Title page
Comparison of Ultrastructure and Lectin Histochemistry on the
Anterior Medial Gland of Nasal Septum in Rat and Gerbil
Chi-Fen Chang 1, Yat-Pang Chau2* and Kuo-Shyan Lu1*
1Department of Anatomy and Cell Biology, College of Medicine, National Taiwan University, and 2Institute of Anatomy and Cell Biology, School of Medicine, National Yang-Ming University
*Corresponding authors:
Kuo-Shyan Lu, Ph.D., Tel: +886-2-23123456#88174; E-mail: lks@ntu.edu.tw and Yat-Pang Chau, Ph.D., Tel: +886-2-28267158; E-mail: leonchau@ym.edu.tw
Department of Anatomy and Cell Biology, College of Medicine, National Taiwan University, 1-1, Jen-Ai Road, Taipei 100, Taiwan, Fax: +886-2-23915292
Institute of Anatomy and Cell Biology, School of Medicine, National Yang-Ming University, 155, 2nd Sec., Li-Nung Street, Shih-Pai, Taipei, 112, Fax: +886-2-28212884,
Abstract
The anterior medial gland (AMG), located in the submucosa of rodent nasal septum, is suggested to provide fluid for humidification of inspired air. Tremendous variation of the environmental air humidity, on which rats and gerbils depend to live, leads us to expect a multiplicity on ultrastructure and various subcellular glycoconjugate distribution within the AMG acinar cells between these two species. Electron microscopy revealed that: (1) The nucleus of AMG cinar cells in rat was irregular-shaped, but that in gerbil was round or elliptical; (2) Secretory granules in rat AMG acinar cells contained homogenous content with various electron density. However, two types of secretory granules in gerbil AMG acinar cells were found: one with lamellated-structure and high electron density, while the others had particulate materials; (3) Myoepithelial cells were present in the acinus of medial and posterior regions in rat AMG, but absent in gerbil; and (4) Nerve terminals were present only in the medial and posterior rat AMG, but in all three regions of the gerbil AMG. Lectin histochemistry demonstrated that: (1) Rat and gerbil AMG acinar cells expressed strong affinity toward Con A and WGA, but neither showed any reactivity toward UEA and PNA; and (2) Varying degrees of reactivity toward different lectins, including DBA, PNA, SBA and EBL, were recognized in rat and gerbil AMG acinar cells.
We confirm the species variation on the ultrastructure and lectin histochemistry of AMG in rats and gerbils, and speculate that these variations may be due to the different living environment.
Introduction
It is well known that most proteins are glycosylated. Glycosylation is an enzymatic process that links saccharides to produce glycan, attached to proteins, lipids or other organic compounds. Glycans in eukaryotic cells can serve a variety of structural and functional roles, such as receptors and cell to cell recognition and adhesion (Ungar, 2009). The glycans of the salivary glycoprotein in oral cavity have been well studied in past studies (Triantafyllou et al., 2004, Ramachandran et al., 2006), but less attention has been paid to that of the eccrine secretion in nasal cavity.
Rat and gerbil are two common laboratory animals used in the biomedical research, including respiratory physiology and cardiovascular studies (Kris-Etherton and Dietschy, 1997, Kucharewicz et al., 2008). However, the rat and the gerbil are two different species of rodent and live in different habitats. Generally, the rat lives in wet and warm areas around the world, while the gerbil (Meriones unguiculatus) lives in dry and sandy areas in western Asia and Africa. The gerbil isa desert rodent and able to tolerate high (38℃) and low (-20℃) ambient temperatures with tremendous variation of air humidity in the living environment. Due to the different climates of the habitats of rat and gerbil, it is believed that there are conspicuous differences in the structure and function of nasal glands in these animals.
the rat middle nasal septum, was first described by Bojsen-Moller (1964). Using whole mounted and osmium-stained translucent preparations, Bojsen-Moller (1964) demonstrated the histological structure and topography of rat AMG. The rat AMG was characterized by the presence of four to five parts and each part consisted of a long main duct connecting to the striated ducts and intercalated ducts on numerous acini. Those openings of glands were located in the vestibular part or the anterior part of the respiratory region. Up to date, the fine structure of AMG in mouse, rat, hamster and human have been studied (Kerjaschki, 1974, Tandler and Bojsen-Moller, 1978, Tandler et al., 2000), but no study has been available concerning the structure and organization of AMG of nasal septum in gerbil.
In light this, we examined the fine structure of AMG in rats and gerbils by light and electron microscopy and detected the regional differences in the composition and distribution of secretory glycoprotein in acinar cells of AMG using lectin histochemistry. We expected that a multiplicity of serous cells and variation of secretorty material would be found between the rat and gerbil AMGs.
Materials and methods
Animals and tissue preparation. A total of 40 male rats (16-24 wk old, 350-400g b.w.) and 40 male gerbils (16-24 wk old, 75-90g b.w.) were purchased from the animal center of National Taiwan University-Hospital and used in the present study. Animals were maintained on a photoperiodicity 14/10 dark/light cycle with a constant temperature (22±1℃) and food and water ad libitum. All animals were anesthetized with sodium-pentobarbital of 80mg/kg b.w, ip., before transcardial perfusion with 4% ice-cold paraformadehyde in 0.1M Phosphate buffer (pH7.4). The nasal septums were removed and placed in fresh fixative overnight. AMGs were dissected out and divided into proximal, middle and distal portions the next day.
Tissue preparation for ultrastructure. Specimens from each portion of AMG were trimmed into 1 mm cubes, washed in 0.1M phosphate buffer (pH7.4), dehydrated through a graded series of ethanol and then embedded in Epon/Araldite mixture. Semithin sections (0.5μm thickness) were cut and stained with 0.5% toluidine blue for light microscopy. Thin sections (60-80nm thickness) were cut and doubly stained with uranyl acetate and lead citrate before being examined in a JOEL 2000 EXΙΙ electron microscope.
Lectin histochemistry. Specimens were washed in 0.1M phosphate buffer, cryoprotected with 30% sucrose, and then embedded in OCT compounds (Tissue Tek,
Miles Inc, Elkhart, IN). Tissue specimens were cut at a thickness of 7 μm in a cryostat. Sections were collected and placed on silane-coated slides. Sections were then washed in 0.05M Tris buffer for 15 minutes and then treated with 0.5% H2O2 in phosphate buffer for 30 minutes in order to remove endogenous peroxidase. S ections were wash ed in 0 .05M Tris buffer for 15 minutes and then incubated with 40 μg/ml
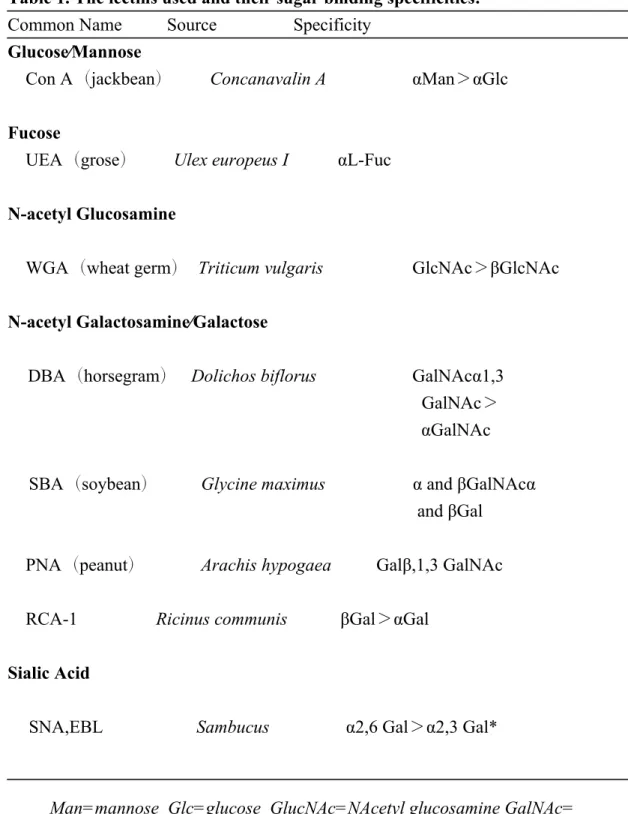
biotinylated lectins, DBA, SBA, PNA, UEA, WGA, RCA-1, ConA and EBL, (Table
1, purchased from Vector Laboratories, Peterborough, UK) in 0.05M Tris buffer for 60 min. After washing in 0.05M Tris buffer for 30 min., tissues were incubated with Avidin-Biotin-complex (Vector Laboratories, Burlingame, CA) for 1 h. The sites of lectin binding were visualized by 0.05% (W/v) DAB in tris buffer, pH7.6, and 6.5 μl/ml H2O2 for 10 min (Walker, 1989.) . Sections were washed, dehydrated, cleared and mounted with Permount (Fischer, U.S.A.) for microscopic examination and photographed with a Leica TCS-2 microscope equipped with a DIX digital camera.
Results
Morphology of AMG. The anterior medial glands (AMG) underlying the submucosa of rat and gerbil nasal septa were pure serous glands consisting of numerous serous acini. The acini secreted their products into short intercalated ducts, striated ducts and then drained into a long excretory duct. According to the spatial location of acinar cells, the AMG in rat and gerbil nasal septum can be divided into proximal, middle and distal portions (Fig. 1A and 1B).
Rat AMG
Fig.2A is a lower power electron micrograph showing the essential characteristics of rat AMG acinus. In rat AMG, acini of the proximal region were about 30-50 m in size. The lumen of acini was small and irregular. Numerous microvilli on the apical surface of acinar cells projected into the lumen of secretory canaliculi. The cytoplasm of acinar cells was packed with accumulate secretory granules containing a homogenous content of various electron density (Type I: high to moderate electron density; Type II: electron lucent or very low electron density; Type III: medium to low electron density). The Golgi complex was usually supranuclear position, accompanied by short and randomly arranged rough endoplasmin reticulum. An irregular, heterochromatic nucleus located in the lower half of the cell and the surrounding basal cytoplasm contained numerous parallel cisternae of rough
endoplasmic reticulum (Fig. 2B). It was noted that absence of myoepithelial cells or nerve fibers existed between the secretory cells.
The morphology of acinar cells in the middle and the distal regions were quite different from that of acinar cells in the proximal region. In these two regions, the apical cytoplasm of acinar cells was crowded with secretory granules that had a homogenous content with high electron density (Fig. 3A). In addition, the rest of the cytoplasm was filled with spherical or rod-like mitochondria and numerous dilated rough endoplasmic reticulum (Fig. 3B). Another distinctive feature of the AMG in these two regions was the presence of the myoepithelial cells. The c ytoplasm of myoepithelial cells contain ed bundles of myofilamants and dense bodies similar to
those in the cytoplasm of smooth muscle cells (Fig. 3C). Furthermore, numerous
cholinergic nerve terminals were found closely surrounding the serous acini and myoepithelial cells (Fig. 3D).
Gerbil AMG.
Three regions (proximal, middle and distal) of the gerbil AMG were also recognized, although the morphology of acinar cells was generally alike. The serous cells had either round or oval nucleus and their nuclei situated centrally . Their cytoplasm contained spherical mitochondria , irregular dilation o f the rough endoplasmic reticulum , and few secretory granules (Fig. 4A). According to the morpholog ical
characteristics of secretory granules , two types of secretory granules were observed. The type I granule was measured 0.7 0.2μm in diameter and contained lamellated structures and dense vesicles (Fig. 4B), while the type II granules were round in profile, about 0.60.2μm, and contained particulate secretory material with moderate electron density (Fig. 4C). Numerous unmyelinated and myelinated nerve terminals were seen in gerbil AMG (Fig. 5A). A number of nerve varicosities, including cholinergic and adrenergic fibers, were located in the intercellular spaces of the acini and showed close contact with the epithelial cells (Fig. 5B and 5C). Furthermore, no myoepithelium was found in the acinus. On this account, it was believed that the secretion of serous cells was directly controlled by neural elements.
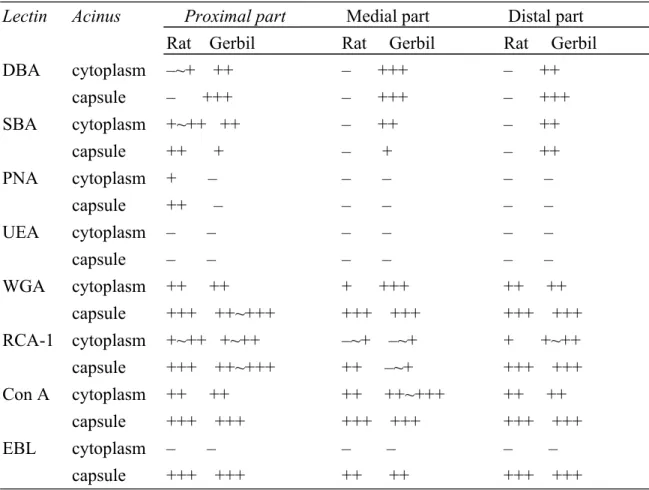
Lectin Histochemisrtry of AMG. The lectins used and their sugar binding specificities are listed in Table 1.With DBA, there was only a weak staining in the acinar cells of rat AMG, but intense staining was found in the acinar cells of gerbil AMG. For PNA, RCA-1 and SBA, the staining results of PNA suggested that PNA lectin did not react with the acinar cells in rat and gerbil AMG. RCA-1 lectin binding showed that the acinar cells in anterior and posterior part of AMG in rat and gerbil have moderate affinity, but weak affinity in the acinar cells in the middle portion of AMG in rat and gerbil. SBA labeling confirmed that the acinar cells in anterior portion of rat AMG and all acinar cells in gerbil AMG had a moderate affinity, but no staining of SBA
lectin was found in the middle and posterior portion of rat AMG. With Con A and WGA, the reactivity from moderate to strong intensity was found in the cytoplasm of acinar cells in both rat and gerbil AMGs. Reactivity of UEA was recognized neither in rat nor in gerbil AMG tissues. Absence of EBL staining was also observed in the cytoplasm of acinar cells in both rat and gerbil AMG (Fig. 6).
The extracellular space surrounding the acinar cells of anterior medial gland also exhibited varied reaction intensities of lectins. With DBA, an intense staining intensity occurred in gerbil AMG, but no labeling of DBA was found in rat AMG. It was also noted that the reaction intensities of WGA, RCA-1, Con A and EBL generally showed a moderate to high intensity of staining around the rat and gerbil AMGs. However, no or weak staining was found with PNA, SBA and UEA on the rat and gerbil AMG (Fig. 7).
Discussion
Ultrastructure of acinar cells in the rat and the gerbil AMG.
Previous studies demonstrated that a large amount of secretory glands were embedded in the lamina propria of mammalian nasal septum, including rat, mouse, hamster, dog and human, respectively. The rat anterior medial gland (AMG), located in the submocosa of the respiratory portion of the mammalian nasal septum, was composed of four to five parts, and each part consisted of a long main duct connecting to the striated ducts and intercalated ducts, on which numerous acini studded. Except the opening of the second main duct into the vestibule, the others opened into the anterior respiratory portion of nasal septum (Bojsen-Moller, 1964, Tandler and Bojsen-Moller, 1978). T he anatomical feature of gerbil and rat AMG are similar except the duct opening at vestibule in gerbil AMG appeared on the third
main duct , while the rat AMG opening at vestibule located on second main duct.
C
onsistent with the previous studies, l ight and electron microscopic observations revealed that both rat and gerbil AMGs were composed of typical serous acini. The
rat AMG acinar cells were filled with s ecretory granules containing homogenous
content with various electron densities. In this study, we found that the gerbil AMG
acinar cells had two types of secretory granules : one type contained packets of lamellae and small dense vesicles , and the other contained close-meshed dense
materials. Although the exact constituents of the secretory product in rat or gerbil AMG serous cells are still unclear, immunostaining for lactoferrin, lysozymes, secretory IgA, neutral endopeptidase and secretory leukoprotease inhibitors was seen exclusively in the serous cells of different mammalian salivary glands (Lee et al., 1990, Castle, 1998, Becerra et al., 2003). To date, it is still controversial whether the morphological structure of secretory granules in serous glandular cells may represent different constituents of secretory product. After comparing the secretory granules in various serous salivary glands from different mammals, Tandler and Philips (1993) concluded that the species-specific patterns of the ultrastucture of salivary gland may exist naturally, but there are major differences between serous granules in different species and noticeable differences exist among serous granules in different major salivary glands in the same organism (Philips et al., 1987, Tandler and Phillips, 1993). Myoepithelial cells are common elements underlying the secretory units of many mammalian exocrine glands such as mammary, sweat, salivary and lacrimal glands (Satoh et al., 1994, Ogawa et al., 1999). The cytoplasm of these cells
containing myofilaments with dense bodies , resemb les the cytoplasmic contents of
smooth muscle cells (Ellis, 1965, Archer and Kao, 1968, Emerman and Vogl, 1986,
Moore et al., 1987, Norberg et al., 1992). Past reports showed that nerve fibers and
Bojsen-Moller, 1978). However, our finding demonstrated that both nerve terminals and myoepithelial cells existed in the medial and distal, but not proximal portions of rat AMG acini. On the contrary, only unmyelinated and myelinated nerve fibers were observed, but none of myoepitheli al cell existed in gerbil AMG. Two types of nerve varicosities, one type contain ing clear vesicles ( probably cholinergic), and the other type having dense cored granules (probably adrenergic or peptidergic) , appeared in close spatial contact with the acinar cells. A number of nerve varicosities were located in the intercellular space of serous cells, indicating a direct innervation of the gerbil AMG acinar cells. The distribution and morphological construction of autonomic innervation has been respectively studied in the nasal gland of human, rat and guinea pig ( Cauna et al., 1972, Grote et al., 1975, Yokoyama et al., 1991). Their findings showed that the epithelium of the glandular acini was only innervated by cholinergic nerve fibers. All intraepithelial nerve fibers were acetylcholinesterase (AChE) positive and contained a lot of round and clear vesicles showing vasoactive intestinal peptide (VIP) and /or neuropeptide Y immunoreactivity. No adrenergic fibers were seen in the nasal glandular cells. It was generally accepted that the secretion of serous gland was under control of the autonomic nerve system. Parasympathetic stimulation greatly enhanced the secretion of acinar cells, while sympathetic stimulation decreased the serous secretion (Garrett and Thulin, 1975,
Delong and Getchell, 1987). In the major salivary glands, including submaxillary and parotid glands in the rat, hamster and guinea pig, both cholinergic and adrenergic axons were found closely surrounding the serous acinar cells. This phenomenon was observed in the gerbil AMG.
Lectin Histochemistry.
According to the linkage of the sugar to the peptide chain, two types of glycoproteins, O- and N-linked glycoproteins, can be found in eukaryotes. In N-linked glycoproteins, the carbohydrates are linked to asparagine residues in Asn-Xaa-Thr/Ser sequons. The Xaa is any amino acid except proline and may be composed of L-fucose, D-mannose, D-galactose and N-acetyl-D-glucosamine (GlcNAc). In O-linked glycoproteins, carbohdrates are linked to the hydroxyl group of serine and theronine by their reducing terminal N- acetyl-D-galactosamine (GalNAc) ( Bretthauer , 2007).
Because lectins have the capacity to bind sugar residues with very high specificity, numerous lectins were commonly used as specific sugar probes to study the glycoconjugate structure in cellular sites, such as plasmmalemma, lysosomes, Golgi cisternae and secretory granules (Schulte and Spicer, 1985, Accili et al., 1992, Menghi and Materazzi, 1994). In the present study, eight lectins were used to investigate the glycoconjugate properties of the serous gland in rat and gerbil AMGs. Table 2 summarized the results of lectin bindings in the present study.
Serous cells are well known to contain abundant N-linked glycoproteins, such as immunoglobulin secretory component, lactotransferrin and lysozymes, by immunohistochemical methods. The strong affinity for ConA, which binds preferentially to mannose, and for WGA, which reacts with N-acetylglucosamine of N-linked glycoproteins, was in agreement with the localization of N-linked glycoproteins in the serous cells of AMG in rat and gerbil. On the other hand, the weak staining of UEA demonstrated their product without terminal fucose. The variation in intensity and affinity of DBA, SBA, PNA, RCA-1 or EBL indicated the presence of GalNAc residues in O-linked glyoproteins from the secretions of AMG acinar cells. The strong binding of RCA-1 and EBL indicated that the galactosyl residues were abundant in the secretion of serous cells in rat and gerbil AMG. PNA has been known to have a carbohydrate specificity towards terminal disaccharide Gal1,3GalNAc. Present results showed a weak staining of PNA for AMG serous cells. The notable difference of lectin labeling in the secretion of AMG serous cells between rat and gerbil were DBA and SBA. The strong staining with DBA and SBA demonstrated the high content of GalNAc in the secretion of gerbil AMG acini, but these observations were not confirmed in rat AMG acini. Generally, the strong staining of DBA and SBA was always noticed in the mucin, i.e., the secretion of mucous glands of respiratory and digestive tracts (Caldero et al., 1988, Kawai et al.,
1988). However, the presence of some O-linked glycoproteins in addition to the N-linked glycoproteins in serous cells was also found in the rat submandular gland (Zhang et al., 1994). The primary purpose of many mucins is to retain the water at surfaces that are exposed to the environment but are not sealed by moisture-impermeable layers. Addition of cluster of sialylated glycan results in strong negative charge in mucin that gives the mucins the capacity to bind large amounts of water.
Because they are highly hydrated structures, the energetic cost of releasing water from mucins reduces the rate of evaporations (Taylor and Drickamer, 2003).
In conclusion, our study has revealed the differences in morphological characteristics and lectin histochemistry of rat and gerbil AMGs and we speculate that
the differences between rat and gerbil AMG cells may be due to the different living
environment of these two rodents, i.e., moderate wet vs. extremely dry. Moreover,
increased concentrations of GalNAc in the gerbil acinar cells compared with those of the rat, may indicate the abundance of GalNAc glycoconjugate enables the gerbil, which lives in the dry desert, to retain water and thus to maintain homeostasis.
Cell Biology, College of Medicine, National Taiwan University) for his excellent technical support. This work was supported in part by grants (NSC98-2628-13-002-085-MY3 to KSL) from the National Science Council, Taiwan.
References
Accili D., Menghi G., Bondi A.M. and Scocco P. (1992). Glycoconjugate composition of mammalian parotid glands elucidated in situ by lectins and glycosidases. Acta Histochem. 92, 196-206.
Archer F.L. and Kao V.C. (1968). Immunohistochemical identification of actomyosin in myoepithelium of human tissues. Lab. Invest. 18, 669-674.
Becerra L., Soares R.V., Bruno L.S., Siqueira C.C., Oppenheim F.G., Offner G.D. and Troxler R.F. (2003). Patterns of secretion of mucins and non-mucin glycoproteins in human submandibular/sublingual secretion. Arch. Oral Biol. 48, 147-154.
Bojsen-Moller F. (1964). Topography of the Nasal Glands in Rats and Some Other Mammals. Anat. Rec. 150, 11-24.
Bretthauer R.K. (2007). Characterization of O-Linked Saccharides on Glycoproteins. In: Pichia Protocols Methods in Molecular Biology, vol 389. 2nd ed. Cregg J.M. (ed). Humana Press Inc. Totowa, NJ. pp107-110.
Caldero J., Campo E., Calomarde X. and Torra M. (1988). Distribution and changes of glycoconjugates in rat colonic mucosa during development. A histochemical study using lectins. Histochemistry 90, 261-270.
Castle J.D. (1998). Protein secretion by rat parotid acinar cells. Pathways and regulation. Ann. N. Y. Acad. Sci. 842, 115-124.
Neurocytol. 1, 49-60.
Delong R.E. and Getchell T.V. (1987). Nasal Respiratory-Function - Vasomotor and Secretory Regulation. Chemical Senses 12, 3-36.
Ellis R.A. (1965). Fine structure of the myoepithelium of the eccrine sweat glands of man. J Cell Biol. 27, 551-563.
Emerman J.T. and Vogl A.W. (1986). Cell size and shape changes in the myoepithelium of the mammary gland during differentiation. Anat. Rec. 216, 405-415.
Garrett J.R. and Thulin A. (1975). Structural changes associated with parotid "degeneration secretion" after post-ganglionic sympathectomy in rats. Cell Tissue Res. 162, 1-12.
Grote J.J., Juijpers W. and Huygen P.L. (1975). Selective denervation of the autonomic nerve supply of the nasal mucosa. Acta Otolaryngol. 79, 124-132.
Kawai T., Greenberg S.D. and Titus J.L. (1988). Lectin histochemistry of normal lung and pulmonary adenocarcinoma. Mod. Pathol. 1, 485-492.
Kerjaschki D. (1974). The anterior medial gland in the mouse nasal septum: an uncommon type of epithelium with abundant innervation. J. Ultrastruct. Res. 46, 466-482.
Kris-Etherton P.M. and Dietschy J. (1997). Design criteria for studies examining individual fatty acid effects on cardiovascular disease risk factors: human and animal
studies. Am. J. Clin. Nutr. 65, 1590S-1596S.
Kucharewicz I., Bodzenta-Lukaszyk A. and Buczko W. (2008). Experimental asthma in rats. Pharmacol. Reports. 60, 783-788.
Lee S.K., Lim C.Y., Chi J.G., Yamada K., Kunikata M., Hashimura K. and Mori M. (1990). Immunohistochemical localization of lysozyme, lactoferrin, alpha 1-antichymotrypsin, and alpha 1-antitrypsin in salivary gland of human fetuses. Acta Histochem. 89, 201-211.
Menghi G. and Materazzi G. (1994). Exoglycosidases and lectins as sequencing approaches of salivary gland oligosaccharides. Histol. Histopathol. 9, 173-183.
Moore D.M., Vogl A.W., Baimbridge K. and Emerman J.T. (1987). Effect of calcium on oxytocin-induced contraction of mammary gland myoepithelium as visualized by NBD-phallacidin. J. Cell Sci. 88 ( Pt 5), 563-569.
Norberg L., Dardick I., Leung R., Burford-Mason A.P. and Rippstein P. (1992). Immunogold localization of actin and cytokeratin filaments in myoepithelium of human parotid salivary gland. Ultrastruct. Pathol. 16, 555-568.
Ogawa Y., Yamauchi S., Ohnishi A., Ito R. and Ijuhin N. (1999). Immunohistochemistry of myoepithelial cells during development of the rat salivary glands. Anat. Embryol. (Berl). 200, 215-228.
Philips C.J., Nagato T. and Tandler B. (1987). Compatative ultrastructure and evolutionary patterns of acinar secretory product of parotid salivary glands in
Neotropical bats. In: Studies in Neotropical Mammalogy: Essay in Honor of Philip Hershkovitz. Patterson B.D. and Timm R.M. (eds). Field Museum of Natural History. Chicago. pp 213-229.
Ramachandran P., Boontheung P., Xie Y., Sondej M., Wong D.T. and Loo J.A. (2006). Identification of N-linked glycoproteins in human saliva by glycoprotein capture and mass spectrometry. J. Proteome Res. 5, 1493-1503.
Satoh Y., Oomori Y., Ishikawa K. and Ono K. (1994). Configuration of myoepithelial cells in various exocrine glands of guinea pigs. Anat. Embryol. (Berl). 189, 227-236. Schulte B.A. and Spicer S.S. (1985). Histochemical methods for characterizing secretory and cell surface sialoglycoconjugates. J. Histochem. Cytochem. 33, 427-438.
Tandler B. and Bojsen-Moller F. (1978). Ultrastructure of the anterior medial glands of the rat nasal septum. Anat. Rec. 191, 147-167.
Tandler B., Edelstein D.R. and Erlandson R.A. (2000). Ultrastructure of submucosal glands in human anterior middle nasal turbinates. J. Anat. 197, 229-237.
Tandler B. and Phillips C.J. (1993). Structure of serous cells in salivary glands. Microsc. Res. Tech. 26, 32-48.
Taylor M.E. and Drickamer K. (2006). O-linked glycosylation. In: Introduction to glycobiology. 2nd ed.Oxford University Press. Oxford. pp 50-68
central acinar cells of the submandibular salivary gland of ferret investigated by lectin histochemistry. Arch. Oral Biol. 49, 697-703.
Ungar D. (2009). Golgi linked protein glycosylation and associated diseases. Semin. Cell Dev. Biol. 20, 762-769.
Walker RA. (1989) The use of lectins in histopathology. Path. Res. Pract. 185, 826-835.
Yokoyama R., Inokuchi T., Takahashi Y. and Watanabe I. (1991). An electron microscopic study of acetylcholinesterase-activity and vasoactive intestinal peptide-and neuro-peptide Y-immunoreactivity of the intraepithelial nerve fibers in the nasal gland of the guinea pig. Arch. Histol. Cytol. 54, 59-67.
Zhang X.S., Proctor G.B., Garrett J.R., Schulte B.A. and Shori D.K. (1994). Use of lectin probes on tissues and sympathetic saliva to study the glycoproteins secreted by rat submandibular glands. J. Histochem. Cytochem. 42, 1261-1269.
Tables.
Table 1. The lectins used and their sugar binding specificities. Common Name Source Specificity
Glucose∕Mannose
Con A(jackbean) Concanavalin A αMan>αGlc Fucose
UEA(grose) Ulex europeus I αL-Fuc N-acetyl Glucosamine
WGA(wheat germ) Triticum vulgaris GlcNAc>βGlcNAc N-acetyl Galactosamine∕Galactose
DBA(horsegram) Dolichos biflorus GalNAcα1,3 GalNAc> αGalNAc
SBA(soybean) Glycine maximus α and βGalNAcα and βGal
PNA(peanut) Arachis hypogaea Galβ,1,3 GalNAc RCA-1 Ricinus communis βGal>αGal
Sialic Acid
SNA,EBL Sambucus α2,6 Gal>α2,3 Gal*
Man=mannose Glc=glucose GlucNAc=NAcetyl glucosamine GalNAc= Nacetyl galactosamine Gal=galactose Fuc=fucose
Walker RA .(1989) The use of lectins in histopathology. Path. Res. Pract. 185, 826-835.
Table 2. Comparative staining of AMG with lectin histochemistry in Rat and Gerbil.
Lectin Acinus Proximal part Medial part Distal part Rat Gerbil Rat Gerbil Rat Gerbil
DBA cytoplasm –~+ ++ – +++ – ++ capsule – +++ – +++ – +++ SBA cytoplasm +~++ ++ – ++ – ++ capsule ++ + – + – ++ PNA cytoplasm + – – – – – capsule ++ – – – – – UEA cytoplasm – – – – – – capsule – – – – – – WGA cytoplasm ++ ++ + +++ ++ ++ capsule +++ ++~+++ +++ +++ +++ +++ RCA-1 cytoplasm +~++ +~++ –~+ –~+ + +~++ capsule +++ ++~+++ ++ –~+ +++ +++ Con A cytoplasm ++ ++ ++ ++~+++ ++ ++ capsule +++ +++ +++ +++ +++ +++ EBL cytoplasm – – – – – – capsule +++ +++ ++ ++ +++ +++
Figure legends
Fig. 1. Schematic diagrams showing the anterior medial gland (AMG) in rat (1A) and gerbil (1B) nasal septa. The anterior medial glands (AMG) underlying the submucosa of rat and gerbil nasal septa are pure serous glands consisting of numerous serous acini. According to the spatial location of acinar cells, the AMG in rat and gerbil nasal septum can be divided into proximal (A), middle (B) and distal regions (C). Note that figure 1A is modified from Tandler and Bojsen-Moller (1978). V: vestibule.
Fig. 2. Electron micrographs of serous acini in the proximal region of rat AMG. (A) Rat AMG acinar cells are arranged around a small central lumen (L). The nucleus (N) with prominent nucleolus is irregular in shape and located at the basal position. Note that numerous secretory granules (Z1-Z3) are usually seen at the apical surface of the cell. Endoplasmic reticulum (ER) and mitochondria are abundant through the cytoplasm. (B) High magnification of electron micrograph of the apical cytoplasm of an acinar cell, showing two types of secretory granules (Z1 and Z2) near the lumen (L). Golgi apparatus (G), mitochondria (m), rough endoplasmic reticulum (ER). Fig. 3. Electron micrographs of acinar cells in the middle regions of rat AMG. (A) A low magnified micrograph showing that AMG acinar cells contain numerous secretory granules which are homogenous with high to moderate (Z1) or medium to low (Z3) electron density, near the luminal surface. Note that numerous processes of
myoepithelial (myo) cells are located surrounding the acini. Rough endoplasmic reticulum (ER), nerve terminal (n). (B) Numerous spherical or rod-like mitochondria (m), dilated rough endoplasmic reticulum (ER) and zymogen granules with high electron density (Z1) are seen in the apical cytoplasm of an acinar cell. (C) Electron micrograph showing a myoepithelial cell process underlying the acinar cell. Note that microfilaments (mf) are evident in the cytoplasm of myoepithelial cell. (D) A nerve terminal which contains neurofilaments and mitochondria (m) is located at the bottom of AMG. G: Golgi ; ER: rough endoplasmic reticulum.
Fig. 4. Electron micrographs of gerbil AMG acinar cells. (A) In the cytoplasm of gerbil AMG acinar cells, two types of secretory granules (Z1 and Z2) and some profiles of rough endoplasmic reticulum (ER) are observed. Note numerous unmyelinated nerve fibers (nf) surrounding the acini of AMG . Nucleus (N), lumen (L). (B, C) High magnification of Electron micrographs depicting the variations in secretory granule substructure. The type I granules (Z1) are characterized with lamellated structures and some dense vesicles (B), while the type II granules contain particulate secretory material with moderate electron density (C). Rough endoplasmic reticulum (ER), mitochondria (m).
Fig. 5. Electron micrographs of the basal aspect of gerbil AMG acinar cells. (A) Myelinated and unmyelinated nerve fibers were shown close to the basement
membrane of AMG. The bundles of unmyelinated nerve fibers were surrounded by thin sheets of Schwann cells. (B, C) It is noted that some nerve terminals (arrows) that contain abundant small clear and a few large dense core vesicles are found within the intercellular space of gerbil AMG acinar cells.
Fig. 6. Light micrographs of the proximal region of rat AMG acinar cells after lectin staining. It is noted that absence of UEA and EBL staining and only weak staining of DBA or PNA are observed in the cytoplasm of AMG acinar cells. The staining of SBA and RCA are weak to moderate. Only the reactivity of Con A and WGA are shown from moderate to strong intensity in the cytoplasm of acinar cells.
Fig.7. Light micrographs of the distal region of gerbil AMG acinar cells after lectin staining. DBA reactivity is moderate to strong in the cytoplasm of acinar cells. The UEA and EBL staining are negative. The reactivity of Con A and WGA staining are seen from moderate to strong in gerbil AMG acinar cells. The staining of SBA and RCA in the cytoplasm of acinar cells is weak to moderate.