1 Corresponding author: wnt@ntu.edu.tw
137
Growth and Habitat Residence History of Migrating Silver
American Eels Transplanted to Taiwan
W
ann-n
ianT
zeng1 andY
u-S
anH
anInstitute of Fisheries Science, College of Life Science, National Taiwan University, Taipei, Taiwan 106, ROC
B
rianM. J
eSSopDepartment of Fisheries and Oceans, Beddford Institute of Oceanography Post Office Box 1006, Dartmonth, Nova Scotia B2Y 4A2, Canada
Abstract.—In Taiwan, there has been a shortage of local Japanese eel Anguilla japon-ica elvers for culture, so culturists have imported Amerjapon-ican eel Anguilla rostrata (Le
Sueur) elvers from North America to meet their needs. From 1999 to 2001, six exotic adult American eels were found in the estuary of the Kaoping River of Taiwan that had escaped from aquaculture ponds as young eels and stayed in the river until silvering. This study compares growth performance and migratory behavior, using otolith stron-tium (Sr)/calcium (Ca) ratios of those six American eels with cohabitating Japanese eels and American eels in North America. Regardless of sex, mean age at maturity of the exotic American eels was greater and mean annual growth rate was less than that of Japanese eels in Taiwan and similar to that of American eels in the southern United States. Sr/Ca ratios at the otolith edge of the six exotic American eels, which recorded their salinity history, increased significantly. Furthermore, four of the six exotic Ameri-can eels spent more than one year in the high-salinity estuary. Their extended residence in the estuary may be due to a delayed spawning migration resulting from a failure to orientate and migrate properly to their native spawning site.
Introduction
Anguillid eels are catadromous fish that spawn in the ocean and grow in freshwater (Tesch 1977). Of the 15 described species and 3 subspecies of anguillid eels in the world (Ege 1939; Castle and Williamson 1974), all are dis-tributed in the Indo-Pacific region except for the European eel Anguilla anguilla (L.) and the American eel A. rostrata (Le Sueur), which occur in the North Atlantic Ocean. In Taiwan, four native anguillid eels exist: Japanese eel A.
japonica Temminck and Schlegel, marbled eel
A. marmorata Quoy and Gaimard, A. bicolor
pacifica Schmidt, and Celebes longfin eel A.
celebesensis Kaup (Tzeng 1982; Tzeng and Tabeta 1983; Han et al. 2001). Of these spe-cies, the Japanese eel is most abundant and is commercially important for aquaculture in Taiwan (Tzeng et al. 1995). The catch of Japa-nese eel elvers is insufficient to meet the aqua-culture demand (Tzeng 1996a), and elvers of exotic eel species, mainly American and Euro-pean eels, have been imported since 1969 and 1977, respectively (Li 1997).
American Fisheries Society Symposium 58:137–147, 2009 © 2009 by the American Fisheries Society
Six exotic American eels—four females (AR1 to AR4) and two males (AR5 and AR6)—were caught in the estuary of the Kaoping River of Taiwan; these were distin-guished from Japanese eels (Han et al. 2002) by their different morphology. These eels had thick snouts, round heads, abundant mesen-teric fat, and unusually large eyes. Vertebral counts, quantified gonadal histology, and phylogenetic analysis of the mitochondrial cytochrome b gene validated them as Ameri-can eels in the silver stage (Han et al. 2002). These eels had escaped from culture ponds early in life and had lived in the Kaoping River for years.
Otolith Sr/Ca ratios, in combination with age data, were used to reconstruct the envi-ronmental history of these exotic American eels and assist interpretation of their life his-tory. The concentration of strontium (Sr) is approximately 100-fold greater in seawater than in freshwater, and the Sr/Ca ratio in oto-liths is positively correlated to ambient salin-ity (Tzeng 1996b; Campana 1999), making the Sr/Ca ratio a valuable tool for analyzing migration behavior of eels (Tzeng et al. 1997, 2002, 2003). In this study, we (1) compared annual growth rate of the exotic American eels with that of Japanese eels collected at the same time in the Kaoping River and with that of American eels in North America and (2) examined their life history traits (e.g., age at maturity and migration).
Materials and Methods
Specimen Collection
A total of 61 silver Japanese eels and six exotic American eels were collected by plastic eel pots in the Kaoping River estu-ary of southern Taiwan from 1999 to 2001 (Figure 1) (Han et al. 2002, 2003). The pots were fixed on the bottom along the riverbank. After collection, eels were immobilized with ice and immediately transferred to the
labora-tory for detailed analysis. Total length (TL ± 0.1 cm) and body weight (BW ± 0.1 g) were measured. Sex of each eel was determined by histological examination of the gonads. Maturation stages were determined, follow-ing Han et al. (2002, 2003), by coloration of the pectoral fins and the dorsal region, as well as by silver color on the belly and gonadal development. Growth and age at maturity of the exotic American eels were compared with those of American eels in North America re-ported in the scientific literature.
Otolith Preparation for Age Determination and Sr/Ca Ratio Analysis
Sagittal otoliths, the largest of the three pairs of otoliths in the inner ear, were used for determining Sr/Ca ratios and ages of the 61 silver Japanese eels and six exotic Ameri-can eels. An electron probe microanalyzer (EPMA, JEOL JXA-8900R) was used to measure otolith strontium (Sr) and calcium (Ca) concentrations. The procedure for pre-paring the otoliths for Sr/Ca ratio analysis followed that of previous studies (Tzeng et al. 1997). Sr and Ca concentrations in the otolith were measured from the primordium to the otolith edge at an interval of 20 µm with an electron beam diameter of 5 µm. The acceler-ating voltage of EPMA was set at 15 kV and probe current at 5 nA. After microchemistry analysis, the otolith was polished to remove the carbon coating and etched 1–2 min with 5% EDTA to reveal the annular marks for age determination (Tzeng et al. 1994, 1997).
Back Calculation of Total Length at Annulus Formation and Growth Rate Measurement
The radii from the primordium to the glass
eel mark (ro), annuli (rn) and otolith edge (R)
were measured to estimate total length at
an-nulus formation (ln) by the Dahl-Lea formula
139 Migrating Silver American Eels
ln = lo + (rn – ro) (R – ro)−1 (L – l
o)
where L is total length of the silver eel at
capture. Total length at the glass eel stage (lo)
was defined as 54 mm, based on Tzeng and Tabeta (1983). Mean absolute annual growth rate (G) for individual silver eels was calcu-lated according to the formula:
G = (ln – ln – 1)
where ln is total length at year n and ln – 1 is
total length at year n –1. Mean annual growth
rates (Ga) for each geographic and sex group
were estimated as Ga = mean TL (mm)/mean
age (yr) to enable comparison of data from other sites.
Statistical Analysis
Differences in morphometric indexes among exotic American eels in Taiwan, na-tive American eels in Nova Scotia, Canada, and native Japanese eels in Taiwan were ex-amined by using analysis of variance (ANO-VA) followed by Tukey’s multiple compari-son test, as appropriate. The variable data for each ANOVA were logarithmically (base 10)
120°E 121°E 122° E 22°N 23°N 24°N 25°N Kaoping River Taiwan Estuary
Figure 1. Sampling site of Japanese and exotic American eels in the estuary of the Kaoping River
transformed to reduce their heterogeneity of variance and nonnormality of distribution (differences were considered significant at α ≤ 0.05).
Results
Comparison of Morphological Indices Among Eel Species and Locations
Length of the female exotic American eels ranged from 515 to 700 mm and the males from 415 to 425 mm, while ages ranged from 8 to 10 years (Table 1). Females were signifi-cantly longer (F = 15.26, df = 1,4, P = 0.017) and heavier (F = 15.63, P = 0.017) than males and grew faster (F = 31.18, P = 0.005) but were similar in age at maturity (F = 0.88, P = 0.40). Mean total length and body weight of the male exotic American eels in the sil-ver stage were larger than those of American eels in Nova Scotia and smaller than those of Japanese eels (male TL: F = 163.3, df = 2,53,
P < 0.001; male BW: F = 158.1, df = 2,53,
P < 0.001) (Table 2a, 2b). Mean TL of the
fe-male exotic American eels was similar to that of Japanese eels and larger than that of American eels from Nova Scotia, while body weight was greater than that of American eels from Nova Scotia and less than Japanese eels (female TL:
F = 51.1, df = 2,69, P < 0.001; female BW: F =
30.6, df = 2,69, P < 0.001). Mean ages of
Amer-ican eels of both sexes in Taiwan were lower than those of eels from Nova Scotia but higher than those of Japanese eels (male: F = 126.5, df = 2,53, P < 0.001; female: F = 127.1, df = 2,69,
P < 0.001) (Table 2c).
Comparison of Mean Annual Growth Rate Among Exotic American Eels
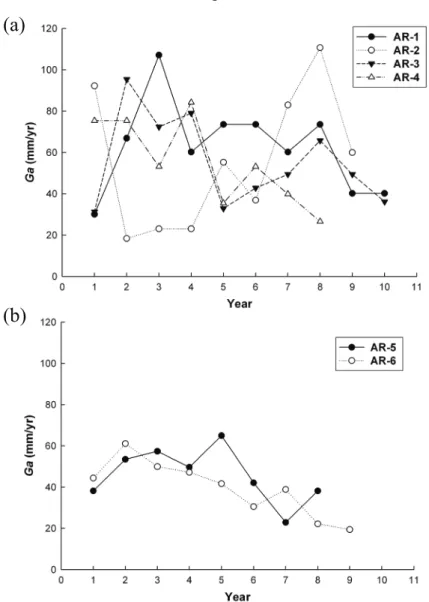
Back-calculated annual growth rate (G) of the six exotic American eels varied greatly (Fig-ure 2). Growth in the first year was not rapid, as is usually observed in cultured eels due to nutrition (Tzeng, personal observation). Alter-natively, if these eels grew slowly in the cul-ture ponds for some reason, their life span in the ponds would not exceed two years because farmers commonly drain the ponds every two years. Because of their older ages at capture, it is obvious that these exotic American eels es-caped from the culture ponds very early in life.
Comparison of Growth Rate Among Eel Species and Locations
For each sex, mean annual growth rate (Ga)
of exotic American eels from Taiwan was much lower than that of Japanese eels from Taiwan but much higher than eels from Nova Scotia (male:
F = 361.7, df = 2,53, P < 0.001, CA<TA<TJ;
female: F = 330.3, df = 2,69, P < 0.001, CA <
Specimen AR-1 AR-2 AR-3 AR-4 AR-5 AR-6
Characters
Date of collection Jul. Jul. Oct. Sep. Feb. Feb. 1999 1999 2000 2000 2001 2001 Total length (mm) 700 612 635 515 425 415 Body weight (g) 454.6 519.2 591.2 236.3 129.4 125.1 Sex F F F F M M Age (yr) 10+ 9+ 10+ 8+ 8+ 9+ Ga (mm/yr) 66.7 64.4 58.8 58.5 52.5 45.6
Table 1. Morphological characters of the six exotic silver American eels (AR1-AR6) collected in
141 Migrating Silver American Eels
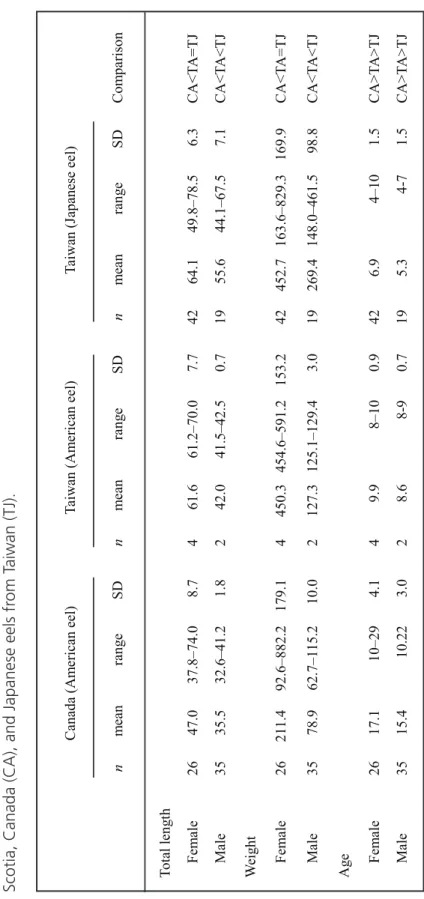
Table 2. Comparison of total length (cm), body weight (g), and age at maturity (yr) of American eels fr om Taiwan (T A) and Nova
Scotia, Canada (CA), and Japanese eels fr
om T aiwan (TJ). Canada (A m erican eel) Ta iw an (A m erica n eel) Ta iw an (J apan ese ee l) n m ean rang e SD n m ean rang e SD n m ean rang e SD C om parison To ta l leng th Fe m al e 26 47.0 37.8–74.0 8.7 4 61.6 61.2–70. 0 7.7 42 64.1 49.8–78.5 6.3 CA<T A =T J Male 35 35.5 32.6–41.2 1.8 2 42.0 41.5–42. 5 0.7 19 55.6 44.1–67.5 7.1 CA<T A <T J W ei ght Femal e 26 21 1. 4 92.6–882.2 179.1 4 450. 3 454.6–591.2 153.2 42 45 2. 7 163.6–829. 3 169.9 CA<T A =T J Male 35 78.9 62.7–1 15 .2 10.0 2 127. 3 125.1–129.4 3.0 19 26 9. 4 148.0–461. 5 98.8 CA<T A <T J A ge Fem al e 26 17.1 10–2 9 4.1 4 9.9 8–10 0.9 42 6.9 4–10 1.5 CA>T A >T J Male 35 15.4 10 .2 2 3.0 2 8.6 8-9 0.7 19 5.3 4-7 1.5 CA>T A >T J
TA < TJ) and slightly lower than that of eels from the southern United States (Table 3). Al-though no statistical comparison can be made,
the mean Ga values are sufficiently similar that
no significant difference likely exists between American eels in Taiwan and those in the southern United States. The Nova Scotia habi-tat has low pH and low productivity and would be expected to have eels with a lower growth rate than those from a more productive site at that latitude.
Mean annual growth rate of native male (r
= –0.94, 95% CI = –1.0 to –0.80, n = 5, P < 0.001, estimated by the bootstrap method) and female (r = –0.69, 95% CI = –0.87 to –0.37, n = 12, P = 0.016) American eels declines signifi-cantly with increasing latitude (Oliveira 1999; Jessop, unpublished data). American eels from a wide range of latitudes also showed a better
significant negative correlation between Ga and
age at maturity for each sex (male: r = –0.95, 95% CI = –1.0 to –0.88, n = 5, P < 0.001; fe-male: r = –0.86, 95% CI = –0.96 to –0.71, n = 12, P < 0.001), while no significant
correla-(a)
(b)
Figure 2. Comparisons of back-calculated annual growth rate for (a) four female and (b) two
143 Migrating Silver American Eels
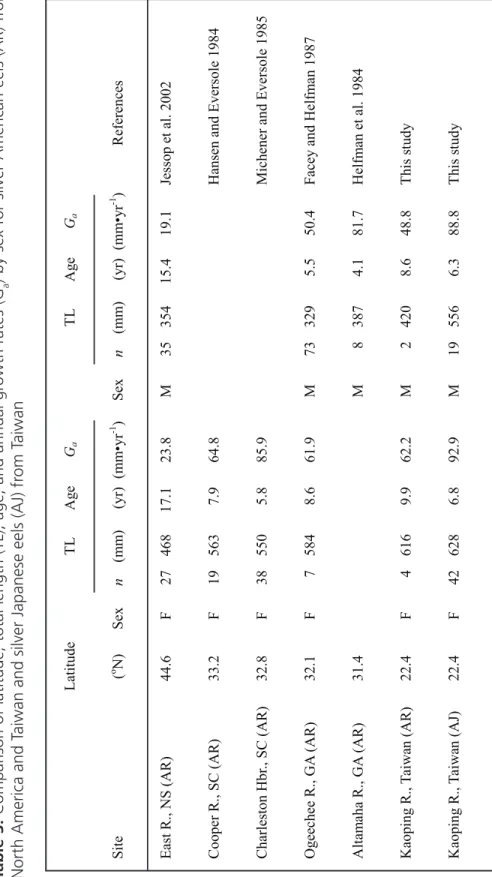
Table 3. Comparison of latitude, total length (TL), age, and annual gr owth rates (Ga ) by sex for silver American eels (AR) fr om
North America and T
aiwan and silver Japanese eels (AJ) fr
om T aiwan Latitude TL A ge Ga TL A ge Ga Si te ( o N ) Sex n (m m ) (y r) (m m •y r -1 ) Sex n (m m ) (y r) (m m •y r -1 ) References East R., NS (AR) 44.6 F 27 468 17.1 23.8 M 35 354 15.4 19.1 Jess op e t al. 2002 Cooper R., SC (AR) 33.2 F 19 563 7.9 64.8
Hansen and Eversole 1984
Charleston Hb r., SC (AR) 32.8 F 38 550 5.8 85.9 Mich ener an d Ev erso le 198 5 Ogeechee R., GA (AR) 32.1 F 7 584 8.6 61.9 M 73 329 5.5 50.4 Face y and Helf m an 1987 A lta m aha R., GA (AR) 31.4 M 8 387 4.1 81.7 Helf m an et al. 1984 Kaop in g R., Ta iw an (AR) 22.4 F 4 616 9.9 62.2 M 2 420 8.6 48.8 Th is st udy Kaop in g R., Ta iw an (A J) 22.4 F 42 628 6.8 92.9 M 19 556 6.3 88.8 Th is st udy
tion was found between Ga and TL at the silver stage for either male (r = 0.32, n = 5, P = 0.60) or female (r = –0.06, n = 12, P = 0.86) eels (Jes-sop, unpublished data).
Sr/Ca Ratios in Otoliths of Exotic American Eels
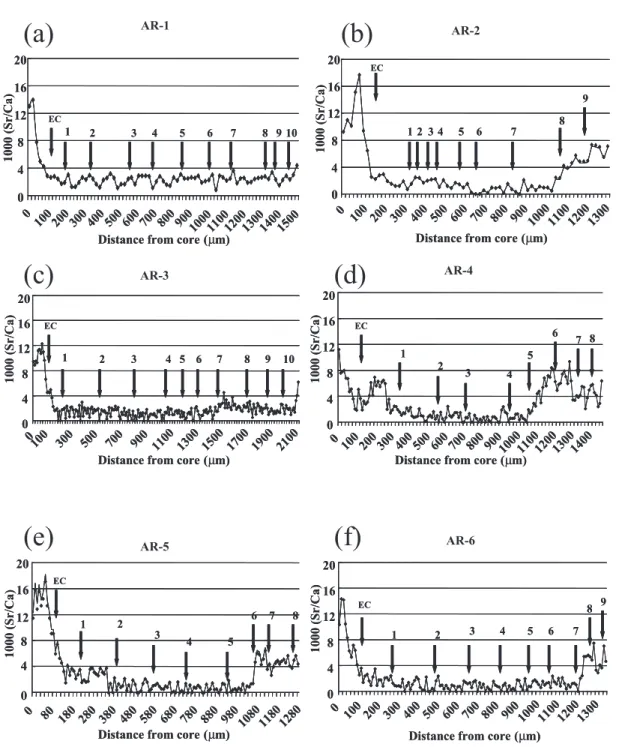
Otolith Sr/Ca ratios of the six exotic sil-ver American eels increased from the primor-dium with a peak at the distance 60–100 µm from the primordium, which corresponded to the timing of metamorphosis from leptoceph-alus to glass eel (Figure 3). Beyond the check associated with metamorphosis, the otolith Sr/Ca ratios decreased rapidly up to the elver check (EC), which is deposited when glass eels enter freshwater in the estuary. Otolith Sr/Ca ratios were greater than 4.0‰ in the early life of five of the six eels. The 4.0‰ Sr/Ca ratio distinguishes between freshwa-ter and seawafreshwa-ter habitats (Tzeng et al. 2002, 2003). Sr/Ca ratios increased, exceeding 4.0‰ only at the very edge of the otolith for two eels (AR-1 and AR-3, Figure 3); this was coincident with silvering. However, Sr/Ca ra-tios at the edge of the otoliths of the other four eels were greater than 4.0‰ for several years (Figure 3). This indicated that these four eels had migrated into and resided in the high-salinity water of the estuary a number of years earlier.
Discussion
In the wild, juvenile eels from northern latitudes grow for a much shorter period of the year than do those from southern latitudes because annual temperatures are lower in the north and above the growth threshold for a shorter period of time. Thus, northern eels are expected to have a lower annual growth rate than more southerly eels. This decreased growth rate may also be reflected in the age of maturity (Vøllestad 1992). Exotic male American eels in Taiwan silvered and migrat-ed to the sea within the range of lengths and
growth rates of emigrants in North America. This was consistent with the observation that size of male American eels is not correlated with latitude (Oliveira 1999). As expected, female silver American eels in Taiwan also migrated at sizes more comparable to those in the southern United States, confirming the negative correlations between latitude, length at migration, and growth rate. These results are consistent with Oliveira’s (1999) conclusion that the panmictic life cycle of the American eel prevents long-term selection of growth rates in different habitats and that growth rates result from environmental con-ditions in a habitat. American eels in Taiwan were older at migration and grew much more slowly than did native Japanese eels, suggest-ing between-species differences in biological characteristics or reduced adaptability to en-vironmental conditions substantially different from those of streams, even in the southern United States.
Sr/Ca ratios at the otolith edges of Japa-nese eels increased significantly from the yellow to the silver stages, confirming that eels migrate from freshwater to high-salinity waters during silvering (Han et al. 2003). For the six exotic American eels, the Sr/Ca ratio had increased at the otolith edge (<4‰) only very recently for two individuals (AR-1 and AR-3) but was elevated for two to three years for the other four eels (Figure 3). If the rise of Sr/Ca ratios at the otolith edges coincided with silvering, then the spawning migration of these exotic American eels must have been interrupted, causing them to remain in the es-tuary.
Silver eels are generally thought not to feed because the digestive tract resorbs (Sinha and Jones 1975; Pankhurst and So-rensen 1984; Han et al. 2003). However, Beulleus et al. (1997) observed that cultured male eels may continue to eat and grow in the silver stage but not feed well. Dollerup and Graver (1985) fed male silver eels in which they had repeatedly induced testes
matura-145 Migrating Silver American Eels
AR-3 AR-4 AR-5 AR-6 AR-1 0 4 8 12 16 20 0 100 200 300 400 500 600700 800 900 100011001200130014001500
Distance from core (µm)
10 00 (S r/ C a) EC 1 2 3 4 5 6 7 8 9 10 0 4 8 12 16 20 0 100 200 300 400 500 600700 800 900 100011001200130014001500
Distance from core (µm)
10 00 (S r/ C a) EC 1 2 3 4 5 6 7 8 9 10
(a)
(b)
(c)
(e)
(d)
(f)
AR-2 EC 1 2 3 4 5 6 7 8 9 0 4 8 12 16 20 0 100 200 300 400 500 600 700 800 900 1000110012001300 10 00 (S r/ C a)Distance from core (µm)
EC 1 2 3 4 5 6 7 8 9 0 4 8 12 16 20 0 100 200 300 400 500 600 700 800 900 1000110012001300 10 00 (S r/ C a)
Distance from core (µm)
0 4 8 12 16 20 0100 300 500 700 900 1100 1300 1500 1700 1900 2100 10 00 (S r/ C a) EC 1 2 3 4 5 6 7 8 9 10
Distance from core (µm) 0 4 8 12 16 20 0100 300 500 700 900 1100 1300 1500 1700 1900 2100 10 00 (S r/ C a) EC 1 2 3 4 5 6 7 8 9 10
Distance from core (µm)
0 4 8 12 16 20 0 100 200 300 400 500 600 700 800 90010001100120013001400 10 00 (S r/ C a) EC 1 2 3 4 5 6 7 8
Distance from core (µm) 0 4 8 12 16 20 0 100 200 300 400 500 600 700 800 90010001100120013001400 10 00 (S r/ C a) EC 1 2 3 4 5 6 7 8
Distance from core (µm)
0 4 8 12 16 20 0 80 180 280 380 480 580 680 780 880 980 108011801280 10 00 (S r/ C a) EC 1 2 3 4 5 6 7 8
Distance from core (µm) 0 4 8 12 16 20 0 80 180 280 380 480 580 680 780 880 980 108011801280 10 00 (S r/ C a) EC 1 2 3 4 5 6 7 8
Distance from core (µm)
0 4 8 12 16 20 0 100 200 300 400 500 600 700 800 900 1000110012001300 10 00 (S r/ C a) EC 1 2 3 4 5 6 7 8 9
Distance from core (µm) 0 4 8 12 16 20 0 100 200 300 400 500 600 700 800 900 1000110012001300 10 00 (S r/ C a) EC 1 2 3 4 5 6 7 8 9
Distance from core (µm)
Figure 3. Otolith Sr/Ca ratio analysis for the six exotic American eels from (a) AR-1 to (f) AR-6,
tion with hCG to stimulate sperm develop-ment. They found that food intake gradually increased and that the eels grew both in size and weight and their atrophied alimentary tracts regenerated. Thus, silver eels might continue growing, but at a slower rate, if their natural spawning emigration was interrupted. When compared with growth in freshwater, the growth rate of exotic American eels AR-2, 4, and 6 decreased after the fish entered high-salinity water (presumably in the silver stage) (Figure 2). By contrast, Jessop et al. (2004) found that American eel growth was usually higher in estuarine waters than in freshwater. Furthermore, if increased Sr/Ca ratios at the otolith edges represented the silvering stage of the exotic American eels, true mean age and total length at maturity would decrease and mean annual growth rate would increase, becoming more similar to that of American eels in the southern USA (Table 2). We be-lieve that exotic American eels AR-2, 4, 5, and 6 had been in the silver stage for more than one year.
In Japan, exotic European eels have been found in Shinjiko Lake, Mikawa Bay (Zhang et al. 1999), and the Uono River (Aoyama et al. 2000). A migrating silver-phase Euro-pean eel has also been captured in the East China Sea (Aoyama et al. 2000), indicating that spawning migration of introduced eels might be possible. Since the life cycle and larval transport routes of American eels and Japanese eels are, in general, quite similar (i.e., North Equatorial Current and Kuroshio Current in the north Pacific for Japanese eels; North Equatorial Current and Gulf Stream in the North Atlantic for American eels) (Cheng and Tzeng 1996; Wang and Tzeng 1998, 2000), interspecific hybridization could oc-cur (Han et al. 2002). However, the silver exotic American eels found in the estuary of the Kaoping River in Taiwan seemed to en-counter some difficulty orienting properly to a potential spawning site. Thus, the threat of hybridization by exotic American eels with
Japanese eels seems unlikely. Further study is needed to assess the ecological importance of in-stream and migratory behaviors of exotic anguillid eels.
Acknowledgments
This study was financially supported by the National Science Council, Taiwan (Grant No. NSC-89–2313-B002–077). The au-thors are grateful to G.H. Cheng for otolith preparation and data processing and to Da-vid Cairns and two anonymous reviewers for helpful comments on an early version of the manuscript.
References
Aoyama, J., S. Watanabe, T. Miyai, S. Sasai, and M. Nishida. 2000. The European eel, Anguilla
anguil-la (L.), in Japanese waters Dana 12:1–5.
Beulleus, K., E. H. Eding, P. Gilson, F. Ollevier, J. Ko-men, and C. J. J. Richter. 1997. Gonadal differen-tiation, intersexuality and sex ratios of European eel (Anguilla Anguilla L.) maintained in captivity. Aquaculture, 153:135–150.
Campana, S. E. 1999. Chemistry and composition of fish otoliths: pathways, mechanism and applica-tions. Marine Ecology Progress Series 188:263– 297.
Castle, P. H. J., and G. R. Williamson. 1974. On the validity of the freshwater eel species Anguilla
an-cestralis Ege from Celebes. Copeia 1974:569–570.
Cheng, P. W., and W. N. Tzeng. 1996. Timing of metamor-phosis and estuarine arrival across the dispersal range of the Japanese eel Anguilla japonica. Marine Ecology Progress Series 131:87–96.
Dollerup, J., and C. M. Graver. 1985. Repeated induction of testicular maturation and spermiation, alternating with periods of feeding and growth in silver eels, Anguilla
anguilla (L.). Dana 4:19–39.
Ege, V. 1939. A revision of the genus Anguilla Shaw, a sys-tematic, phylogenetic and geographical study. Dana Report 16:1–256.
Facey, D. E., and G. S. Helfman. 1987. Reproductive migra-tions of American eels in Georgia. Proceedings of the Annual Conference of the Southeastern Association of Fish and Wildlife Agencies 39:132–138.
Francis, R. I. C. C. 1990. Back-calculation of fish length: a critical review. Journal of Fish Biology 36:883–902. Han, Y. S., C. W. Chang, J. T. He, and W. N. Tzeng. 2001.
147 Migrating Silver American Eels
Validation of the occurrence of short-finned eel
Anguil-la bicolor pacifica in the natural waters of Taiwan. Acta
Zoologica Taiwanica 12:9–19.
Han, Y. S., I. C. Liao, Y. S. Huang, J. T. He, C. W. Chang, and W. N. Tzeng. 2003. Synchronous changes of morphol-ogy and gonadal development of silvering Japanese eel
Anguilla japonica. Aquaculture 219:783–796.
Han, Y. S., C. H. Yu, H. T. Yu, C. W. Chang, I. C. Liao, and W. N. Tzeng. 2002. The exotic American eel in Tai-wan: Ecological implications. Journal of Fish Biology 60:1608–1612.
Hansen, R. A., and A. G. Eversole. 1984. Age, growth, and sex ratio of American eel in brackish-water portions of a South Carolina river. Transactions of the American Fisheries Society 113:744–749.
Helfman, G. S., E. L. Bozeman, and E. B. Brothers. 1984. Size, age, and sex of American eels in a Georgia River. Transactions of the American Fisheries Society 113:132–141.
Jessop, B. M., J. C. Shiao, Y. Iizuka, and W. N. Tzeng. 2002. Migratory behaviour and habitat use by American eels
Anguilla rostrata as revealed by otolith microchemistry.
Marine Ecology Progress Series 233:217–229. Jessop, B. M., J. C. Shiao, Y. Iizuka, and W. N. Tzeng. 2004.
Variation in the annual growth, by sex and migration history, of silver American eels Anguilla rostrata. Ma-rine Ecology Progress Series 272:231–244.
Li, L.S. 1997. Eel aquaculture. Page 84 in K. S. Chang, edi-tor. Aquaculture. Chien-Cheng Publishing Company, Kaohsiung, Taiwan.
Michener, W. K., and A. G. Eversole. 1985. Age, growth, and sex ratio of American eels in Charleston Harbor, South Carolina. 1983 Proceedings of the Annual Conference Southeastern Association of Fish and Wildlife Agencies 37:422–431.
Oliveira, K. 1999. Life history characteristics and strategies of the American eel, Anguilla rostrata. Canadian Jour-nal of Fisheries and Aquatic Sciences 56:795–802. Pankhurst, N. W., and P. W. Sorensen. 1984. Degeneration
of the alimentary tract in sexually maturing European
Anguilla anguilla (L.) and American eels Anguilla rostrata (Le Sueur). Canadian Journal of Zoology
62:1143–1149.
Sinha, V. R. P., and J. W. Jones, 1975. The European freshwa-ter eel. Liverpool University Press, Liverpool, UK. Tesch, F. W. 1977. The eel: biology and management of
an-guillid eels. Chapman and Hall, London.
Tzeng, W. N. 1982. Newly record of the elver, Anguilla
cele-besensis Kaup, from Taiwan. Bioscience 19:57–66.
Tzeng, W. N. 1996a. Short- and long- term fluctuations in catches of elvers of the Japanese eel, Anguilla japonica, in Taiwan. Pages 85–89 in D. A. Hancock, D.C. Smith,
A. Grant, and J. B. Beumer, editors. Developing and sustaining world fisheries resources: the state of sci-ence and management. 2nd World Fisheries Congres, Australia.
Tzeng, W. N. 1996b. Effects of salinity and ontogenetic movements on strontium:calcium ratios in the oto-liths of Japanese eel Anguilla japonica Temminck and Schlegel. Journal of Experimental Marine Biology and Ecology 199:111–122.
Tzeng, W. N., P. W. Cheng, and F. Y. Lin. 1995. Relative abundance, sex ratio and population structure of the Japanese eel Anguilla japonica in the Tanshui River system of northern Taiwan. Journal of Fish Biology 46:183–201.
Tzeng, W. N., Y. Iizuka, J. C. Shiao, Y. Yamada, and H. P. Oka. 2003. Identification and growth rates comparison of divergent migratory contingents of Japanese eel
(An-guilla japonica). Aquaculture 216:77–86.
Tzeng, W. N., K. P. Severin, and H. Wickström. 1997. Use of otolith microchemistry to investigate the environmental history of European eel Anguilla anguilla. Marine Ecol-ogy Progress Series 149:73–81.
Tzeng, W. N., J. C. Shiao, and Y. Iizuka. 2002. Use of otolith Sr:Ca ratios to study the riverine migratory behaviors of Japanese eel Anguilla japonica. Marine Ecology Prog-ress Series 245:213–221.
Tzeng, W. N. and O. Tabeta. 1983. First record of the short-finned eel Anguilla bicolor pacifica elvers from Taiwan. Bulletin of the Japanese Society of Scientific Fisheries 49:27–32.
Tzeng, W. N., H. F. Wu, and H. Wickström. 1994. Scanning electron microscopic analysis of annulus microstructure in otolith of European eel Anguilla anguilla. Journal of Fish Biology 45:479–492.
Vøllestad, L. A. 1992. Geographic variation in age and length at metamorphosis of maturing European eel: environ-mental effects and phenotypic plasticity. Journal of Ani-mal Ecology 61:41–48.
Wang, C. H., and W. N. Tzeng. 1998. Interpretation of geo-graphic variation in size of American eel, Anguilla
ros-trata (Le Sueur), elvers on the Atlantic coast of North
America by their life history and otolith ageing. Marine Ecology Progress Series 168:35–43.
Wang, C. H., and W. N. Tzeng. 2000. The timing of meta-morphosis and growth rates of American and European eel leptocephali: A mechanism of larval segregative mi-gration. Fisheries Research 46:191–205.
Zhang, H., N. Mikawa, Y. Yamada, N. Horie, A. Okamura, T. Utoh, S. Tanaka, and T. Motonobu. 1999. Foreign eel species in the natural waters of Japan detected by poly-merase chain reaction of mitochondrial cytochrome b region. Fisheries Science 65:684–686.