Benzene Adsorption
Short Original Communications
Short Original Communications
Kinetics of Benzene Adsorption onto Activated Carbon
Chia-Line Chuang 1, Pen-Chi Chiang 1 and E.E. Chang 2z Graduate Institute of Environmental Engineering, National Taiwan University, Taipei, Taiwan z Department of Biochemistry, Taipei Medical University, Taipei, Taiwan
Corresponding author: Chia-Line Chuang; e-mail: clc chuang@ms69.url.com.tw
DOh httD://dx.doi.oroJ10.1065/esDr2001.10.098
Abstract. A n activated carbon bed adsorption process is influ- enced by the adsorbents' characteristics, volatile organic com- pound (VOC) characteristics, and process conditions. In the lit- eratures, the adsorption processes of the adsorbents and VOCs were usually considered to be in equilibrium. In this study, the VOC adsorption processes by activated carbon were consid- ered to be a kinetic process, i.e. they are not in equilibrium. Then, isothermal adsorption curves and a small column experi- ment were simulated.
Keywords: Activated carbon; adsorption; benzene; desorption; kinetics
IIIF~I'I
Introduction
Activated carbon (AC) has been used extensively by indus- tries as an adsorbate for the removal of hazardous air pol- lutants among other applications. Major parameters that may affect the adsorption process include the surface prop- erty of the AC, characteristics and concentration of VOC, and temperature. As far as the modeling of adsorption iso- therm is concerned, three types of model can be recognized: the kinetic model, i.e. the Langmuir model [1], the thermo- dynamic model, i.e. the Gibbs adsorption isotherm equa- tion [2], and the statistical thermodynamic model, i.e. the Dubinin equation [3]. In the AC bed adsorption study, the usual models involve an isothermal plug-flow trace-compo- nent system with a linear equilibrium isotherm equation [4,5], a homogeneous surface diffusion model (HSDM) [6], and an ideal adsorbed solution theory (IAST) [7]. They con- sider adsorption to be an irreversible reaction or each solute to be in equilibrium.
In the actual adsorption process, the adsorption is not at an equilibrium condition and both adsorption and desorption processes occur at the same time. The objective of this in- vestigation is intended to develop a thermodynamic model with a non-linear driving force in conjunction with the Lang- muir model for predicting the effect of temperature and gas- eous concentration on activated carbon adsorption.
1 Model Development
1.1 Kinetic adsorption/desorption model
In 1916, Langmuir [1] developed an isotherm equation, ac- cording to the Langmuir hypothesis and considering the fol- lowing adsorption/desorption reaction condition of VOC
(C6H6) on AC. Initially, time = 0, the adsorption ratio on AC is 0; after reaction time t, the adsorption ratio is X; fi- nally, the equilibrium adsorption ratio is X~, and the VOC concentration is always maintained at C.
From the adsorption reaction rate and desorption reaction rate equilibrium, it can thus be integrated to
k a x Cx t = X e x In [Xff(X~-X)] (1)
(ka x C + k a) x t = In [Xff(X~-X)] (2)
Subsequently, the k., ka, and K can be solved, where K is the reaction constant equal to ka/k a.
1.2 Activated carbon bed adsorption simulation
Based on the above hypothesis, the physicochemical param- eters including ka, ka, and K, determined from the kinetic adsorption/desorption studies, can be used for the AC bed adsorption process. Assuming the adsorption process is a series of continuous-flow stirred tank reactors (CFSTRs), it can be simulated by the numerical method as:
AX~,~,i/At = k~ x (1 - Xs,t,i) X C g , t , i - k a x Xs,t, i
(3)
ACg,t,i/At = (AXs,t,i TM • q0 x M/n)/[(L x A - M/d)/n] (4)where Xs,t, i is the adsorption ratio on AC in the i-th unit CSTR at time, t, in the solid phase, q0 is the unit-layer ad- sorption weight per AC, M is the weight of AC, L is the length of the column, A is the cross-sectional area, and d is the density of AC.
2 Experimental Method
2.1 Kinetic adsorption/desorption studies
In each reaction, about 25 to 40 mg of treated AC was mea- sured and placed in a small scale that was 0.8 cm in diam- eter. Then, the scale was placed in a reaction chamber and the treated benzene vapor, which was controlled at the same temperature and concentration, passed through the reaction chamber. The measured weight change every 10 seconds was collected by a personal computer.
2.2 Activated carbon bed tests
A small column about 6 cm in length and 1 cm in diameter was used in this test. The reaction temperature was con- trolled by a thermostat at 303~ the weight of packed AC
6
ESPR - Environ Sci & Pollut Res 10 (1) 6 - 8 (2003)Short Original Communications
Benzene Adsorption
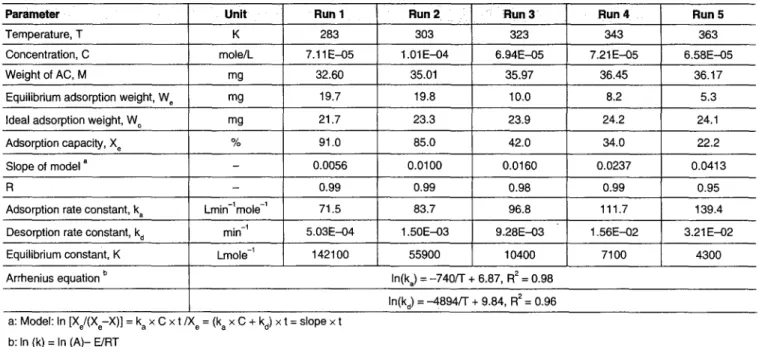
Table 1 9 Adsorption conditions and results of kinetic tests performed at different temperatures
P a r a m e ~ r Unit
Desorption rate constant, k d
Run I
min -I
Run 2 Run 3 Run 4 323 6.94E-05 35.97 10.0 23.9 42.0 0.0160 0.98 96.8 9.28E-03 10400 Run 5 Temperature, T K 283 303 343 363
Concentration, C mole/L 7.11E-05 1.01 E-04 7.21E-05 6.58E-05
Weight of AC, M mg 32.60 35.01 36.45 36.17
Equilibrium adsorption weight, W e mg 19.7 19.8 8.2 5.3
Ideal adsorption weight, W o mg 21.7 23.3 24.2 24.1
Adsorption capacity, X e % 91.0 85.0 34.0 22.2
Slope of model a - 0.0056 0.0100 0.0237 0.0413
R - 0.99 0.99 0.99 0.95
Adsorption rate constant, k a Lmin-lmole -1 71.5 83.7 111.7 139.4
5.03E-04 1.50E-03 1.56E-02 3.21E-02
Equilibrium constant, K Lmole -1 142100 55900 7100 4300
Arrhenius equation b In(ka) = -740/T + 6.87, R 2 = 0.98
In(kd) = -4894/T + 9.84, R 2 = 0.96 a: Model: In [Xe/(Xe-X)] = k a x C x t / I X e = (k a x C + kd) x t = slope x t
b: In (k) = In (A)- E/RT
was about 2,500 mg, and the outflow gas concentration was measured every 15 min by GC-FID.
3 Results and Discussion
3.1 Determinations of adsorption reaction rate (k~) and Desorption reaction rate (kd)
Table 1 shows the adsorption conditions and calculated pa- rameters of C6H 6 by AC at different temperatures in the ki- netic adsorption/desorption studies. It illustrates when the tem- perature rose from 283~ to 363~ and the benzene concentrations were almost the same, X~ declined from 91% to 22%, indicating that adsorption was influenced by tem- perature, and when the temperature increases, ka and k d also increase, but the reaction equilibrium constant (K) decreases. Based on Arrhenius' equation, physicochemical parameters can be calculated. Since, the slope of ln(k~) is -740, the adsorption activated energy, Ea, is equal to 1470 cal mole -1. A similar ob- servation shows that the desorption activated energy, E d, is 9725
calmole -1. Therefore, the heat of adsorption of C t H 6 can be determined as ~ = Ea - Ed, i.e., -8255 calmole -1.
3.2 Influence of physicochemical parameters
In this study, we can obtain the physicochemical parameters (Ea, Ed, Aa, Aa, and K) of C t H 6 adsorption on AC from ki- netic adsorption/desorption studies, and these parameters can be used to predict the results under different conditions. Fig. 1 presents the effect of the temperature on adsorption isotherm, and that has about 5-10% error between predict data and experimental data in this study. The above simula- tions and kinetic parameters indicate that the adsorption efficiency has a high relationship with temperature. With increasing temperature, the adsorption rate and desorption rate rise, but the adsorption weight decreases. At high ben- zene concentrations, the temperature effect is not signifi-
70 60 5o
g 4o
~ ao
~" 20 10 0 "1" = 283K . . . . -- -. = - - . - ~ ; , " 9 / / / J 1 ~ - - 3 6 3 K ' / , f z a . i i I / / fr # ; / "',i
[ 9 - 283K - - - 3 0 3 K . _ 1I
3 2 3 K . . . . 3 4 3 K . . . . 363K 0 0.0005 0.001 C o n c . ( m o l e / L )Fig. 1 : Effect of temperature on the adsorption isotherm curve
cant for the equilibrium adsorption weight. That is, during treatment of high benzene concentrations, raising the reac- tion temperature can increase the reaction rate and reduce the treatment time. On the other hand, if the benzene con- centration is low, raising the reaction temperature will de- crease the efficiency of AC adsorption.
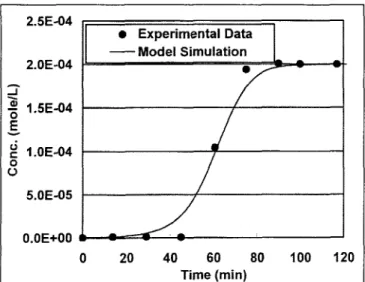
3.3 Simulation of adsorption breakthrough curve
Fig. 2 illustrates the simulation results of a breakthrough curve of the AC bed adsorption process. The results show that the adsorption process can be simulated. The simula- tion breakthrough curve did not fit very well with experi- mental data, and may be caused by the simulation param- eters (ka, kd, Aa, and Aa) error between kinetic study and real column reaction. That is, because all of the simulation
Benzene Adsorption
Short Original Communications
2.0E-04 ~-~ 1.5E-04~ 1.0E4~4
5.0E-05 0.0E+00 A w w v 9 Experimental Data 2.5E-04 Model Simulation 20 40 60 80 100 120 Time (min)Fig. 2: Result of breakthrough curve of experimental data and model simulation
parameters were derived from the kinetic adsorption/des- orption studies, there may be other reasons that were not completely considered in this model, but it could be a pre- liminary predictor of AC bed adsorption.
4 Conclusion
An AC bed adsorption process is influenced by the surface characteristics of adsorbent, characteristics and concentra- tion of VOC, humidity and flow rate. Temperature is a very important parameter. In this study, a new adsorption/des- orption model is developed, it can be used to simulation the
adsorption isotherm and the column adsorption process, and model simulation shows that a high reaction temperature will cause both the adsorption reaction rate and desorption reaction rate to rise, and the adsorption efficiency to de- crease. But at high VOC concentrations, a high temperature effect of adsorption efficiency is evidently less.
References
[1] Langmuir IJ (1916): The Constitution and Fundamental Prop- erties of Solids and Liquids. Amer Chem Soc 38, 2221-2295 [2] Myers AL, Prausnitz JM (1965): Thermodynamics of Mixed-
Gas Adsorption. MChE J 11,121-126
[3] Dubinin MM (1989): Fundamentals of The Theory of Ad- sorption in Micropores of Carbon Adsorbents: Characteris- tics of Their Adsorption Properties and Microporous Struc- tures. Carbon 27, 457-467
[4] Malek A, Farooq S (1997): Kinetics of Hydrocarbon Adsorp- tion on Activated Carbon and Silica Gel. MChE J 43, 761- 776
[5] Malek A, Faroog S, Rathor MN, Hidajat K (1995): Effect of Velocity Variation due to Adsorption-desorption on Equilib- rium Data from Breakthrough Experiments. Chem Eng Sci 50, 737-740
[6] Crittenden JC, Weber WJ (1978): Predictive Model for De- sign of Fixed-Bed Adsorbers: Single-Component Model Veri- fication. J Environ Eng 104, 433-443
[7] Pigram PJ, Lamb RN, Hibbert DB, Collins RE (1994): Mod- eling of the Desorption Behavior of Microporous Amorphous Hydrogenated Carbon Films. Langmuir 10, 142-147
Received: October 24th, 2000 Accepted: October 21 st, 2001
OnlineFirst: October 25th, 2001
Chia-Line Chuang is a Ph.D. Candidate at the Graduate Institute of Environmental Engineering, Na-
tional Taiwan University, Taipei. His researches include air pollution control and advanced treatment
processes of water supply systems. Currently (2001), his interests focus on the study of activated carbon adsorption/desorption.