269.
[114] Kahlert C and Kalluri R. Exosomes in tumor mi- croenvironment influence cancer progression and metastasis. J Mol Med (Berl) 2013; 91:
431-437.
[115] EL Andaloussi S, Mäger I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov 2013; 12: 347-357.
[116] Thery C, Ostrowski M and Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol 2009; 9: 581-593.
[117] Perez-Torres M, Valle BL, Maihle NJ, Negron- Vega L, Nieves-Alicea R and Cora EM. Shed- ding of epidermal growth factor receptor is a regulated process that occurs with overexpres-sion in malignant cells. Exp Cell Res 2008;
314: 2907-2918.
[118] Zhen Y, Caprioli RM and Staros JV. Character- ization of glycosylation sites of the epidermal growth factor receptor. Biochemistry 2003; 42:
5478-5492.
[119] Arscott WT and Camphausen KA. EGFR iso- forms in exosomes as a novel method for bio- marker discovery in pancreatic cancer. Bio-mark Med 2011; 5: 821.
[120] Yamashita T, Kamada H, Kanasaki S, Maeda Y, Nagano K, Abe Y, Inoue M, Yoshioka Y, Tsut- sumi Y, Katayama S, Inoue M and Tsunoda S. Epidermal growth factor receptor localized to exosome membranes as a possible biomarker for lung cancer diagnosis. Pharmazie 2013;
68: 969-973.
[121] Park JO, Choi DY, Choi DS, Kim HJ, Kang JW, Jung JH, Lee JH, Kim J, Freeman MR, Lee KY, Gho YS and Kim KP. Identification and charac- terization of proteins isolated from microvesi-cles derived from human lung cancer pleural effusions. Proteomics 2013; 13: 2125-2134.
[122] Welton JL, Khanna S, Giles PJ, Brennan P, Brewis IA, Staffurth J, Mason MD and Clayton A. Proteomics analysis of bladder cancer exo- somes. Mol Cell Proteomics 2010; 9: 1324-1338.
[123] Choi DS, Park JO, Jang SC, Yoon YJ, Jung JW, Choi DY, Kim JW, Kang JS, Park J, Hwang D, Lee KH, Park SH, Kim YK, Desiderio DM, Kim KP and Gho YS. Proteomic analysis of microvesi- cles derived from human colorectal cancer as- cites. Proteomics 2011; 11: 2745-2751.
[124] Demory Beckler M, Higginbotham JN, Franklin JL, Ham AJ, Halvey PJ, Imasuen IE, Whitwell C, Li M, Liebler DC and Coffey RJ. Proteomic anal- ysis of exosomes from mutant KRAS colon can-cer cells identifies intercellular transfer of mu- tant KRAS. Mol Cell Proteomics 2013; 12:
343-355.
[125] Graner MW, Alzate O, Dechkovskaia AM, Keene JD, Sampson JH, Mitchell DA and Bigner DD. Proteomic and immunologic analyses of brain tumor exosomes. FASEB J 2009; 23: 1541-1557.
[126] Al-Nedawi K, Meehan B, Kerbel RS, Allison AC and Rak J. Endothelial expression of autocrine VEGF upon the uptake of tumor-derived mi-crovesicles containing oncogenic EGFR. Proc Natl Acad Sci U S A 2009; 106: 3794-3799.
[127] Meckes DG Jr, Shair KH, Marquitz AR, Kung CP, Edwards RH and Raab-Traub N. Human tumor virus utilizes exosomes for intercellular com-munication. Proc Natl Acad Sci U S A 2010;
107: 20370-20375.
[128] Raposo G, Nijman HW, Stoorvogel W, Liejen- dekker R, Harding CV, Melief CJ and Geuze HJ. B lymphocytes secrete antigen-presenting ves- icles. J Exp Med 1996; 183: 1161-1172.
[129] Filipazzi P, Burdek M, Villa A, Rivoltini L and Hu- ber V. Recent advances on the role of tumor exosomes in immunosuppression and disease
progression. Semin Cancer Biol 2012; 22:
342-349.
[130] Gutierrez-Vazquez C, Villarroya-Beltri C, Mittel- brunn M and Sanchez-Madrid F. Transfer of extracellular vesicles during immune cell-cell interactions. Immunol Rev 2013; 251:
125-142.
[131] Huang SH, Li Y, Zhang J, Rong J and Ye S. Epi- dermal growth factor receptor-containing exo- somes induce tumor-specific regulatory T cells. Cancer Invest 2013; 31: 330-335.