Abstract The effects of AlCl3 on growth and polyamine levels of rice roots were investigated. When rice roots were treated with AlCl3, root growth was markedly inhibited. AlCl3 treatment resulted in a higher putrescine content and lower spermidine and spermine contents in rice roots. D-Argnine and a-methylornithine, inhibi-tors of putrescine biosynthesis, caused a reduced content of putrescine in rice roots under Al stress. AlCl3 treatment also resulted in a decrease in diamine oxidase activity in rice roots. The growth of rice roots in the presence of AlCl3 was recovered after the addition of D-arginine or a-methylornithine. The protective effect of D-arginine or a-methylornithine in counteracting AlCl3-inhibited growth of rice roots is unlikely caused by reduction of Al uptake. Further-more, the effect of the growth recovery in AlCl3-treated rice roots by D-arginine or a-methylornithine was reversed by the addition of putrescine. Our results strongly suggest that putrescine accumulation is a factor causing growth inhibition of rice roots under Al tress. Evidence is also presented to show that
lignification is responsible for putrescine- and AlCl3-inhibited growth of rice roots.
Keywords Aluminum Æ Growth Æ Putrescine Æ Rice
Introduction
The polyamines putrescine (PUT), spermidine (SPD), and spermine (SPM) are polycationic cellular molecules and are present in all living organisms. The involvement of polyamines in the growth of prokaryotic and eukaryotic cells was established through a series of mutants in Escherichia coli and Saccharumyces cerevisiae (Tabor and Tabor 1984). Bertosi et al. (1965) reported that polyamines stimulated the growth of explants from dormant tubers of Helianthus tuberous. Subsequently, polyamine contents and plant growth rates have been positively correlated in a wide variety of conditions in which high contents of polyamines are associated with rap-idly growing tissues (Evans and Malmberg1989). In response to various types of environmental stress, plants accumulate polyamines, especially PUT (Boucherau et al.1999; Galston et al.1997; Kao 1997). K+ deficiency may be the first stress condition that has been shown to increase PUT levels in plants (Richards and Coleman 1952;
J.-W. Wang Æ C. H. Kao (&)
Department of Agronomy, National Taiwan University, Taipei, Taiwan, ROC
e-mail: kaoch@ntu.edu.tw DOI 10.1007/s11104-006-9127-y
O R I G I N A L P A P E R
Aluminum-inhibited root growth of rice seedlings
is mediated through putrescine accumulation
Jen-Wu Wang Æ Ching Huei KaoReceived: 14 March 2006 / Accepted: 8 September 2006 / Published online: 6 October 2006
Young and Galston 1984). Watson and Malm-berg (1996) have shown that arginine decarbox-ylase (ADC), an enzyme critical for inducible PUT synthesis, is activated upon K+deficiency in Arabidopsis. Phosphate deprivation also in-creases PUT contents in tobacco, barley, and rice plants (Shih and Kao 1996; Sinclair 1967; Takahashi and Yoshida1960). It has been shown that feeding PUT to cut leaves of barley produced the same symptoms found in K+ deficient plant (Coleman and Richards 1956). It has also been demonstrated that PUT accumu-lation as a factor causing growth inhibition of suspension-cultured rice cells under K+ defi-ciency and phosphate deprivation (Shih and Kao 1996; Sung et al. 1994). An inhibition of root growth has been described in genetically modified tobacco plants with high contents of PUT (Masgrau et al. 1997; Panicot et al. 2002). Moreover, Watson et al. (1998) reported that Arabidopsis mutants with low content of PUT had higher root growth rate than did wild-type plants. More recently, Camacho-Cristo´bal et al. (2005) found that the inhibition of root growth caused by boron deprivation was associated high content of PUT in tobacco plants. All these results seem to suggest that high content of endogenous PUT is toxic for plant growth.
Aluminum (Al) does not exert any known function in plant metabolism and belongs to the non-essential metal. Under neutral soil condi-tions, it exists in the non-phytotoxic insoluble form, whereas acidification of soil and soil water below pH 4.5 dramatically enhances release of the phytotoxic aluminum ion (MacDonald and Martin 1988). Since acid soils occupy up to 40% of world arable land (Kochian 1995), Al phyto-toxicity may be considered as one of the major limiting factors of crop productivity in the world (Matsumoto 2000). The primary effect of Al toxicity is the inhibition of root growth; however, the mechanisms involved in this toxicity are far from clear (Matsumoto 2000). It is not known whether PUT accumulates in roots under Al stress. Neither do we know the relation between the PUT content and Al-inhibited root growth. In this paper, we described the possible role of PUT in Al-inhibited root growth of rice seedlings.
Materials and methods Plant material
Rice (Oryza sativa L., cv. Taichung Native 1) seeds were sterilized with 2.5% sodium chlorite for 15 min and washed extensively with distilled water. In order to get more uniformly germinated seeds, rice seeds in Petri dish (20 cm) containing distilled water were pre-treated at 37°C for 1-day under dark condition. Uniformly germinated seeds were then selected and transferred to a Petri dish (9 cm) containing filter paper moist-ened with 10 ml of distilled water and were allowed to grow at 27°C in darkness. Then, 2-day-old seedlings were treated with distilled water, AlCl3 (0.5 mM) or test solutions at the desired concentrations as specified in the individ-ual experiments. Root growth of rice seedlings grown in distilled water is similar to that grown in medium containing inorganic salts and so seed-lings grown in distilled water were used as the controls. Each Petri dish contained 10 seedlings and each treatment was replicated four times. Al determination
For the determination of Al, roots were dried at 65°C for 48 h. Dried material was ashed at 550°C for 20 h. Ash residue was incubated with 31% HNO3 and 17.5% H2O2 at 70°C for 12 h, and dissolved in 0.1 M HCl. Al was then quantified using an atomic absorption spectrophotometer (Model AA-680, Shimadzu, Kyoto, Japan). Polyamine determination
Roots were homogenized in 5 ml of 5% (w/v) perchloric acid. Polyamine contents were deter-mined using high performance liquid chromatog-raphy (Waters 484, Milford, USA) after benzoylation as described previously (Chen and Kao1991).
Extraction and assay of diamine oxidase (DAO)
For extraction of DAO, roots were homogenized with ice-cold phosphate buffer (50 mM, pH 7.8)
using a pestle and mortar. The homogenate was centrifuged at 10,000g for 20 min at 4°C. DAO activity was measured by the method of Naik et al. (1981). The incubation mixture contained 50 mM phosphate buffer (pH 7.8), 10 mM PUT, 0.1 mM pyridoxal phosphate and enzyme extract in a total volume of 4 ml. After incubation at 30°C for 1 h, the reaction was terminated using 1 ml 20% (w/v) trichloroacetic acid. After 30 min, the incubation mixture was centrifuged at 5,000g for 15 min. One ml of ninhydrin mixture (250 mg ninhydrin in 6 ml acetic acid and 4 ml phosphoric acid) was added to the supernatant. Color was developed at 100°C for 30 min. After adding 1 ml of acetic acid, absorbance was measured at 510 nm. In controls, trichloroacetic acid was added prior to the enzyme solutions. One unit of DAO was defined as increase of A510 per h.
Lignin determination
The lignin content in roots was measured by the Sasaki et al. (1996) method, a method originally described by Morrison (1972). Roots were homogenized with a pestle and mortar in 95% ethanol. The homogenate was centrifuged at 1,000g for 5 min. The pellet was washed three times with 95% ethanol and twice with a mixture of ethanol and hexane (1:2, v/v). The material was allowed to air dry and its lignin content measured. The dried sample was washed one time with 2 ml acetyl bromide in acetic acid (1:3, v/v). Then 1 ml acetyl bromide in acetic acid (1:3, v/v) was added to the pellet and incubated at 70°C for 30 min. After cooling of the mixture to room tempera-ture, 0.9 ml of 2 N NaOH and 0.1 ml 7.5 M hydroxylamine hydrochloride were added, and the volume was made up to 10 ml with acetic acid. After centrifugation at 1,000g for 5 min, the absorbance of the supernatant was measured at 280 nm (A280).
Statistical analysis
Statistical differences between measurements (n = 4) on different treatment or on different times were analyzed by Duncan’s multiple range test or Student’s t-test.
Results and discussion
AlCl3inhibits growth of rice roots
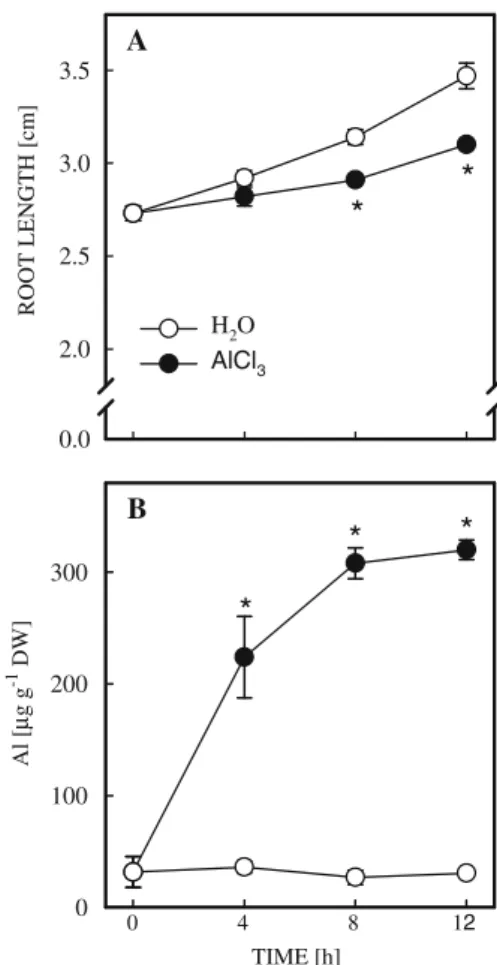
In previous work, we observed that increasing concentration of AlCl3from 0.25 to 0.5 mM at pH 4.0 progressively decreased root growth of rice seedlings and no further decrease was observed at 0.75 and 1 mM AlCl3 (Wang and Kao 2004). Thus, 0.5 mM AlCl3 was used in the present investigation. The reduction of root growth by AlCl3was evident 8 h after treatment (Fig.1A). Al concentration in the control roots remained unchanged during 12 h of incubation (Fig.1B). However, Al concentration in AlCl3-treated roots increased with increasing duration of incubation
ROOT LENGTH [cm] 0.0 2.0 2.5 3.0 3.5
*
*
A TIME [h] Al [ µ g g -1 DW] 0 100 200 300*
*
*
B H2O AlCl3 12 8 4 0Fig. 1 Changes in root length (A) and Al concentration (B) in rice roots treated with AlCl3(0.5 mM, pH 4.0) or
H2O (pH 4.0). Means ± SE (n = 4). Asterisks indicate
values that are significantly different between H2O and
(Fig. 1B). The increase in Al concentration in AlCl3-treated roots was evident 4 h after treatment (Fig. 1B).
Changes of polyamines in AlCl3-treated rice roots
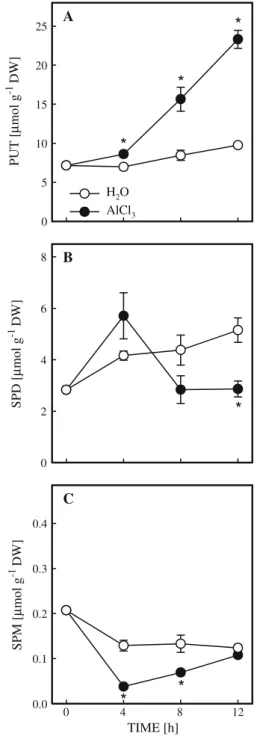
To characterize the role of polyamines in the growth of rice roots under Al stress, the contents of polyamines in rice roots in the presence or absence of AlCl3were determined. The chroma-tography analysis indicated the presence of PUT, SPD, and SPM in rice roots. The content of PUT in the control roots remained unchanged for the first 8 h of treatment but increased slightly at 12 h (Fig. 2A). AlCl3-treated rice roots had a higher content of PUT than the control roots at 4, 8 and 12 h after treatment (Fig. 2A). In contrast, AlCl3 treatment had a lower content of SPD than the control roots at 12 h (Fig. 2B), which occurred after the onset of growth inhibition of rice roots caused by AlCl3(Fig.1A). Thus, it is unlikely that SPD plays any role in Al-inhibited root growth. The content of SPM in AlCl3-treated roots was also lower than that in the control roots at 4 and 8 h (Fig.2C). If the lower content of SPM is responsible for the growth inhibition in rice roots under Al stress, then AlCl3-treated rice roots might be expected to recover their growth by the addition of SPM. However, no such recovery was observed (data not shown).
Since PUT accumulation in rice roots under Al stress is preceded root growth inhibition, PUT may be a factor causing growth inhibition in rice roots. If this suggestion is correct, PUT is expected to inhibit the growth in the control rice roots. Addition of PUT to the control rice roots indeed resulted in a reduction of root growth (Fig. 3). This agrees with the already reported toxic effect of exogenous PUT treatments to various organ-isms including plants (Davis and Ristow 1991; DiTomaso et al. 1989; Guarino and Cohen1979; Shevyakova1966; Strogonov et al.1972).
AlCl3affects the biosynthesis and catabolism of PUT
The PUT may be formed in plants directly from ornithine via ornithine decarboxylase (ODC) or
indirectly from the decarboxylation of arginine via arginine decarboxylase (ADC). To under-stand whether PUT accumulation in rice roots
SPD [ µ mol g -1 DW] 0 2 4 6 8 PUT [ µ mol g -1 DW] 0 5 10 15 20 25
*
*
A*
H2O AlCl3 B TIME [h] SPM [ µ mol g -1 DW] 0.0 12 8 4 0 0.1 0.2 0.3 0.4*
*
C*
Fig. 2 Changes in the contents of polyamines in rice roots treated with AlCl3 (0.5 mM, pH 4.0) or H2O (pH 4.0).
Means ± SE (n = 4). Asterisks indicate values that are significantly different between H2O and AlCl3treatments
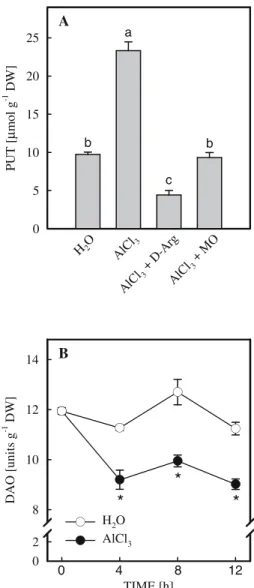
under Al stress is a result of an enhancement in PUT biosynthesis, we tested the effect of inhib-itors of PUT biosynthesis (D-Arg and MO) on the content of PUT in rice roots under Al stress. Both D-Arg and MO decreased the content of PUT induced by AlCl3(Fig. 4A). Although the activ-ities of ADC and ODC, enzymes responsible for the biosynthesis of PUT (Evans and Malmberg 1989), were not measured, the results suggest, although indirectly, that AlCl3 may affect the biosynthesis of PUT.
PUT content can also be regulated by the catabolism of PUT. Diamine oxidase (DAO) catalyzes the catabolism of PUT to pyrroline, hydrogen peroxide, and ammonia (Boucherau et al.1999). However, it is not known whether the increase in PUT content under Al stress is attributable to the decrease in DAO activity. The presence of DAO in rice roots has been demonstrated previously (Lin and Kao 2002). In the present study, we observed that AlCl3 treat-ment indeed resulted in a reduction of DAO activity in rice roots (Fig.4B), indicating that PUT content in rice roots under Al stress is also regulated by DAO activity. We believe that this is the first report showing that both biosynthesis and catabolism of PUT are responsible for PUT accumulation in rice roots under Al stress.
Effect ofD-Arg and MO on the growth of AlCl3-treated rice roots
To investigate the role of PUT in root growth, the effect of D-Arg and MO on the growth of rice roots treated with or without Al was examined. Under normal condition without Al, D-Arg and
R O O T LENGTH [cm] 0 1 2 3 4 a b b c PUT [mM] 0 1 3 5
Fig. 3 Effect of exogenous PUT on root growth of rice seedlings. Two-day-old rice seedlings were treated with various concentrations of polyamines for 12 h. Means ± SE (n = 4). Values with the same letter are not signifi-cantly different at P < 0.05 TIME [h] DA O [un its g -1 DW ] 0 2 8 10 12 14
*
*
*
H2O AlCl3 PUT [ µ mo l g -1 DW ] 0 5 10 15 20 25 A B b a c b H2O AlCl 3 AlC l3+ D-Arg AlCl 3+ MO 12 8 4 0Fig. 4 Effect ofD-Arg and MO on the content of PUT (A)
in rice roots treated with AlCl3 (0.5 mM, pH 4.0) and
changes in DAO activity (B) in the roots of rice seedlings in the presence or absence AlCl3(0.5 mM, pH 4.0). The
concentrations of D-Arg and MO were 5 mM. PUT
content was determined 12 h after treatment. Means ± SE (n = 4). Values with the same letter are not significantly different at P < 0.05. Asterisks indicate values that are significantly different between H2O and AlCl3treatments
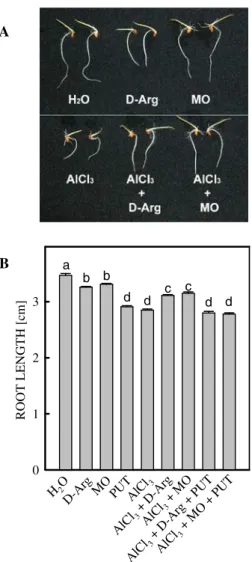
MO caused an inhibition of root growth (Fig. 5A). BothD-Arg and MO, which decreased PUT content induced by AlCl3(Fig. 4A), signif-icantly enhanced growth of roots treated with AlCl3for 12 h (Fig.5B). This protective effect of AlCl3-inhibited root growth was also observed in a long (48 h) D-Arg or MO treatment (Fig.5A). These results suggest that PUT accumulation is a factor related to growth inhibition of rice roots under Al stress. Furthermore, the growth recovery
in AlCl3-treated rice roots by D-Arg or MO was reversed by the addition of PUT (Fig.5B).
SinceD-Arg or MO was added simultaneously with AlCl3, thus D-Arg or MO-reduced growth inhibition of roots caused by AlCl3 may be mediated through binding Al to carboxyl group ofD-Arg or MO, which in turn reduces the uptake of Al. Al concentration in roots treated with AlCl3 was not significantly different from that treated with AlCl3+D-Arg or AlCl3+ MO (Fig.6). Thus, the protective role of D-Arg or MO in counteracting AlCl3-inhibited growth of roots is unlikely caused by reduction of Al uptake. This conclusion is supported further by the observations that the protective effect of D-Arg or MO was also observed when rice roots were exposed to D-Arg or MO and AlCl3 sepa-rately (Fig.7).
The presence of D-Arg under Al stress decreased PUT content less than the control roots (Fig.4A). However, root growth inhibition by Al was not recovered to the control level in the presence ofD-Arg (Fig.5). These results indicate that Al-inhibited root growth of rice cannot be explained solely by PUT accumulation, other possibilities cannot be completely excluded.
ROOT LENGTH [cm] 0 1 2 3 H2O D-Ar g MO PUTAlCl3 AlC l3+ D-Ar g AlCl 3+ MO AlC l3+ D-Ar g+ PUT AlCl 3+ MO +PU T a b b d d c c d d A B
Fig. 5 Effect ofD-Arg, MO, and PUT on root growth of
rice seedlings treated with or without AlCl3(0.5 mM, pH
4.0). The concentrations of D-Arg, MO, and PUT were
5 mM. Picture was taken 48 h after treatment (A) and root growth was measured 12 h after treatment (B). Mean-s ± SE (n = 4). ValueMean-s with the Mean-same letter are not significantly different at P < 0.05 Al [ µ g g -1 DW] 0 100 200 300 H2O AlCl 3 D-Arg + AlCl 3 MO + AlCl 3 a a a b
Fig. 6 Effect ofD-Arg, MO, and PUT on Al concentration
in rice roots treated with or without AlCl3(0.5 mM, pH
4.0). The concentrations of D-Arg, MO, and PUT were
0.5 mM. Al concentration was measured 12 h after treatment. Means ± SE (n = 4). Values with the same letter are not significantly different at P < 0.05
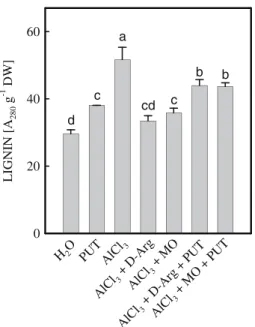
PUT and AlCl3increase the content of lignin Lignification, the process of collective formation of phenylpropanoid macromolecules and poly-merization of cinnamyl alcohols, is part of cell differentiation and irreversibly inhibits cell elon-gation. It has been shown that lignification in the elongation region coincided with the extent of inhibition of root growth by Al in two wheat cultivars that differed in their sensitivity to Al (Sasaki et al. 1996). Here, we show that AlCl3 treatment resulted in an increase in lignin content (Fig. 8). Adding PUT, which inhibited growth of rice roots (Fig.3), significantly increased lignin content in rice roots (Fig.8). Similarly, adding D-Arg and MO, which counteracted growth inhi-bition of rice roots caused by AlCl3 (Fig. 5), prevented increase in lignin content in AlCl3 -treated rice roots (Fig. 8). Furthermore, the decrease in lignin content in AlCl3-treated rice roots by D-Arg or MO was reversed by the addition of PUT (Fig.8). It appears that lignifi-cation is responsible for PUT- and AlCl3-inhibited growth of rice roots. The facts thatD-Arg almost
completely reduced the increase in lignin content caused by AlCl3but did not completely recover the root growth (Figs.5A, 8) suggest that ligni-fication is not the only mechanism for Al-inhib-ited growth of rice roots. Our results do not explain the mechanism of Al- or PUT-induced lignin formation. Further study in this direction may allow better understanding of the toxic effect of Al on rice roots.
Conclusion
It has been shown that PUT accumulation is a factor causing growth inhibition of suspension-cultured rice cells under K+deficiency and phos-phate deprivation (Sung et al.1994; Shih and Kao 1996). Here, we show that AlCl3-inhibited growth of rice roots is mediated though PUT accumula-tion. Clearly, PUT in excess of the content normally found in rice cells could be a factor, among others, inhibiting growth of rice cells. Our results are also consistent with the findings that the abnormal phenotypes induced by boron
ROOT LENGTH [cm]
0
1
2
3
4
H2O H2O D-Ar g H2O H2O AlC l3 D-A rg AlC l3 MO AlC l3 a b b d c c MO H2OFig. 7 Effect of pre-treatment ofD-Arg and MO on root
growth of rice seedlings treated with or without AlCl3
(0.5 mM, pH 4.0). Two-day-old rice seedlings were pre-treated with distilled water (pH 4.0), 0.5 mM D-Arg (pH 4.0) and 0.5 mM MO (pH 4.0), respectively, for 12 h and then treated with distilled water or 0.5 mM AlCl3(pH 4.0)
for 12 h. Means ± SE (n = 4). Values with the same letter are not significantly different at P < 0.05
LIGNIN [A 280 g -1 DW ] 0 20 40 60 H2O AlC l3 PUT AlCl 3+ D-A rg AlC l3+ MO AlC l3+ D-Ar g+ PUT AlC l3+ MO +PU T d a c c cd b b
Fig. 8 Effect ofD-Arg, MO, and PUT on the content of
lignin in the roots of rice seedlings treated with AlCl3
(0.5 mM, pH 4.0). The concentrations ofD-Arg, MO, and
PUT were 5 mM. Lignin level was measured 12 h after treatment. Means ± SE (n = 4). Values with the same letter are not significantly different at P < 0.05
deficiency (Camacho-Cristo´bal et al. 2005) or by genetic manipulation, such as over-expression of ADC (Masgrau et al. 1997; Watson et al. 1998; Panicot et al.2002), are linked to high contents of endogenous PUT. Although other possibilities cannot be completely ruled out, the results of this investigation indicate that the observed growth reduction of rice roots under Al stress is due to, at least in part, PUT accumulation via ADC, ODC, and DAO. Under normal condition without Al, D-Arg and MO treatments lead to the inhibition of rice root growth (Fig.5A), suggesting that at least some amount of PUT seems to be necessary for normal growth. Judging from the data pre-sented here, PUT could be both a necessary factor and an inhibitory factor for the growth of rice roots, which may be depending on the content of PUT in rice roots.
References
Bertosi F, Bagni N, Moruzzi G, Calderera CM (1965) Spermines as a new growth-promoting substance for Helianthus tuberous explants (Jerusalem artichoke) in vitro. Experientia 21:80–81
Boucherau A, Aziz A, Larther F, Martin-Tanguy J (1999) Polyamines and environmental challenges: recent development. Plant Sci 140:103–125
Camacho-Cristo´bal JJ, Maldonado JM, Gonza´lez-Fontes A (2005) Boron deficiency increases putrescine levels in tobacco plants. J Plant Physiol 162:921–928 Chen CT, Kao CH (1991) Senescence of rice leaves. XXX.
Levels of endogenous polyamines and dark-induced senescence of rice leaves. Plant Cell Physiol 32:935– 941
Coleman RG, Richards FJ (1956) Physiological studies in plant nutrition. XVIII. Some aspects of nitrogen metabolism in barley and other plants in relation to potassium deficiency. Ann Bot 20:393–409
Davis RH, Ristow JL (1991) Polyamine toxicity in Neurospora crassa: protective role of the vacuole. Arch Biochem Biophys 285:306–311
DiTomaso JM, Shaff JE, Kochian LV (1989) Putrescine-induced wounding and its effects on membrane integrity and ion transport processes in roots of intact corn seedlings. Plant Physiol 90:988–995
Evans PT, Malmberg RL (1989) Do polyamines have roles in plant development? Annu Rev Plant Physiol Plant Mol Biol 40:235–269
Galston AW, Kaur-Sawhney R, Altabella T, Tiburcio AF (1997) Plant polyamines in reproductive activity and response to abiotic stress. Bot Acta 110:197– 207
Guarino LA, Cohen SS (1979) Mechanism of toxicity of putrescine in Anacystis nidulans. Proc Natl Acad Sci USA 76:3660–3664
Kao CH (1997) Physiological significance of stress-induced changes in polyamines in plants. Bot Bull Acad Sin 38:141–144
Kochian LV (1995) Cellular mechanism of aluminum toxicity and resistance in plants. Annu Rev Plant Physiol Plant Mol Biol 46:237–260
Lin CC, Kao CH (2002) NaCl-induced changes in putres-cine content and diamine oxidase activity in roots of rice seedlings. Biol Plant 45:633–636
MacDonald T, Martin RB (1988) Al ion in biological systems. Trends Biol Sci 13:15–19
Masgrau C, Altabella T, Farra´s R, Flores D, Thompson AJ, Besford RT, Tiburcio AF (1997) Inducible overexpression of oat arginine decarboxylase in transgenic tobacco plants. Plant J 11:465–473 Matsumoto H (2000) Cell biology of aluminum toxicity
and tolerance in higher plants. Int Rev Cytol 200:1– 46
Morrison IM (1972) A semi-micro method for the deter-mination of lignin and its use in predicting the digestibility of forage crops. J Sci Food Agric 23:455–463
Naik BL, Goswami RG, Srivastava SK (1981) A rapid and sensitive colorimetric assay of amine oxidase. Anal Biochem 111:146–148
Panicot M, Masgrau C, Borrel A, Cordeiro A, Tiburcio AF (2002) Effect of putrescine accumulation in tobacco transgenic plants with different expression levels of oat arginine decarboxylase. Physiol Plant 114:281–287
Richards FJ, Coleman RG (1952) Occurrence of putrescine in potassium-dificient barley. Nature 170:460
Sasaki M, Yamamoto Y, Matsumoto H (1996) Lignin deposition induced by aluminum in wheat (Triticum aestivum) roots. Physiol Plant 96:193–198
Shevyakova NI (1966) On the stimulating and toxic affects of diamines in plants. Fizol Rast 13:522–524
Shih C-Y, Kao CH (1996) Growth inhibition in suspen-sion-cultured rice cells under phosphate deprivation is mediated through putrescine accumulation. Plant Physiol 111:721–724
Sinclair C (1967) Relationship between mineral deficiency and amine synthesis in barley. Nature 213:214–215 Strogonov BP, Shevyakova NI, Kavanov VV (1972)
Diamines in plant metabolism under conditions of salinization. Fizol Rast 19:1098–1104
Sung H-I, Liu L-F, Kao CH (1994) Putrescine accumula-tion is associated with growth inhibiaccumula-tion in suspen-sion-cultured rice cells under potassium deficiency. Plant Cell Physiol 35:313–316
Tabor CW, Tabor H (1984) Polyamines. Annu Rev Biochem 53:749–790
Takahashi T, Yoshida D (1960) Relationship between the accumulation of putrescine and the nutrition of tobacco plant. J Soil Sci Manure Jpn 31:39–41
Wang J-W, Kao CH (2004) Reduction of aluminum-inhibited root growth of rice seedlings with supple-mental calcium, magnesium and organic acid. Crop Environ Bioinfo 1:191–198
Watson MB, Malmberg RL (1996) Regulation of Arabid-opsis thaliana (L.) heynh arginine decarboxylase by potassium deficiency stress. Plant Physiol 111:1077–1083
Watson MB, Emory KK, Piatak RM, Malmberg RL (1998) Arginine decarboxylase (polyamine synthesis) mutants of Arabidopsis thaliana exhibited altered root growth. Plant J 13:231–239
Young ND, Galston W (1984) Physiological control of arginine decarboxylase activity in potassium deficient oat shoots. Plant Physiol 76:331–335