December 2010 Note 1639
Momordica charantia L., a slender-stemmed tendril climber, belongs to the family Cucurbitaceae and is com-monly known as bitter gourd or bitter melon. It is wildly cul-tivated as a vegetable crop in tropical and subtropical areas, including Asia, East Africa, and South America. Tissues of this plant have extensively been used as folk medicine for the treatment of diabetes and diseases of liver in Taiwan. The previous pharmacological studies have demonstrated that the extracts or constituents of tissues of M. charantia possess anti-diabetic and anti-inflammatory activities.1—3) The Cu-curbitaceae plants have been recognized as rich sources of cucurbitane-type triterpenoids possessing anti-diabetic activ-ity.3) More than seventy cucurbitane-type triterpenoids have been identified from the fruits,3—10)seeds,11,12)roots,13)leaves and vines14,15)of M. charantia. As part of our program aimed at the discovery of the cucurbitane-type triterpenes from M. charantia originated in Taiwan, we have reported the isola-tion and structure elucidaisola-tion of 21 cucurbitane-type triter-penoids from the MeOH extract of the stems of this plant.16—18) In our continuing investigation on the same ex-tract, we further isolated two novel pentanorcucurbitane triterpenes, 22-hydroxy-23,24,25,26,27-pentanorcucurbit-5-en-3-one (1) and 3,7-dioxo-23,24,25,26,27-pentanorcucurbit-5-en-22-oic acid (2), as well as a novel trinorcucurbitane triterpene, 25,26,27-trinorcucurbit-5-ene-3,7,23-trione (3) (Fig. 1). In this paper, we describe the isolation and structure elucidation of compounds 1—3 and the cytoprotective activi-ties of compounds 2 and 3 in tert-butyl hydroperoxide (t-BHP)-induced hepatotoxicity of HepG2 cells.
Results and Discussion
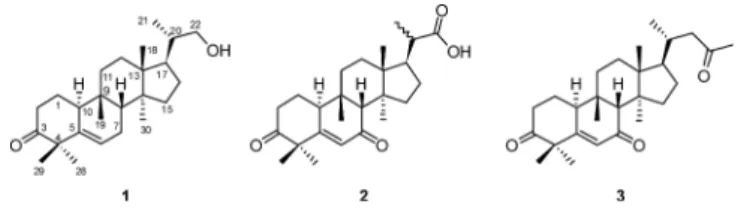
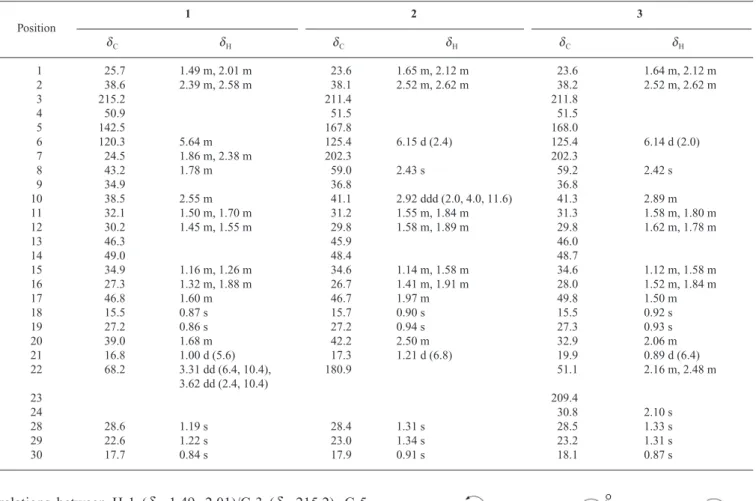
Compound 1 was obtained as a white amorphous powder. The IR spectrum suggested the presence of hydroxy (3422 cm⫺1), double bond (3075, 1654 cm⫺1), and isolated ketone (1708 cm⫺1) absorptions. The 1H- and 13C-NMR spectra of 1 (Table 1) contained signals for five methyl sin-glets [dH 0.84, 0.86, 0.87, 1.19, 1.22 (3H each, s)], one methyl doublet [dH1.00 (3H, d, J⫽5.6 Hz)], a trisubstituted double bond [dH5.64 (1H, m); dC120.3 (d), 142.5 (s)], and an ABX splitting pattern of secondary alcohol [3.31 (1H, d, J⫽6.4, 10.4 Hz), 3.62 (1H, d, J⫽2.4, 10.4 Hz); dC68.2 (t)]. The 13C-NMR spectrum of 1 revealed 25 carbon signals, which were assigned by the distortionless enhancement by polarization transfer (DEPT) experiments as six aliphatic methyl, seven aliphatic methylene, four aliphatic methine, four aliphatic quaternary, one oxygenated methylene, one olefinic methine, one quaternary olefinic, and one isolated ketone carbonyl carbons. Its high resolution electron impact mass spectrum (HR-EI-MS) exhibited a molecular ion at m/z 372.3048, consistent with the molecular formula, C25H40O2, which indicated six degrees of unsaturation. Two degrees of unsaturation were attributable to a trisubstituted olefin and a ketone group, and the remaining four units of unsaturation were accounted for the tetracyclic skeleton. On the basis of the fact that the major tetracyclic triterpenoids presenting in the genus Momordica plants are cucurbitane-type triterpenes, compound 1 was tentatively proposed to be a 23,24,25,26,27-pentanorcucurbitacin derivative. By comparison of the 1 H-and 13C-NMR data with those of the known compound, (23E)-25-hydroxycucurbita-5,23-diene-3,7-dione,16) indicated that both compounds exhibited identical structure in rings A—D of the tetracyclic skeleton and the 13C-NMR signals of C-23—C-27 of the side chain were absent in 1. Thus, com-pound 1 was considered as a 23,24,25,26,27-pentanorcucur-bitacin triterpene. The planar structure of 1 was constructed by the 1H–1H correlated spectroscopy (COSY), heteronuclear multiple-quantum coherence (HMQC), and heteronuclear multiple bond coherence (HMBC) spectra. The HMBC
cor-Cucurbitane Triterpenoids from Momordica charantia and Their
Cytoprotective Activity in tert-Butyl Hydroperoxide-Induced
Hepatotoxicity of HepG2 Cells
Chiy-Rong CHEN,aYun-Wen LIAO,bLai WANG,bYueh-Hsiung KUO,c,#Hung-Jen LIU,d
Wen-Ling SHIH,bHsueh-Ling CHENG,band Chi-I CHANG*,b,#
aDepartment of Biological Science and Technology, Meiho University; Pingtung 91201, Taiwan: bGraduate Institute of
Biotechnology, National Pingtung University of Science and Technology; Pingtung 91201, Taiwan: cTsuzuki Institute for
Traditional Medicine, College of Pharmacy, China Medical University; Taichung 40402, Taiwan: and dInstitute of
Molecular Biology, National Chung-Hsing University; Taichung 40227, Taiwan.
Received June 6, 2010; accepted September 2, 2010; published online September 6, 2010
Two novel pentanorcucurbitane triterpenes, 22-hydroxy-23,24,25,26,27-pentanorcucurbit-5-en-3-one (1) and 3,7-dioxo-23,24,25,26,27-pentanorcucurbit-5-en-22-oic acid (2) together with a new trinorcucurbitane triterpene, 25,26,27-trinorcucurbit-5-ene-3,7,23-trione (3) were isolated from the methyl alcohol extract of the stems of
Momordica charantia. The structures of the new compounds were elucidated by spectroscopic methods.
Com-pounds 2 and 3 showed potent cytoprotective activity in tert-butyl hydroperoxide (t-BHP)-induced hepatotoxicity of HepG2 cells.
Key words Chinese herb; Momordica charantia; Cucurbitaceae; pentanorcucurbitane; trinorcucurbitane; cytoprotective
Chem. Pharm. Bull. 58(12) 1639—1642 (2010)
© 2010 Pharmaceutical Society of Japan ∗ To whom correspondence should be addressed. e-mail: changchii@mail.npust.edu.tw
#These authors contributed equally to this work.
relations between H-1 (dH 1.49, 2.01)/C-3 (dC 215.2), C-5 (dC142.5), C-10 (dC38.5); H-6 (dH5.64)/C-4 (dC50.9), C-5, C-7 (dC24.5), C-8 (dC43.2), C-10 (Fig. 2) confirmed that the isolated ketone and the trisubstituted double bond were located at C-3 and C-5,6 positions, respectively. The hydroxy group was attached on C-22 was assured by the HMBC cor-relations between H-22 (dH3.31, 3.62)/C-17 (dC46.8), C-20 (dC39.0), C-21 (dC16.8) (Fig. 2). The relative configurations of sterogenic C-atoms in the tetracyclic rings were deter-mined by significant nuclear Overhauser effect (NOE) corre-lations between H-8 (dH 1.78)/Me-18 (dH 0.87), Me-19 (dH 0.86); H-10/Me-28 (dH 1.19), Me-30 (dH 0.84); H-17 (dH 1.60)/Me-30 (dH 0.84) in the nuclear Overhauser enhance-ment exchange spectroscopy (NOESY) spectrum (Fig. 3). The NOESY correlations between H-20 (dH 1.68)/Me-18; Me-21 (dH 1.00)/H-12 (dH 1.55); H-17 (dH1.60)/H-22 (dH 3.31, 3.62) allowed us to assure the configuration at C-20 as R.19—21)Furthermore, the electron impact mass spectrum (EI-MS) of 1 showed the fragment ion at m/z 313 [M⫺C3H7O (side chain)]⫹ derived from the loss of side chain by the cleavage of C-17/C-20 bond. In addition, the fragment ion at m/z 222 [M⫺C10H14O]⫹ was corresponded to retro-Diels-Alder (RDA) cleavage between A and B rings. From the above evidence, compound 1 was determined as 22-hydroxy-23,24,25,26,27-pentanorcucurbit-5-en-3-one, namely penta-norcucurbitacin A. Complete 1H- and 13C-NMR chemical shifts were established by 1H–1H COSY, HMQC, HMBC, and NOESY spectra.
The HR-EI-MS of 2 showed a molecular ion at m/z 400.2606, which corresponded to the molecular formula, C25H36O4, indicating eight degrees of unsaturation. The IR
spectrum indicated the presence of an isolated ketone, a car-boxylic acid, a conjugated ketone, and a conjugated double bond. The presence of an a,b-unsaturated ketone system was further confirmed by the significant UV absorption at
1640 Vol. 58, No. 12
Table 1. 1H- and 13C-NMR Data for 1—3 (400, 100 MHz in CDCl 3) Position 1 2 3 dC dH dC dH dC dH 1 25.7 1.49 m, 2.01 m 23.6 1.65 m, 2.12 m 23.6 1.64 m, 2.12 m 2 38.6 2.39 m, 2.58 m 38.1 2.52 m, 2.62 m 38.2 2.52 m, 2.62 m 3 215.2 211.4 211.8 4 50.9 51.5 51.5 5 142.5 167.8 168.0 6 120.3 5.64 m 125.4 6.15 d (2.4) 125.4 6.14 d (2.0) 7 24.5 1.86 m, 2.38 m 202.3 202.3 8 43.2 1.78 m 59.0 2.43 s 59.2 2.42 s 9 34.9 36.8 36.8 10 38.5 2.55 m 41.1 2.92 ddd (2.0, 4.0, 11.6) 41.3 2.89 m 11 32.1 1.50 m, 1.70 m 31.2 1.55 m, 1.84 m 31.3 1.58 m, 1.80 m 12 30.2 1.45 m, 1.55 m 29.8 1.58 m, 1.89 m 29.8 1.62 m, 1.78 m 13 46.3 45.9 46.0 14 49.0 48.4 48.7 15 34.9 1.16 m, 1.26 m 34.6 1.14 m, 1.58 m 34.6 1.12 m, 1.58 m 16 27.3 1.32 m, 1.88 m 26.7 1.41 m, 1.91 m 28.0 1.52 m, 1.84 m 17 46.8 1.60 m 46.7 1.97 m 49.8 1.50 m 18 15.5 0.87 s 15.7 0.90 s 15.5 0.92 s 19 27.2 0.86 s 27.2 0.94 s 27.3 0.93 s 20 39.0 1.68 m 42.2 2.50 m 32.9 2.06 m 21 16.8 1.00 d (5.6) 17.3 1.21 d (6.8) 19.9 0.89 d (6.4) 22 68.2 3.31 dd (6.4, 10.4), 180.9 51.1 2.16 m, 2.48 m 3.62 dd (2.4, 10.4) 23 209.4 24 30.8 2.10 s 28 28.6 1.19 s 28.4 1.31 s 28.5 1.33 s 29 22.6 1.22 s 23.0 1.34 s 23.2 1.31 s 30 17.7 0.84 s 17.9 0.91 s 18.1 0.87 s
Fig. 2. Main HMBC Correlations of 1—3
247 nm. The 1H- and 13C-NMR spectra of 2 (Table 1) dis-played signals for five methyl singlets [dH 0.90, 0.91, 0.94, 1.31, 1.34 (3H each, s)], one set of a,b-unsaturated carbonyl system [dH6.15 (1H, d, J⫽2.4 Hz); dC125.4 (d), 167.8 (s), 202.3 (s)]. The 13C-NMR spectrum of 2 revealed 25 reso-nances. The 13C-NMR data of 2 showed close resemblance with those of the known compound, (23E)-25-hydroxycucur-bita-5,23-diene-3,7-dione,16) indicated that both compounds exhibited identical structure in rings A—D of the tetracyclic skeleton and the 13C-NMR signals of C-23—C-27 of the side chain were absent in 2. Compound 2 was tentatively pro-posed to exhibit a basic skeleton of 23,24,25,26,27-pentanor-cucurbitacin. In turn, the downfield methine signal at dH2.50 (H-20) as well as the HMBC correlations between H-17 (dH 1.97)/C-20 (dC 42.2); H-20 (dH 2.50)/C-17 (dC 46.7); H-20/C-22 (dC 180.9); Me-21 (dH 1.21)/C-22 (Fig. 2) con-firmed that the carboxylic acid group was located at C-22 po-sition. The relative configurations of sterogenic C-atoms in the tetracyclic rings were determined by significant NOE cor-relations between H-8 (dH2.43)/Me-18 (dH0.90), Me-19 (dH 0.94); H-10 (dH2.92)/Me-28 (dH1.31), Me-30 (dH0.91); H-17 (dH1.97)/Me-30 (dH0.91) in the NOESY spectrum (Fig. 3). The configuration at C-20 was considered as a racemic center due to locating at a-position of carbonyl functionality. The EI-MS spectrum of 2 showed the fragment ion at m/z 327 [M⫺C3H5O2(side chain)]⫹derived from the loss of side chain by the cleavage of C-17 and C-20 bond. In addition, the fragment ion at m/z 164 [M⫺C15H24O2]⫹ was corre-sponded to flavone like RDA cleavage. Thus, compound 2 was elucidated as 3,7-dioxo-23,24,25,26,27-pentanorcucur-bit-5-en-22-oic acid, namely pentanorcucurbitacin B.
Compound 3 was obtained as a white amorphous powder. The molecular formula was determined as C27H40O3from the HR-EI-MS [M]⫹ m/z 412.2981. In the UV spectrum, a sig-nificant absorption maximum at 246 nm suggested the pres-ence of an a,b-unsaturated ketone. The IR spectrum dis-played absorption bands for a conjugated ketone, a conju-gated double, and an isolated cyclohexanone. The 1H- and 13
C-NMR spectra of 3 (Table 1) exhibited signals for the presence of five methyl singlets [dH 0.87, 0.92, 0.93, 1.31, 1.33 (3H each, s)], a secondary methyl [dH 0.89 (3H, d, J⫽6.4 Hz)], an acetyl methyl [dH2.10 (s)], and one a,b-un-saturated carbonyl system [dH 6.14 (1H, d, J⫽2.0 Hz); dC 125.4 (d), 168.0 (s), 202.3 (s)]. In turn, the 13C-NMR spec-trum of 3 revealed 27 carbon signals. By comparison of the 1H- and 13C-NMR data with those of the known compound, (23E)-cucurbita-5,24-diene-3,7,23-trione,17) indicated that both compounds exhibited identical structure in rings A—D and the C-25—C-27 NMR signals of side chain were absent in 3. Thus, compound 3 was considered as a 25,26,27-trinor-cucurbitacin triterpene with a C5 side chain containing an isolated ketone functionality. The NMR signals for an acetyl methyl [dH2.10 (s); dC30.8 (s)] as well as the HMBC corre-lations between H-20 (dH2.06)/C-23 (dC209.4); Me-21 (dH 0.89)/C-22 (51.1); H-24 (dH 2.10)/C-23 confirmed that the isolated ketone was located at C-23 (Fig. 2). The EI-MS spectrum of 3 showed the fragment ion at m/z 369 [M⫺COCH3]⫹derived from the loss of an acetyl group. The relative configurations of sterogenic C-atoms in the tetra-cyclic rings were determined by significant NOE correlations between H-8/Me-18 (dH 0.92), Me-19 (dH 0.93);
H-10/Me-28 (dH1.33), Me-30 (dH0.87); H-17 (dH1.50)/Me-30 in the NOESY spectrum (Fig. 3). The configuration at C-20 was determined as R on the basis of the NOESY correlations be-tween H-20 (dH 2.06)/Me-18; Me-21 (dH 0.89)/H-12 (dH 1.62); H-22 (dH 2.16)/H-17 (dH1.50).19—21) From the above evidence, compound 3 was characterized as 25,26,27-trinor-cucurbit-5-ene-3,7,23-trione. Complete 1H- and 13C-NMR chemical shifts were established by 1H–1H COSY, HMQC, HMBC, and NOESY spectra.
Compounds 2 and 3 were tested for their cytotoxic activity toward human hepatoma HepG2 cells with fluorouracil (5-FU) as a positive control (IC50⫽1.9 mM) by using the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bro-mide] colorimetric method based on the described proce-dures.18)The two compounds were not cytotoxic against the HepG2 cell line up to 100mM. The cytoprotective effects of compounds 2 and 3 on HepG2 cells injured by tert-butyl hy-droperoxide (t-BHP) were performed in an assay described earlier.22)The pretreatment of compounds 2 and 3 on HepG2 cells inhibited t-BHP-induced cytotoxicity. Compound 2 at a dose of 5mM protected the t-BHP-induced cytotoxicity of HepG2 to 55.2% of control group. Compound 3 at a dose of 10mMprotected the t-BHP-induced cytotoxicity of HepG2 to 56.9% of control group. The protective effects of compounds 2 and 3 were similar to that of silybin, a commercial agent, which protected the t-BHP-induced cytotoxicity of HepG2 to 52.5% of control group at a dose of 10mM(Fig. 4).
Experimental
General Experimental Procedures Optical rotations were measured on a JASCO DIP-180 digital spectropolarimeter. UV spectra were measured in MeOH using a Shimadzu UV-1601PC spectrophotometer. IR spectra were obtained on a Nicolet 510P FT-IR spectrometer. NMR spectra were recorded in CDCl3at room temperature on a Varian Mercury plus 400 NMR
spec-trometer, and the solvent resonance was used as internal shift reference [tetramethyl silane (TMS) as standard]. The 2D NMR spectra were recorded by using standard pulse sequences. EI-MS and HR-EI-MS were recorded on Finnigan TSQ-700 and JEOL SX-102A mass spectrometers, respectively. TLC was carried out on silica gel 60 F254plates (Merck, Germany). Column
chromatography was performed on silica gel (230—400 mesh ASTM, Merck). HPLC was performed on a Hitachi L-7000 chromatograph with a Lichrosorb Si gel 60 (5mm) column (250⫻10 mm).
Plant Material The stems of Momordica charantia were collected in Pingtung County, Taiwan in July, 2003. Identification of the voucher speci-mens was done by Prof. Sheng-Zehn Yang, Curator of Herbarium, National
December 2010 1641
Fig. 4. Cytoprotective Effect of 2 and 3 on t-BHP-Toxicified HepG2 Cells
#Statistically significant compared with normal data (p⬍0.05). ∗ Statistically
Pingtung University of Science and Technology. A voucher specimen (no. 2013) was deposited at the Herbarium of National Pingtung University of Science and Technology.
Extraction and Isolation The air-dried stems (18 kg) of M. charantia were extracted with MeOH (3⫻30 l) at room temperature (7 d each). The combined MeOH extract was evaporated under reduced pressure to afford a black residue, which was suspended in H2O (3 l), and then partitioned
se-quentially, using EtOAc and n-BuOH (3⫻2 l) as solvent. The EtOAc fraction (386 g) was chromatographed on a silica gel column (120⫻10 cm), using solvent mixtures of n-hexane and EtOAc with increasing polarity as eluents. Eleven fractions were collected as follows: 1 [5000 ml, n-hexane], 2 [4000 ml, n-hexane–EtOAc (49 : 1)], 3 [4000 ml, n-hexane–EtOAc (19 : 1)], 4 [4000 ml, n-hexane–EtOAc (9 : 1)], 5 [4000 ml, n-hexane–EtOAc (17 : 3)], 6 [4000 ml, n-hexane–EtOAc (8 : 2)], 7 [4000 ml, n-hexane–EtOAc (7 : 3)], 8 [3000 ml, n-hexane–EtOAc (5 : 5)], 9 [3000 ml, n-hexane–EtOAc (4 : 6)], 10 [3000 ml, n-hexane–EtOAc (2 : 8)], and 11 (6000 ml, EtOAc). Fraction 6 was further chromatographed on a silica gel column (5⫻45 cm), eluted with CH2Cl2–EtOAc (8 : 1 to 0 : 1) to give seven fractions (each about 700 ml),
6A—6G. Fr. 6E was subjected to column chromatographed over silica gel eluted with n-hexane–CH2Cl2–EtOAc (5 : 3 : 1) and semipreparative HPLC
eluted with n-hexane–EtOAc (7 : 3) to yield 1 (1.1 mg). Fraction 7 was fur-ther chromatographed on a silica gel column (5⫻45 cm), eluted with CH2Cl2–EtOAc (8 : 1 to 0 : 1) to resolve into seven fractions (each about
600 ml), 7A—7G. Fr. 7C was subjected to column chromatography over sil-ica gel eluted with CH2Cl2–EtOAc (15 : 1) and semipreparative HPLC eluted
with n-hexane–EtOAc (7 : 3) to yield 3 (6 mg). Fraction 8 was further puri-fied through a silica gel column (5⫻45 cm), eluted with CH2Cl2–EtOAc
(7 : 1) to obtain six fractions (each about 500 ml), 8A—8F. Fr. 8F was sub-jected to column chromatographed over Si gel eluted with
n-hexane–CH2Cl2–EtOAc (3 : 3 : 1) and semipreparative HPLC eluted with
CH2Cl2–EtOAc (3 : 2) to yield 2 (4.5 mg).
22-Hydroxy-23,24,25,26,27-pentanorcucurbit-5-en-3-one (1): Amorphous white powder; [a]D
25⫹36.7 (c⫽0.10, MeOH); 1H- and 13C-NMR data, see
Table 1; IR (KBr) nmax3422, 3075, 2950, 2872, 1708, 1654, 1465, 1382,
1270, 1031, 977, 734, 705 cm⫺1; EI-MS m/z: 372 [M]⫹(1), 357 (2), 354 (1), 313 (1), 271 (3), 222 (88), 207 (52), 189 (19), 163 (100), 133 (30), 123 (58), 105 (62), 91 (60); HR-EI-MS m/z: 372.3048 (Calcd for C25H40O2372.3030).
3,7-Dioxo-23,24,25,26,27-pentanorcucurbit-5-en-22-oic Acid (2): Amor-phous white powder; [a]D25⫹38.7 (c⫽0.36, MeOH); 1H- and 13C-NMR data,
see Table 1; IR (KBr) nmax3427, 3072, 2950, 2872, 1693, 1645, 1460, 1377,
1260, 1216, 1124, 1036, 886, 842 cm⫺1; UV (MeOH) lmax(loge) 247 (3.53)
nm; EI-MS m/z: 400 (M⫹, 100), 385 (68), 372 (19), 357 (15), 339 (20), 327 (14), 311 (21), 263 (30), 205 (34), 164 (45), 136 (38), 121 (20); HR-EI-MS
m/z: 400.2606 (Calcd for C25H36O4400.2614).
25,26,27-Trinorcucurbit-5-ene-3,7,23-trione (3): Amorphous white pow-der; [a]D
25⫹86.2 (c⫽0.37, MeOH); 1H- and 13C-NMR data, see Table 1; IR
(KBr) cm⫺1: 3041, 2955, 2882, 1718, 1645, 1616, 1465, 1382, 1353, 1294, 1250, 1158, 886; UV (MeOH) lmax(loge) 203 (4.21), 246 (3.55) nm;
EI-MS m/z: 412 [M]⫹(4), 369 (7), 355 (16), 339 (8), 205 (24), 149 (25), 136 (42), 121 (100), 107 (69), 93 (89), 79 (76), 67 (73); HR-EI-MS m/z: 412.2981 (Calcd for C27H40O3412.2978).
Cytoprotective Assay HepG2 cells (hepatocellular carcinoma cell line) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supple-mented with 10% fetal bovine serum, L-glutamine 2 mM, 1%
penicillin/strep-tomycin (penicillin 10000 U/ml and streppenicillin/strep-tomycin 10 mg/ml) in a humidified atmosphere of 5% CO2at 37 °C. The protective effect of compounds 2 and 3
on HepG2 cells injured by t-BHP was measured using the MTT colorimetric assay based on the described procedures with some modifications.22)Briefly,
HepG2 cells were plated on a 96-well plate with 1⫻104cells per well. The
cells were treated with different concentrations of test compounds. After preincubated for 2 h, the cultured media were changed to the media contain-ing t-BHP (100 mM), incubated for 3 h and then rinsed with
phosphate-buffered saline. Subsequently, the wells were incubated with the MTT (100ml/well concentrated at 5 mg/ml) at 37 °C for 4 h. After removing the supernatant, 200ml of dimethyl sulfoxide (DMSO) was added to redissolve the formazan crystals. Absorbance at 550 nm was measured to estimate
sur-vived cells.
Statistic Analysis The significance of various treatments was deter-mined by the Student’s t-test. The results were expressed as mean⫾S.E.M. Differents were considered significant if p⬍0.05.
Acknowledgements This research was supported by Grants to Chi-I Chang from the National Science Council of Taiwan (NSC 97-2317-B-020-002 and NSC 98-2622-B-020-97-2317-B-020-002-CC1). This study was supported in part by Taiwan Department of Health Clinical Trial and Research Center of Ex-cellence (DOH99-TD-B-111-004) and Cancer Research Center of Excel-lence (DOH99-TD-C-111-005). We thank Ms. Shu-Yun Sun and Ms. Lih-Mei Sheu for the EI-MS and HR-EI-MS measurement in the Instrumenta-tion Center of the College of Science, NaInstrumenta-tional Taiwan University and Na-tional Chung Hsing University. We are also grateful to the NaNa-tional Center for High-Performance Computing for computer time and facilities.
References
1) Rathi S. S., Grover J. K., Vats V., Phytother. Res., 16, 236—243 (2002).
2) Kobori M., Nakayama H., Fukushima K., Ohnishi-Kameyama M., Ono H., Fukushima T., Akimoto Y., Masumoto S., Yukizaki C., Hoshi Y., Deguchi T., Yoshida M., J. Agric. Food Chem., 56, 4004—4011 (2008).
3) Harinantenaina L., Tanaka M., Takaoka S., Oda M., Mogami O., Uchida M., Asakawa Y., Chem. Pharm. Bull., 54, 1017—1021 (2006). 4) Fatope M. O., Takeda Y., Yamashita H., Okabe H., Yamauchi T., J. Nat.
Prod., 53, 1491—1497 (1990).
5) Kimura Y., Akihisa T., Yuasa N., Ukiya M., Suzuki T., Toriyama M., Motohashi S., Tokuda H., J. Nat. Prod., 68, 807—809 (2005). 6) Nakamura S., Murakami T., Nakamura J., Kobayashi H., Matsuda H.,
Yoshikawa M., Chem. Pharm. Bull., 54, 1545—1550 (2006). 7) Li Q. Y., Chen H. B., Liu Z. M., Wang B., Zhao Y. Y., Magn. Reson.
Chem., 45, 451—456 (2007).
8) Akihisa T., Higo N., Tokuda H., Ukiya M., Akazawa H., Tochigi Y., Kimura Y., Suzuki T., Nishino H., J. Nat. Prod., 70, 1233—1239 (2007).
9) Matsuda H., Nakamura S., Murakami T., Yoshikawa M., Heterocycles,
71, 331—341 (2007).
10) Liu Y., Ali Z., Khan I. A., Planta Med., 74, 1291—1294 (2008). 11) Miyahara Y., Okabe H., Yamauchi T., Chem. Pharm. Bull., 29, 1561—
1566 (1981).
12) Okabe H., Miyahara Y., Yamauchi T., Miyahara K., Kawasaki T.,
Chem. Pharm. Bull., 28, 2753—2762 (1980).
13) Chen J. C., Tian R. R., Qiu M. H., Lu L., Zheng Y. T., Zhang Z. Q.,
Phytochemistry, 69, 1043—1048 (2008).
14) Yasuda M., Iwamoto M., Okabe H., Yamauchi T., Chem. Pharm. Bull.,
32, 2044—2047 (1984).
15) Chen J. C., Liu W. Q., Lu L., Qiu M. H., Zheng Y. T., Yang L. M., Zhang X. M., Zhou L., Li Z. R., Phytochemistry, 70, 133—140 (2009). 16) Chang C. I., Chen C. R., Liao Y. W., Cheng H. L., Chen Y. C., Chou
C. H., J. Nat. Prod., 69, 1168—1171 (2006).
17) Chang C. I., Chen C. R., Liao Y. W., Cheng H. L., Chen Y. C., Chou C. H., J. Nat. Prod., 71, 1327—1330 (2008).
18) Chang C. I., Chen C. R., Liao Y. W., Shih W. L., Cheng H. L., Tzeng C. Y., Li J. W., Kung M. T., Chem. Pharm. Bull., 58, 225—229 (2010). 19) Antonov A. S., Afiyatullov S. S., Kalinovsky A. I., Ponomarenko L. P., Dmitrenok P. S., Aminin D. L., Agafonova I. G., Stonik V. A., J. Nat.
Prod., 66, 1082—1088 (2003).
20) Antonov A. S., Kalinovsky A. I., Stonik V. A., Afiyatullov S. S., Aminin D. L., Dmitrenok P. S., Mollo E., Cimino G., J. Nat. Prod., 70, 169—178 (2007).
21) Mansoor T. A., Hong J., Lee C. O., Bae S. J., Im K. S., Jung J. H., J.
Nat. Prod., 68, 331—336 (2005).
22) Lee H. U., Bae E. A., Han M. J., Kim D. H., Biol. Pharm. Bull., 28, 1992—1994 (2005).