STUDIES ON THE ANTIOXIDANT COMPONENTS AND ACTIVITIES OF THE METHANOL EXTRACTS OF COMMERCIALLY GROWN HEMEROCALLIS FULVA L.
(DAYLILY) IN TAIWAN
LI-YUN LIU1, LIN-YING CHANG2, SHIN-SHOU CHOU3, YU-LIN HSIAO1and
YI-WEN CHIEN4,5 1Department of Food Science Nutrition and Nutraceutical Biotechnology
Shih Chien University Taipei, Taiwan 2Department of Food Science National Taiwan Ocean University
Keelung, Taiwan
3Bureau of Food and Drug Analysis Department of Health
Taipei, Taiwan
4Department of Nutrition and Health Science Taipei Medical University
250 Wu-Hsing Street Taipei 11014, Taiwan
Accepted for Publication September 29, 2008
ABSTRACT
We explored the antioxidant components and activities of daylily
Hemerocallis fulva L. flowers among two growth areas, three flower ages and
two processing methods. The results showed that the growth area, flower age and processing method all significantly influenced the functional components and antioxidant activities grown in mountainous areas of Taiwan. The total phenols, flavonoids and total chlorophylls of the methanol extracts of D3DF were 59.51, 70.76 and 5.67% of the respective values of F3DF. The total phenols and anthocyanins of the methanol extracts of F1DF were 2.64 mg GAE/100 g·dried basis (db) and 0.102mmole/100 g·db, which were
signifi-cantly higher than the others. The total flavonoid contents of F1DF, F2DF,
5Corresponding author. TEL: +886-2-27361661 ext. 6556; FAX: +886-2-27373112; EMAIL:
ychien@tmu.edu.tw
DOI: 10.1111/j.1745-4514.2009.00306.x
Journal of Food Biochemistry 34 (2010) 90–104.
© 2010, Wiley Periodicals, Inc.
F3DF and D3DF were 20.83, 29.67, 31.65 and 22.30 mg QE/100 g·db, respectively, with the F1DF level as the lowest. The reducing power showed that both the fresh and dried flowers were very weak. The amounts of most flavonoids in the flowers from Hualien were greater than those from Taidong.
PRACTICAL APPLICATIONS
The aim of this research was to discuss the antioxidant activities and the main components of Hemerocallis fulva L. (day lily); for example, the content of total phenols, flavonoids and total chlorophylls. In particular, the study examines the content of 10 common types of flavonoids. The research used different solvents to extract H. fulva L. grown at different time and origins. The findings provide consumers with a better understanding of the functions of H.
fulva L. It increases the H. fulva L. uptake and improves consumers’ health.
Consequently, it increases farmers’ income.
INTRODUCTION
Flowers of Hemerocallis fulva L. (daylily; jin chen or golden needle in Chinese) contain several antioxidant compounds such as anthocyanins, fla-vonoids, carotenoids, chlorophylls and a potential agent for the inhibition of lipid oxidation, which may prevent atherosclerosis (Thu et al. 2004).
Flavonoids are polyphenol compounds of secondary metabolic com-pounds in plants, and their structure is a 2-phenyl-benzo-a-pyrone (Table 1). There are more than 4,000 kinds of structures in the heterocyclic ring because the substituting group of the ring produces different kinds of structures. Flavonoids in plants combine with carbohydrates to form glycosides (Hollman
TABLE 1.
OCCURRENCE OF FLAVONOIDS IN COMMON FOODS Flavonoid subgroup Major foods Examples of foods
Flavones herbs parsley, thyme
Flavonols vegetables, fruits, beverages onions, kale, broccoli, apple, cherries, berries, tea, red wine
Flavanones fruits citrus
Catechins fruits, beverages apples, tea
Anthocyanidins fruits cherries, grapes
Isoflavones vegetables soy beans, legumes
et al. 1996). Research has shown (Bailleul and Trotin 2000; Luo et al. 2002)
that flavonoids have free radical-scavenging activity against superoxide anions, hydroxide free radicals and peroxide, and inhibit radical damage.
According to Lin (2002), farmers in Taiwan always divide the flowers according to the maturity of the flower bud that means the days before to flower, as 1-, 2-, 3-, and 4-day flowers, indicating the length of time the bud has been open. The polyphenol contents of daylily flowers are affected by the method of processing (Hsu et al. 2000). When H. fulva flowers are freeze dried, there are higher amounts of polyphenols, so they have greater reducing power, Fe2+-chelating activity, DPPH-scavenging activity and lipid peroxida-tion inhibiperoxida-tion power.
There are eight kinds of glycosides based on the character of the structure (Fig. 1): flavones (apigenin, luteolin, kaempherol, rutin, quercetin, galangin and chrysin) and flavanols (myricetin, morin); flavanonols (hesperidin, naringin, naringenin, pinocembrin); and flavanones, including chalcones
O OH + O O OH O O O OH O O O O OH O O O
FIG. 1. EIGHT KINDS OF GLYCOSIDES BASED ON THE CHARACTER OF THE STRUCTURE
(catechin, epicatechin, epigallocatechin and gallate), isoflavones (daidzein, daidzin, genistein and genistin) and anthocyanidins (cyaniding, delphinidin, malvidin, pelargonidin, peonidin and petunidin) (Table 2). The types and con-tents of flavonoids are not exactly similar in different plants (Li et al. 1998). Flavonoids are used as oxidation inhibitors, and the main functions are: (1) as a metal-chelating agent and -reducing agent; (2) to eliminate active oxygen species; (3) to end the chain-reaction of free radicals; and (4) to end the formation of singlet oxygen. In addition, flavonoids can restrict the reaction of the fat oxidation enzyme and the NADPH oxidation enzyme in the oxidizing reaction, thus preventing tumors. Their antioxidant activities have close rela-tionships with their structures.
There are many methods for analyzing flavonoids, such as high-performance liquid chromatography (HPLC), spectrophotometry, thin-layer chromatography and capillary electrophoresis (CE). The amount needed for a sample is small, the analysis time is short and the separation effect is good for the CE method (Hilhorst et al. 1998). So it is suitable for analyzing the contents of flavonoids. This research used CE to examine the compositions of flavonoids in daylily flowers. The objectives of this study was to determine the effects of the growth area, the age of the flower, and the processing method on the antioxidant components and their activities.
MATERIALS AND METHODS Sources of Samples
Fresh Daylily Flowers. One- to 3-day-before to flower were purchased from Hualien’s Ye-Lie Mt. and 3-day-before to flower (F3DF) from a moun-tain area in Taidong.
TABLE 2.
FLAVONOL AND FLAVONE CONTENTS OF COMMON VEGETABLES, FRUITS AND BEVERAGES
Flavonol and flavone contents* Foods Low
(<10 mg/kg or <10 mg/L)
cabbage, spinach, carrots, peas, mushrooms, peaches, strawberries, orange juice, white wine, brewed coffee
Medium
(<50 mg/kg or <50 mg/L)
lettuce, broad beans, red pepper, tomatoes, apples, grapes, cherries, tomato juice, red wine, tea beverages
High
(>50 mg/kg or >50 mg/L)
broccoli, endive, kale, French beans, celery, onions, cranberries
* Represents the sum of quercetin, kaempferol, myricetin, luteolin and apigenin. Source of the data: Hilhorst et al. (1998).
Dried Daylily Flowers. Three-day-old daylily flowers were purchased from Hualien’s Ye-Lie market.
Sample Pretreatment. The flowers were dried in a freeze dryer (40C, 80 mm Hg), ground into a powder with a mortar, and the powder was stored in a refrigerator at 4C.
Reagents
Boric Acid-Methyl Alcohol Solution. A 0.1-M boric acid solution and caustic soda solution were mixed and adjusted to pH 9.5. Methyl alcohol was added in a 9:1 proportion to the boric acid-methyl alcohol solution, and this was filtered through a 0.45-mm filter.
Others. DPPH (1,1-biphenyl-2-picry-hydrazyl), potassium ferricyanide (K3Fe(CN)6), 3-(2-pyridyl-5,6-bis(4-phenyl-sulfuric acid)-1,2,4-trizine (fer-rozine), ethylene diaminetetraacetic acid (EDTA), folin-Ciocalteau’s reagent, gallic acid and quercetin were purchased from Sigma Chemical (St. Louis, MO). CuSO4and KH2PO4were purchased from Shimakyu Chemical (Osaka, Japan). FeCl2·4H2O, FeCl3·6H2O, NaCl, NaBr, Na2CO3, AlCl3·6H2O, acetic acid, trichloroacetic acid (TCA) and 2-thiobarbituric acid (TBA) were pur-chased from Kanto Chemical (Tokyo, Japan). Acetonitrile, methanol, dichlo-romethane, petroleum ether, ethyl acetate, chloroform, acetone, ether, 25% ammonia water and sulfuric acid were purchased from Merck (Darmstadt, Germany).
Methods
Preparation of Methanol Extracts of H. fulva Flowers. The powdered flowers (0.30 g) were placed into an extractor at 45C, and spun at 150 rpm for 20 min with 10-mL methanol; and then this mixture was centrifuged at 3,500 rpm for 15 min. We collected the supernatant in 25-mL test tubes. Methanol (5 mL) was added to the residue and duplicated two times. The supernatant and filtrate were mixed. The filtrate was collected in 25-mL flasks. Finally, methanol was used to bring the volume to 25 mL.
Determination of Total Phenols and Flavonoids. According to the method of Taga et al. (1984), 0.2 mL of each test solution was mixed with 0.8 mL of a 7.5% Na2CO3solution. Next, 1 mL of Folin–Ciocalteau’s phenol reagent was added to the mixture. The mixture was placed at room temperature for 30 min in the dark, and then the absorbance at 765 nm was measured. Total phenols of the sample were expressed in gallic acid equivalents (GAE) (mg/g
extracted dry powder). According to the method of Quettier-Deleu et al. (2000), 1 mL of each test solution was mixed with 1 mL of a 2% methanolic AlCl3·6H2O solution. The solution was placed at room temperature for 10 min, and then the absorbance at 430 nm was measured. Total flavonoids were expressed as quercetin equivalents (QE) (mg/g extracted dry powder).
Determination of Total Anthocyanins. According to the method of Padmavati et al. (1997), 0.5–2 g of powdered flowers was mixed with 10 mL of a methanol solution (containing 1% hydrochloric acid) and placed into an extractor at 45C and 200 rpm for 30 min in the dark. Then this mixture was centrifuged at 3,000 rpm and 4C for 15 min. We collected the upper trans-parent liquid in a 50-mL flack. A methanol solution (10 mL) was added to the residue and duplicated two times. The supernatant and methanol solution (containing 1% hydrochloric acid) were combined to bring the volume to 50 mL, then the absorbances at 627 and 530 nm were individually measured, and the absorbance coefficient was 31.6. The following equation was used to measure the content of total anthocyanins:
Total anthocyanins (mmole/100 g) = (Absorbance530 - 0.33Absobance657)/ 31.6¥ volume (mL) ¥ dilution/sample weight (g) ¥ 100.
Determination of Antioxidant Activity
Determination of DPPH-Scavenging Activity. According to the method
of Shimada et al. (1992), 2 mL of four concentrations (1, 2.5, 5 and 10 mg/ mL) of methanol extracts from powdered daylily flowers was separately mixed with 2 mL of a 1 mM DPPH solution. The test sample solution replaced by methanol was used as the control. Methanol was used to replace the DPPH solution as the blank. Each mixture was allowed to stand for 30 min in the dark, and then the absorbance at 517 nm was measured. The percentage of the DPPH-scavenging activity was expressed as (1 – 0[absorbance of the test sample – absorbance of the blank]/absorbance of the control)¥ 100%.
Determination of the Reducing Power. Using Oyaizu’s (1986) method,
0.5 mL of 0.2 M Na3PO4 buffer (pH 6.6) was added to 0.1 mL of the sample extract solution. Next, 0.2 mL of 1% K3Fe (CN)6was added. Each mixture was incubated at 50C for 20 min, and then it was rapidly cooled down with ice cubes. Next, 0.5 mL of 10% CCl3COOH was added to the solution. The solution was centrifuged at 3,000 rpm for 10 min, and 0.5 mL of supernatant was mixed with 0.5 mL deionized water and 0.1 mL 1%FeCl3·6H2O. The mixture was placed in the dark for 10 min, and then the absorbance at 700 nm was measured. The reducing power was expressed as the absorbance of the test sample – the absorbance of the control.
Determination of Fe2+-Chelating Activity. The Fe2+-chelating activity of the extracts of powdered flowers was measured according to the method of Dinis et al. (1994). Two milliliter of four concentrations (1, 2.5, 5 and 10 mg/ mL) of methanol extracts of powdered flowers was separately mixed with 0.1 mL of 1 mM FeCl2.4H2O and allowed to stand for 30 s. Each reaction mixture was vigorously mixed with 0.2 mL of 2.5 mM ferrozine solution and incubated for 10 min in the dark, and then the absorbance at 562 nm was measured. The percentage of ferrous ion-chelating activity was expressed as (1 – [absorbance of the test sample – absorbance of the blank]/absorbance of the control)¥ 100%.
Determination of the Flavonoid Contents by CE
Standard Solution. Ten grams of each sample (naringin, chrysin,
pinocanbrin, hesperidin, daidzein, rutin, naringenin, isoqurcetin, kaempferol, myricetin, quercetin and morin) was precisely weighed, and then dissolved in 10 mL of 80% ethyl alcohol to make the standards. The standards were diluted by 80% ethyl alcohol to 8, 12, 16 and 20mg/mL to produce standard solutions before use.
The Boric Acid-Methyl Alcohol Solution. A 0.1-M boric acid solution
was mixed with a caustic soda solution to adjust the pH to 9.5. Methyl alcohol was added in a 9:1 proportion to obtain the boric acid–methyl alcohol solution; it was filtered through a 0.45-mm microfilter.
CE Conditions. The correct amount of clear liquid was filtered through a
0.45-mm microfilter, as the examined sample, and analyzed with CE.
The total length of the analysis tube was 60 cm; the effective length was 50 cm i.d. of a 50-mm glass-capillary column, with an untreated surface, a voltage of 18 kV of voltage, a temperature of 25C and a wavelength of 214 nm. A sample was poured into the high-pressure nitrogen for 5 s, and the material was recorded and processed using P/ACE System Gold V8.0 software (Beckman Instrument, Fullerton, CA).
Determination of the Concentration. Individual volumes of samples and
standard solutions were injected into the CE instrument. Peaks were identified by comparing the retention times and spectra of samples with those of the standard solutions. The following formula was used to calculate the amounts of flavonoid in the test samples:Amount of flavonoid (ppm)= C ¥ V/W, where C is the flavonoid concentration (mg/mL) calculated by the standard curve, V is the volume of the sample solution (mL) and W is the weight of the sample (g).
Statistical Analysis. Results were expressed in terms of means and standard deviations. For all measurements, results were considered statistically significant at P< 0.05. All statistical analyses were conducted by using the method of Statsoft (Kim and Kohact 1975); the comparisons were made by analysis of variance.
RESULTS AND DISCUSSION
Antioxidant Components and the Yield of H. fulva Methanol Extracts As shown in Table 3, the yields of the methanol extracts of H. fulva flowers were about 29.47–30.78%. There were no significant differences between the sources and among the ages of the flowers. Total phenols and total chlorophylls of the methanol extract of D3DF (dried 3-day-before to flower) were 1.22 mg GAE/100 g·dried basis (db) and 0.013mg/100 g·db, which were significantly lower than those of the fresh flower methanol extracts. Values of the yield, total phenols and total chlorophylls of D3DF were 40.49, 92.73 and 94.32% lower compared with the respective values for F3DF The total phenols and anthocyanins of the methanol extracts of F1DF (fresh 1-day-old flowers) were 2.64 mg GAE/100 g·db and 0.102mmole/100 g·db, which were signifi-cantly higher than the others. The total flavonoid contents of F1DF, F2DF, F3DF and D3DF were 20.83, 29.67, 31.65 and 22.30 mg QE/100 g·db, respec-tively, with F1DF being the lowest. The results showed that the antioxidant components differed because of the age of the flower.
Distribution of Antioxidant Activities
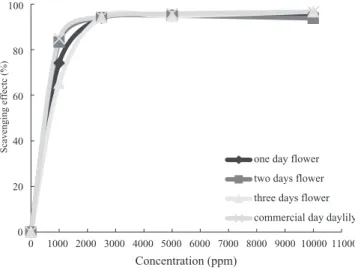
The DPPH-scavenging activities of none of the tested methanol extracts of H. fulva flowers significantly differed. The IC50 values were about 0.500~0.625 mg/mL (Table 4), while the concentration was 2,375 ppm for both methanol extracts of the tested flowers which produced 95% DPPH-scavenging activity (Fig. 2).
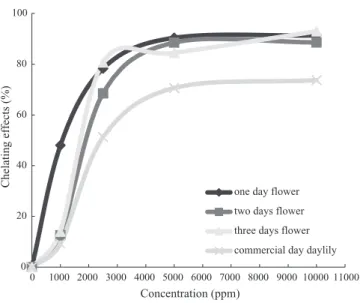
The Fe2+-chelating activity of the methanol extracts showed that D3DF was significantly lower than the others, while F1DF was the highest (Fig. 3). The IC50of D3DF was 2.560 mg/mL, while that of F1DF was 1.125 mg/mL (Table 4). This suggests that the total phenol, flavonoid and chlorophyll con-tents of daylily flowers decreased during the drying process. Those compo-nents had Fe2+-chelating activity. That was the reason the Fe2+-chelating activity of D3DF was significantly lower.
The reducing power of the methanol extracts of H. fulva flowers was examined. All of the test samples exhibited very low reducing powers when the
T ABLE 3. YIELDS AND ANTIOXIDANT CONTENTS OF THE METHANOL EXTRACTS OF HEMEROCALLIS FUL V A L FLOWERS Y ield (% db) T otal phenols (mg GAE/100 g·db) Fla v onoids (mg QE/100 g·db) Anthoc yanins (m mole/100 g·db) T o tal chlorophylls (m g/100 g·db) F1F1DF 30.38 ⫾ 0.23 2.64 ⫾ 0.1 1 a* 20.83 ⫾ 1.21 d 0.102 ⫾ 0.007 a 0.201 ⫾ 0.034 a F2DF 30.13 ⫾ 0.67 2.16 ⫾ 0.07 b 29.67 ⫾ 1.15 b 0.087 ⫾ 0.004 b 0.221 ⫾ 0.012 a F3DF 31.78 ⫾ 0.51 2.05 ⫾ 0.03 c 31.65 ⫾ 0.55 a 0.071 ⫾ 0.003 c 0.229 ⫾ 0.001 a D#D3DF 29.47 ⫾ 0.14 1.22 ⫾ 0.01 d 22.30 ⫾ 0.25 c 0.084 ⫾ 0.002 b 0.013 ⫾ 0.002 b * E ach v alue is the mean ⫾ standard de viation of triplicate analysis; v alues in the same column w ith dif ferent superscripts significantly dif fer at P < 0.05. F1DF , fresh 1 day before to flo wer; F2DF , fresh 2 d ays before to flo wer; F3DF , fresh 3 days before to flo wer; D3DF , dried 3 days before to flo wer; GAE, gallic acid equi v alents; QE, quercetin equi v alents; db, dried basis.
concentration was 10,000 ppm. Both F3DF and D3DF were nearly zero, while F1DF and F2DF were only 0.17 and 0.14 (Fig. 4).
Flavonoids Content Analysis
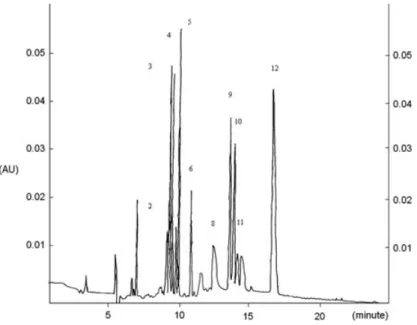
CE Spectrum of H. fulva Flavonoids. There were 12 different fla-vonoids separated by CE (Fig. 5), including naringin, chrysin, pinocembrin, hesperidin, daidzein, rutin, naringenin, isoquercetin, kaempherol, myricetin, quercetin and morin.
TABLE 4.
ANTIOXIDANT ACTIVITY OF METHANOL EXTRACTS OF HEMEROCALLIS FULVA FLOWERS DPPH-scavenging activity IC50(mg/mL) Fe+2-chelating activity IC 50 (mg/mL) F1DF 0.595⫾ 0.04* 1.125⫾ 0.04c F2DF 0.580⫾ 0.24 1.920⫾ 0.72b F3DF 0.625⫾ 0.16 1.670⫾ 0.51b D3DF 0.500⫾ 0.21 2.560⫾ 0.83a
* Each value is the mean⫾ standard deviation of triplicate analysis; values in the same column with different superscripts significantly differ at P< 0.05.
F1DF, fresh 1 day before to flower; F2DF, fresh 2 days before to flower; F3DF, fresh 3 days before to flower; D3DF, dried 3 days before to flower.
Concentration (ppm)
Scavenging effectc (%)
one day flower two days flower three days flower commercial day daylily
0 1000 100 80 60 40 20 0 2000 3000 4000 5000 6000 7000 8000 9000 10000 11000
Standard Curve of Flavorids. The concentration of the flavorid stan-dards were 4–20mg/mL, after individual CE. We used the concentration of flavorid standards against the absorbance and calculated the correlation coef-ficient (R2), which were 0.9845–0.9988, indicating quite-good linear relation-ships as shown in Table 5.
0 20 40 60 80 100 0 1000 2000 3000 4000 5000 6000 7000 8000 9000 10000 11000 Concentration (ppm) Chelating effects (%)
one day flower two days flower three days flower commercial day daylily
FIG. 3. EFFECTS ON THE FERROUS ION-CHELATING ABILITY OF THE METHANOL EXTRACTS OF DAYLILY FLOWERS
0 0.05 0.1 0.15 0.2 0 1000 2000 3000 4000 5000 6000 7000 8000 9000 10000 11000 Concentration (ppm) Absorbance at 700nm
one day flower two days flower three days flower commercial day daylily
Detection Limits. Based on a signal/noise (S/N) ratio of 3 as the detec-tion limit, the following detecdetec-tion limits were obtained (Table 6).
Flavonoid Contents of the Methanol Extracts of H. fulva Flowers. Table 7 shows that the flavonoid contents of daylily flowers signifi-cantly differed because of the growth area and the age of the flowers. Although
FIG. 5. CAPILLARY ELECTROPHORESIS SPECTRUM OF HEMEROCALLIS
FULVA FLAVONOIDS
1. naringin, 2. chrysin, 3. pinocembrin, 4. hesperidin, 5. daidzein, 6. rutin, 7. naringenin, 8. isoquercetin, 9. kaempherol, 10. myricetin, 11. quercetin, 12. morin.
TABLE 5.
CORRELATION COEFFICIENTS (R2) OF FLAVORIDS BY
CAPILLARY ELECTROPHORESIS ANALYSIS
Component R2 Component R2 Luteolin 0.9979 Vitexin 0.9980 Chrysin 0.9985 Kaempherol 0.9976 Galangin 0.9964 Naringin 0.9962 Apigenin 0.9988 (⫾)Naringenin 0.9943 Quercetin 0.9978 Morin 0.9983 Rutin 0.9980 Myricetin 0.9845 Isoquercetin 0.9980 Hesperidin 0.9845 Pinocembrin 0.9982 Daidzein 0.9981
the total flavonoid contents of 3-day-old flowers were higher than 1- or 2-day-old flowers, that of the 3-day-2-day-old flowers grown in Hualien were significantly higher than that grown in the Taidong area; the total flavonoid content from Hualien was 1,517.76 ppm, while that from Taidong was 1,301.79 ppm; that from Hualien area was significantly 16.5% higher. The total flavonoid contents of flowers grown in Hualien area were also in the order of 3-day-old> 2-day-old> 1-day-old flowers; the total flavonoid contents of 3-day-old flowers were significantly 394% higher than 1-day-old flowers and 127% higher than 2-day-old flowers.
TABLE 6.
THE DETECTION LIMITS OF FLAVORIDS BY CAPILLARY ELECTROPHORESIS ANALYSIS
Component Detection limitsmg/mL Component Detection limitsmg/mL
Luteolin 0.10 Vitexin 0.12 Chrysin 0.17 Kaempherol 0.1 Galangin 0.15 Naringin 0.3 Apigenin 0.08 (⫾)Naringenin 0.08 Quercetin 0.14 Morin 0.3 Rutin 0.25 Myricetin 0.2 Isoquercetin 0.15 Hesperidin 0.18 Pinocembrin 0.13 Daidzein 0.2 TABLE 7.
FLAVONOID CONTENTS OF THE METHANOL EXTRACTS OF HEMEROCALLIS FULVA FLOWERS (PPM) Flavonoid TD-F3DF HL-F1DF HL-F2DF HL-F3DF Naringin — — — 1.86⫾ 0.45 Chrysin 167.80⫾ 10.21a* — 26.51⫾ 2.78c 38.01⫾ 2.76b Pinocembrin — — — 5.59⫾ 1.69 Hesperidin 107.59⫾ 9.34a 5.12⫾ 1.35d 31.64⫾ 1.76c 57.51⫾ 2.54b Rutin 81.55⫾ 1.24 — — — Naringenin — 42.92⫾ 3.98 — — Kaempherol 360.21⫾ 8.69 — — Myricetin 110.59⫾ 8.57c 103.92⫾ 9.47c 142.14⫾ 10.34b 651.48⫾ 8.67a Quercetin 475.05⫾ 13.56b 154.82⫾ 10.45c 468.15⫾ 8.98b 763.32⫾ 9.76a Total 1301.79⫾ 12.53b 306.78⫾ 10.54d 668.44⫾ 10.66c 1517.76⫾ 10.02a
* Each value is the mean⫾ standard deviation of triplicate analysis; values in the same row with different superscripts significantly differ at P< 0.05.
TD-F3DF, Taidong fresh 3 days before to flower; HL-F1DF, Hualien fresh 1 day before to flower; HL-F2DF, Hualien fresh 2 days before to flower; HL-F3DF, Hualien fresh 3 days before to flower.
As to the individual flavonoid contents, we found that the content of 1-day-old flowers from Hualien was highest in quercetin (154.82 mg/kg), followed by myricetin (103.92 mg/kg) and naringenin (42.92 mg/kg); the 2- and 3-day-old flowers were highest in quercetin (468.15 and 763.32 mg/kg), followed by myricetin (142.14 and 651.48 mg/kg) and hesperidin (31.64 and 57.51 mg/kg); but the 3-day-old flowers from Taidong were highest in quer-cetin (475.05 mg/kg), followed by kaempferol (360.21 mg/kg) and chrysin (167.80 mg/kg). Chu (1998) once pointed out that the Dutch scholar used hydrolysis to extract carbohydrates and used HPLC to examine the composi-tion of flavonoids in vegetables. The results showed that the main flavonoids in the vegetable part were quercetin, followed by kaempferol and quercetin which was quite high in the onion (347 mg/kg).
CONCLUSIONS
The results showed that the growth area, the age of the flower and the processing method significantly influenced the functional components and antioxidant activities of the flowers of H. fulva grown in mountainous areas of Taiwan. The amounts of most flavonoids from Hualien-grown flowers were greater than those of Taidong-grown ones. Total phenols, anthocyanins of the methanol extract of fresh 1 day before to flower were significantly higher than those of 2- and 3 day before to flower. The Fe2+-chelating activity of the methanol extracts of fresh H. fulva L. flowers was 2,250mg, while that of dried flowers was 5,120mg. The reducing powers of both fresh and dried flowers were very weak.
ACKNOWLEDGMENT
The authors gratefully acknowledge the financial support of the National Science Council of the ROC (NSC91-2745-p-158-003).
REFERENCES
BAILLEUL, F. and TROTIN, F. 2000. Phenolic compounds and antioxidant activities of buckwheat, hulls and flour. J. Ethnopharmacol. 72, 35–42. CHU, Y.-H. 1998. Introduction to flavonoids. J. Food Ind. 30, 1–5.
DINIS, T.C.P., MADEIRA, V.M.C. and ALMERIDE, L.M. 1994. Action of phenolic derivatives (acetaminophen, salicylate and 5-aminosalicylate) as
inhibitors of membrane lipid peroxidation and as peroxyl radical scaven-gers. Arch. Biochem. Biophys. 315, 161–169.
HILHORST, M.J., SOMSEN, W.G. and JONG, G.J. 1998. Potential of capil-lary electrophoresis for the profiling of propolis. J. High Resol. Chro-matogr. 21, 608–612.
HOLLMAN, P.C.H., HERTOG, M.G.L. and KATAN, M.B. 1996. Analysis and health effects of flavonoids. Food Chem. 57, 43–46.
HSU, H.F., CHANG, C.L. and CHU, Y.H. 2000. Flavonoid contents and antioxidative activities of several vegetables. Taiwan J. Agric. Chem. Food Sci. 38, 377–387.
KIM, X.X. and KOHACT, F.J. 1975. Analysis of Variance and Covariance
Subprograms ANOVA and One Way in SPSS Statistical Package for the Social Science, 2nd Ed., pp. 98–430, McGraw-Hill, New York, NY.
LI, W., ASADA, Y. and YOSHIKAWA, T. 1998. Antimicrobial flavonoids from Glycyrrhiza glabra hairy root cultures. Planta Med. 64, 746–747. LIN, X.S. 2002. The specialty of Hua-Lian Hemerocallis fulva L fresh bud. J.
Agric. World 223, 98–101.
LUO, X.D., BASILE, M.J. and KENNELLY, E.J. 2002. Poly-phenolic anti-oxidant from the fruits of Chrysophyllum cainito L. (star apple). J. Agric. Food Chem. 50, 1379–1382.
OYAIZU, M. 1986. Anti-oxidative activity of browsing products of gluco-mamine fractional by organic solvent and thin layer chromatograph. Nippon Shokuhin Kogyo Gakkaish 35, 771–775.
PADMAVATI, M., SAKTHIVEL, N., THARA, K.V. and REDDY, A.R. 1997. Differential sensitivity of rice pathogens to growth inhibition by fla-vonoids. Phytochemistry 46, 499–502.
QUETTIER-DELEU, C., GRESSIER, B., VASSEUR, J., DINE, T., BRUNET, C., LUYCKX, M., CAZIN, M., CAZIN, J.C., BAILLEUL, F. and TROTIN, F. 2000. Phenolic compounds and antioxidant activities of buckwheat (Fagopyrum esculentum Moench) hulls and flour. J. Ethnop-harmacol. 72, 35–42.
SHIMADA, K., FUJIKAWA, K., YAHARA, K. and NAKAMWZA, T. 1992. Anti-oxidation properties of xanthan on the auto-oxidation of soybean oil in cyclo-dextrin emulsion. J. Agric. Food Chem. 40, 945–948.
TAGA, M.S., MILLER, E.E. and PRATT, D.E. 1984. China seeds as a source of natural antioxidants. J. Am. Oil Chem. Soc. 61, 928–931.
THU, N.N., SAKURAI, C., UTO, H., VAN, C.N., LIEN, T.K., YAMAMOTO, S., OHMORI, R. and KONDO, K. 2004. The poly-phenol content and anti-oxidant activities of the main edible vegetables in Northern Vietnam. J. Nutr. Sci. Vitam. (Tokyo) 50, 203–210.