J
OURNAL OFC
LINICALM
ICROBIOLOGY, Dec. 2007, p. 3992–3995
Vol. 45, No. 12
0095-1137/07/$08.00
⫹0 doi:10.1128/JCM.01202-07
Copyright © 2007, American Society for Microbiology. All Rights Reserved.
Prevalence of Methicillin-Resistant Staphylococcus aureus Nasal
Colonization among Taiwanese Children in 2005 and 2006
䌤
Yhu-Chering Huang,
1,3* Kao-Pin Hwang,
2,3Po-Yen Chen,
4Chih-Jung Chen,
1,3and Tzou-Yien Lin
1,3Division of Pediatric Infectious Diseases, Chang Gung Children’s Hospital and Chang Gung Memorial Hospital at Linko
1and
Kaohsiung,
2Taiwan; College of Medicine, Chang Gung University, Taoyuan, Taiwan
3; and Department of Pediatrics,
Taichung Veterans General Hospital, Taichung, Taiwan
4Received 15 June 2007/Returned for modification 20 August 2007/Accepted 4 October 2007
From July 2005 to October 2006, a total of 3,046 children, of ages between 2 months and 5 years,
presented for a well-child health care visit to one of three medical centers, which are located in the
northern, central, and southern parts of Taiwan, and were surveyed for nasal carriage of
methicillin-resistant Staphylococcus aureus (MRSA). The overall prevalences of S. aureus and MRSA nasal carriage
among the children were 23% and 7.3%, respectively (18% and 4.8% in the central region, 25% and 6.7%
in the southern region, and 27% and 9.5% in the northern region). Of the 212 MRSA isolates (96%)
available for analysis, a total of 10 pulsed-field gel electrophoresis (PFGE) patterns with two major
patterns (C [61%] and D [28%]) were identified. One hundred forty-nine isolates (70%) contained type IV
staphylococcal cassette chromosome mec (SCCmec) DNA, and 55 isolates (26%) contained SCCmec V
T.
The presence of Panton-Valentine Leukocidin (PVL) genes was detected in 60 isolates (28%). Most MRSA
isolates belonged to one of two major clones, characterized as sequence type 59 (ST59)/PFGE C/SCCmec
IV/absence of PVL genes (59%) and ST59/PFGE D/SCCmec V
T/presence of PVL genes (25%). We concluded
that between 2005 and 2006, 7.3% of healthy Taiwanese children were colonized by MRSA in nares. MRSA
harbored in healthy children indicates an accelerated spread in the community.
Recent reports indicate that community-acquired
methicil-lin-resistant Staphylococcus aureus (CA-MRSA) infections are
increasing worldwide and may now involve persons without
risk factors predisposing them for acquisition (2, 11–14, 20,
24). Asymptomatic CA-MRSA colonization has been
docu-mented in healthy children attending the emergency
depart-ments and outpatient clinics of children’s hospitals (6, 19, 25,
27, 28).
Carriage of S. aureus, including MRSA, is well known to be
a significant risk factor for subsequent infection (7, 29), and the
anterior nares are the most consistent sites of colonization.
The presence of S. aureus nasal colonization can provide an
indication of a high risk for subsequent infection.
In Taiwan, previous reports (1, 3, 9, 15, 17, 23, 31) have
indicated that during the period from 1997 to 2003, MRSA
accounted for 9.8% to 36% of CA S. aureus infections in
children without risk factors and the MRSA colonization rate
in the general population ranged from 1.9% to
⬃3.3% for
school children and 5.3% for healthy children presented for
health care visits to 10.8% for health care workers and 13.6%
for contacts of CA-MRSA infection. It is noteworthy, however,
that most of these studies were conducted in the northern part
of Taiwan and no island-wide survey has yet been conducted to
elucidate this issue. To estimate the extent of MRSA in the
community in Taiwan and to assess if there is an increasing
trend of MRSA nasal colonization in healthy children during
the past 5 years, we conducted this island-wide survey between
2005 and 2006. All collected MRSA isolates were also further
characterized by molecular methods.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board of the Chang Gung Memorial Hospital. From July 2005 to October 2006, all children of ages be-tween 2 months and 5 years who presented for a well-child health care visit to any one of three medical centers in Taiwan were invited to participate in this study. The three medical centers involved were the Chang Gung Children’s Hospitals at Linko (hospital A) and Kaohsiung (hospital C) and Taichung Veterans Gen-eral Hospital (hospital B), which are situated, respectively, in northern, southern, and central parts of Taiwan. In each hospital, around 80 subjects were recruited for study for each month, and the ages of the subjects were evenly distributed in seven separate age ranges, which included⬎2 to 6 months, ⬎6 to 12 months, ⬎12 to 18 months, ⬎18 to 24 months, ⬎2 to 3 years, ⬎3 to 4 years, and ⬎4 to 5 years. A culture from the anterior nares for the detection of MRSA was obtained from each subject after written consent was obtained from their par-ents/guardians.
Survey specimens for culture were obtained with a cotton swab, placed in the transport medium (Venturi Transystem; Copan Innovation Ltd., Limerick, Ire-land), and then brought to and processed in the microbiological laboratories within 4 hours of the sampling. All S. aureus isolates were sent to Chang Gung Memorial Hospital at Linko for microbiological characterization. Identification of MRSA was confirmed according to Clinical and Laboratory Standards Insti-tute 2005 guidelines (5). Pulsed-field gel electrophoresis (PFGE) with SmaI digestion was used in this study to fingerprint the MRSA isolates and was performed according to procedures described previously (3, 16, 18). The geno-types were designated in alphabetical order, as in our previous studies (3, 15–18); any new genotype, if identified, was designated consecutively. PFGE patterns with fewer than four band differences from an existing genotype were defined as subtypes of that genotype.
SCCmec typing of isolates was done using a multiplex PCR strategy described previously (26). Control strains for SCCmec types I, II, III, and IVa, kindly provided by Keiichi Hiramatsu, were as follows: type I, NCTC10442; type II, N315; type III, 85/2082; and type IVa, JCSC4744. SCCmec typing for type VTwas determined by using a particular primer described elsewhere (1), and strain TSGH-17, kindly provided by Chi-Chien Wang, was used as a control. However, the SCCmec typing method for type VTyielded inconsistent results; thus, an
* Corresponding author. Mailing address: Chang Gung Children’s
Hospital, Department of Pediatrics, 5, Fu-Shin Street, Kweishan,
Taoyuan, Taiwan. Phone: 3281200, ext. 8202. Fax:
886-3-3288957. E-mail: ychuang@adm.cgmh.org.tw.
䌤
Published ahead of print on 17 October 2007.
alternative method was used. The appearance of an isolate with only two bands (414 bp and 243 bp) in the multiplex PCR analysis may have indicated that the isolate contained SCCmec VT. To confirm their identities, a novel pair of prim-ers, ccrC-5F (5⬘-CAC TTA ATC CAT TGA CAC AG-3⬘) and ccrC-5R (5⬘-AAA GAT TGA GGG ATA AGA CT-3⬘), was designed according to the published sequence (GenBank accession no. AY894416) of the ccrC gene of a Taiwanese strain, S. aureus TSGH-17. Amplification of a specific 1,081-bp DNA fragment, which was subjected to further sequence analysis for some representative isolates in preliminary experiments, confirmed that the isolates contained SCCmec VT. The presence of Panton-Valentine leukocidin (PVL) genes was determined by a PCR strategy described previously (22). Some isolates of representative PFGE patterns were selected and underwent multilocus sequence typing (MLST) as described elsewhere (8). The allelic profiles were assigned through comparison of the sequences at each locus with those of the known alleles in the S. aureus MLST database and were defined as sequence types accordingly.
RESULTS
During the study period, 1,279 subjects were recruited from
hospital A, 1,011 subjects from hospital B (from July 2005 to
June 2006), and 756 subjects from hospital C (from October
2005 to June 2006). All the children enrolled are Taiwanese.
The number of subjects enrolled in each age group ranged
from 430 for children of ages
⬎6 to 12 months to 443 for
children of ages
⬎2 to 3 years. Of the total of 3,046 subjects
enrolled in this study, 713 (23%) were colonized with S. aureus.
Of the 713 isolates, 221 (31%) were demonstrated to be
MRSA. The details of the nasal MRSA colonization
preva-lence for subjects in the different parts of Taiwan are shown in
Table 1. The MRSA colonization rate in northern Taiwan was
significantly higher than that in the central (P
⬍ 0.001) and
southern (P
⬍ 0.039) parts of Taiwan. The nasal MRSA
col-onization prevalences for the subjects in each age group were
8.4% for the children of ages
⬎2 to 6 months and 6.3%, 3.2%,
3.9%, 9.0%, 9.5%, and 10.1% for children of ages
⬎6 to 12
months,
⬎12 to 18 months, ⬎18 to 24 months, ⬎2 to 3 years,
⬎3 to 4 years, and ⬎4 to 5 years, respectively. For those less
than 18 months of age, the carriage rate decreased with
in-creasing age (P
⫽ 0.0011; Mantel-Haenszel test for trend),
while for those older than 12 months of age, the carriage rate
increased with increasing age (P
⬍ 0.0001).
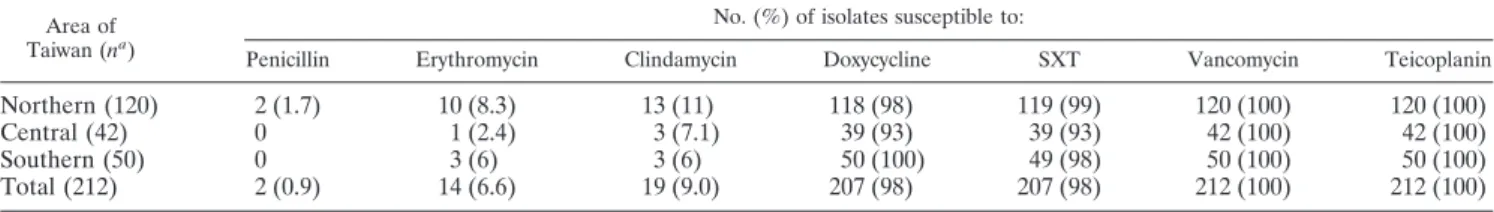
Of the 221 MRSA isolates, 212 isolates were available for
analysis. All of these 212 isolates were sensitive to vancomycin
and teicoplanin. All but two of the isolates identified from
hospital A were resistant to penicillin. Most isolates were
re-sistant to erythromycin and clindamycin but sensitive to
tri-methoprim-sulfamethoxazole (SXT) and doxycycline. The
de-tailed susceptibility distribution of various antibiotics for the
isolates is shown in Table 2. No significant difference in
anti-biotic susceptibility patterns was noted among the isolates
from the three different regions of Taiwan.
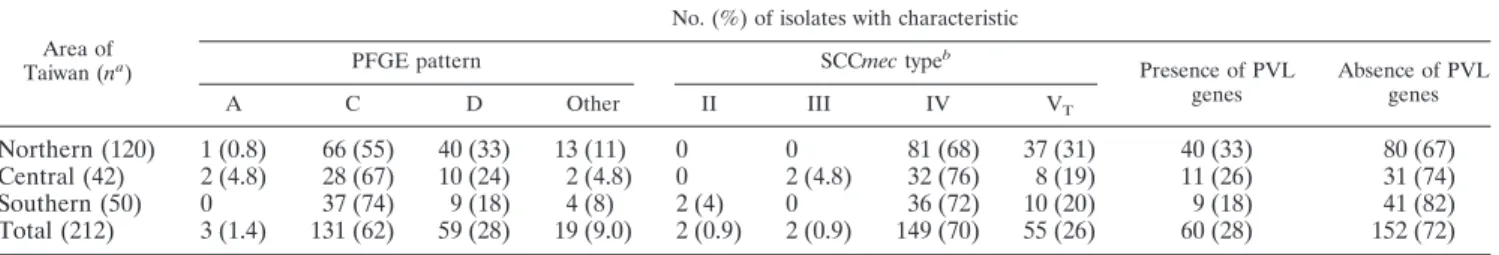
Table 3 illustrates the detailed distribution of PFGE
pat-terns, SCCmec types, and the presence/absence of PVL genes
among these isolates. A total of 10 PFGE patterns were
iden-tified. Patterns C and D were the two most common patterns
and accounted for 62% and 28% of the isolates analyzed,
respectively. The distribution of PFGE patterns among the
three regions showed a trend for a difference (P
⫽ 0.09 by a
log-likelihood contingency test). Four types (types II, III, IV,
and V
T) of SCCmec genes were identified among the isolates,
with type IV (70%) being the predominant type, followed by
type V
T(26%). The distribution of SCCmec types among the
three regions was significantly different (P
⫽ 0.03). Four
iso-lates of the AF PFGE pattern were untypeable by the methods
used in this study. PVL genes were present in 60 isolates
(28%). Twenty-five isolates underwent MLST, and eight
se-quence types were identified. Sese-quence type 59 (ST59) was the
most common sequence type and accounted for 9 of 10 PFGE
type C isolates, 4 of 6 PFGE type D isolates, and the isolate of
PFGE type AN. The other two isolates of PFGE type D were
ST338, which is a single-locus variant of ST59 (a single
nucle-otide difference in the gmk locus). The remaining isolate of
PFGE type C belonged to a new sequence type, which is a
single-locus variant of ST59 (a single nucleotide difference in
the pta locus). One isolate of PFGE type F also belonged to a
new sequence type, which is also a single-locus variant of ST9
(a single nucleotide difference in the gmk locus). The detailed
association of PFGE patterns with sequence types and SCCmec
types and the presence of the PVL gene of these isolates are
shown in Table 4. The MRSA isolates characterized by ST59/
PFGE type C/SCCmec IV/absence of PVL genes and ST59/
PFGE type D/SCCmec V
T/presence of PVL genes were the
two most common clones and accounted for 59% and 25% of
the isolates analyzed, respectively.
TABLE 1. Nasal carriage of MRSA among infants and children
presented for a well-child health care visit in Taiwan
Area of Taiwan No. of subjects No. (%) with S. aureusa No. (%) with MRSAbNorthern
1,279
344 (26.9)
121 (9.5)
Central
1,011
180 (17.8)
49 (4.8)
Southern
756
189 (25)
51 (6.7)
Total
3,046
713 (23.4)
221 (7.3)
aThe rate of carriage of S. aureus among children in the central region was
significantly lower than those among children in the northern and southern regions (P⬍ 0.001).
bThe rate of carriage of MRSA among children in the northern region was
significantly higher than those among children in the central (P⬍ 0.001) and southern (P⫽ 0.039) regions.
TABLE 2. Antibiotic susceptibility rates of 212 colonizing MRSA isolates from children in Taiwan
Area of Taiwan (na)
No. (%) of isolates susceptible to:
Penicillin Erythromycin Clindamycin Doxycycline SXT Vancomycin Teicoplanin
Northern (120)
2 (1.7)
10 (8.3)
13 (11)
118 (98)
119 (99)
120 (100)
120 (100)
Central (42)
0
1 (2.4)
3 (7.1)
39 (93)
39 (93)
42 (100)
42 (100)
Southern (50)
0
3 (6)
3 (6)
50 (100)
49 (98)
50 (100)
50 (100)
Total (212)
2 (0.9)
14 (6.6)
19 (9.0)
207 (98)
207 (98)
212 (100)
212 (100)
an, no. of isolates.
DISCUSSION
Results from this study indicate that the national prevalence
of nasal MRSA colonization among otherwise healthy children
in Taiwan was 7.3% during the period from July 2005 to
Oc-tober 2006 inclusively, with values ranging from 4.8% in the
central region of Taiwan to 9.5% in the northern region of
Taiwan. Compared with those among the healthy children
dur-ing the period of 2001 to 2002 (1, 17, 23) (Table 5), though the
study population was different for these studies, the nasal
MRSA colonization prevalence among healthy children in
Tai-wan increased significantly, from 1.9% in 2001 to 9.5% (P
⬍
0.0001 by chi-square test) during the period of 2005 to 2006 for
northern Taiwan and significantly from 3.3% to 6.7% for
southern Taiwan (P
⬍ 0.001 by chi-square test). This
increas-ing trend of nasal MRSA colonization prevalence might
ac-count for the increasing incidence of CA-MRSA infection in
children in Taiwan (3, 9, 31). In the United States, where
CA-MRSA is also being increasingly reported, the MRSA
col-onization prevalence for the general population appeared to
have been relatively low until the year 2002 (19, 21, 25, 27, 28).
In a survey (21) involving 9,622 persons conducted between
2001 and 2002, national S. aureus and MRSA nasal
coloniza-tion prevalence estimates were 32.4% and 0.8%, respectively.
For healthy children, the nasal colonization rates ranged from
0.2% to 2.2% (19, 25, 27, 28), as reported in several pediatric
studies; however, an increasing trend in this regard has been
noted in certain areas of the United States recently (6). Creech
et al. (6) reported that the nasal MRSA colonization rate
among healthy children in Nashville, TN, increased
signifi-cantly from 0.8% in 2001 to 9.2% in 2004, a picture not
dis-similar to what we show in the present study from Taiwan.
In the United States, CA-MRSA strains have been
recog-nized as representing a novel pathogen which was genetically
different from the nosocomial MRSA strains (14, 24). They
have limited antibiotic resistance (except to
-lactams), have
two common PFGE patterns (USA 300 and USA 400), possess
different exotoxin gene profiles (e.g., PVL), and contain SCCmec
DNA (10). In contrast, the CA-MRSA clinical isolates in
Tai-wan were multiresistant and shared two common PFGE
pat-terns (patpat-terns D and C in this study) (1, 3, 4, 30). In the
current study, more than 90% of the MRSA colonization
iso-lates were multiresistant to erythromycin and clindamycin but
sensitive to SXT and doxycycline. In addition, most
coloniza-tion isolates shared common molecular characteristics, and
more than 80% of the isolates belonged to one of two major
clones, characterized by ST59/PFGE type C/SCCmec
IV/ab-sence of PVL genes or ST59/PFGE type D/SCCmec V
T/pres-ence of PVL genes. However, among the clinical isolates, the
clone characterized by ST59/PFGE type D/SCCmec V
T/pres-ence of PVL genes was the dominant clone (1, 3, 30), while
among the colonized isolates, the clone characterized by ST59/
PFGE type C/SCCmec IV/absence of PVL genes was
domi-nant. It seemed that PVL genes, reported to be a virulence
factor associated with necrotizing pneumonia and abscesses
(22), may be associated with the ability of a PVL-positive clone
to cause infection.
There existed several limitations in the current study. First,
the demographic characteristics and the risk factors associated
with MRSA acquisition were not analyzed and compared
be-tween the children with and without CA-MRSA colonization,
though all the children were healthy and presented for health
care visits. Living with a family member who works in a
hos-pital or clinic and demographic characteristics (e.g., age and
gender) were reported to be associated with an increased risk
of MRSA colonization (6, 21, 25). Second, the persistence of
MRSA carriage in the subjects could not be determined and
the incidence of subsequent MRSA infection in the subjects
could not be measured in this cross-sectional analysis of
MRSA nasal colonization prevalence.
In summary, 7.3% of healthy children in Taiwan were
colo-nized by MRSA in the nares during the period from 2005 to
2006. MRSA carriage in the children may accelerate the
spread in the community. Two major CA-MRSA clones were
TABLE 3. Distribution of PFGE patterns, SCCmec types, and presence of PVL genes among 212 colonizing MRSA isolates
Area of Taiwan (na)
No. (%) of isolates with characteristic
PFGE pattern SCCmec typeb
Presence of PVL genes Absence of PVL genes A C D Other II III IV VT
Northern (120)
1 (0.8)
66 (55)
40 (33)
13 (11)
0
0
81 (68)
37 (31)
40 (33)
80 (67)
Central (42)
2 (4.8)
28 (67)
10 (24)
2 (4.8)
0
2 (4.8)
32 (76)
8 (19)
11 (26)
31 (74)
Southern (50)
0
37 (74)
9 (18)
4 (8)
2 (4)
0
36 (72)
10 (20)
9 (18)
41 (82)
Total (212)
3 (1.4)
131 (62)
59 (28)
19 (9.0)
2 (0.9)
2 (0.9)
149 (70)
55 (26)
60 (28)
152 (72)
an, no. of isolates.bFour isolates were untypeable. The distributions of SCCmec types were significantly different among the three regions (P⫽ 0.03).
TABLE 4. Association of PFGE patterns with MLST, SCCmec
types, and presence of PVL genes for 212 MRSA isolates
PFGE pattern (na) No. of subtypes SCCmec type (n) Status of PVLgenes (n) Sequence type
b
A (3)
3
III (2), IV (1)
Absent
ST239
C (131)
25
IV (129), V
T(2)
Absent (129),
present (2)
ST59, new*
D (59)
13
V
T(53), IV
(6)
Present (57),
absent (2)
ST59, ST338*
F (3)
2
II (2), V
T(1)
Absent
ST5, new*
AF (4)
1
Untypeable
Absent
ST89
AK (2)
2
IV
Absent
ST508
AN (3)
2
IV
Absent
ST59
AQ (5)
3
IV
Absent
ST508
AR (1)
1
IV
Absent
ST15
BA (1)
1
IV
Absent
ST5
an, no. of isolates.bEach of the three sequence types marked with an asterisk is a single-locus
variant of ST59, but they differ from each other.
identified and would appear to have spread island-wide.
Fur-ther studies are needed to determine the host factors of
colo-nization and to develop strategies to disrupt transmission of
CA-MRSA to susceptible hosts.
ACKNOWLEDGMENTS
This study was supported by a grant from National Science
Coun-seling of Executive Yuan of Taiwan (NSC95-2314-B182A-145).
We have no financial relationships relevant to this article to disclose.
REFERENCES
1. Boyle-Vavra, S., B. Ereshefsky, C. C. Wang, and R. S. Daum. 2005. Success-ful multiresistant community-associated methicillin-resistant Staphylococcus
aureus lineage from Taipei, Taiwan, that carries either the novel
staphylo-coccal chromosome cassette mec (SCCmec) type VTor SCCmec type IV. J. Clin. Microbiol. 43:4719–4730.
2. Chambers, H. F. 2001. The changing epidemiology of Staphylococcus aureus? Emerg. Infect. Dis. 7:178–182.
3. Chen, C. J., Y. C. Huang, C. H. Chiu, L. H. Su, and T. Y. Lin. 2005. Clinical features and genotyping analysis of community-acquired methicillin-resistant
Staphylococcus aureus infections in Taiwanese children. Pediatr. Infect. Dis.
J. 24:40–45.
4. Chen, F. J., T. L. Lauderdale, I. W. Huang, et al. 2005. Methicillin-resistant
Staphylococcus aureus in Taiwan. Emerg. Infect. Dis. 11:1761–1763.
5. Clinical and Laboratory Standards Institute. 2005. Performance standards for antimicrobial disk diffusion susceptibility testings: 15th informational supplement. Clinical and Laboratory Standards Institute, Wayne, PA. 6. Creech, C. B., II, D. S. Kernodle, A. Alsentzer, C. Wilson, and K. M.
Edwards. 2005. Increasing rates of nasal carriage of methicillin-resistant
Staphylococcus aureus in healthy children. Pediatr. Infect. Dis. J. 24:617–621.
7. Ellis, M. W., D. R. Hospenthal, D. P. Dooley, P. J. Gray, and C. K. Murray. 2004. Natural history of community-acquired methicillin-resistant
Staphylo-coccus aureus colonization and infection in soldiers. Clin. Infect. Dis. 39:
971–979.
8. Enright, M. C., N. P. Day, C. E. Davies, S. J. Peacock, and B. G. Spratt. 2000. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol.
38:1008–1015.
9. Fang, Y. H., P. R. Hsueh, J. J. Hu, P. I. Lee, J. M. Chen, C. Y. Lee, et al. 2004. Community-acquired methicillin-resistant Staphylococcus aureus in children in northern Taiwan. J. Microbiol. Immunol. Infect. 37:29–34.
10. Fey, P. D., B. Said-Salim, M. E. Rupp, S. H. Hinrichs, D. J. Boxrud, C. C.
Davis, et al.2003. Comparative molecular analysis of community- or hospi-tal-acquired methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 47:196–203.
11. Frank, A. L., J. F. Marcinak, P. D. Mangat, and P. C. Schreckenberger. 1999. Community-acquired and clindamycin-susceptible methicillin-resistant
Staphylococcus aureus in children. Pediatr. Infect. Dis. J. 18:993–1000.
12. Gonzalez, B. E., G. Martinez-Aguilar, K. G. Hulten, W. A. Hammerman, J.
Coss-Bu, A. valos-Mishaan, et al.2005. Severe staphylococcal sepsis in adolescents in the era of community-acquired methicillin-resistant
Staphylo-coccus aureus. Pediatrics 115:642–648.
13. Gorak, E. J., S. M. Yamada, and J. D. Brown. 1999. Community-acquired methicillin-resistant Staphylococcus aureus in hospitalized adults and chil-dren without known risk factors. Clin. Infect. Dis. 29:797–800.
14. Herold, B. C., L. C. Immergluck, M. C. Maranan, D. S. Lauderdale, R. E.
Gaskin, S. Boyle-Vavra, et al.1998. Community-acquired
methicillin-resis-tant Staphylococcus aureus in children with no identified predisposing risk. JAMA 279:593–598.
15. Huang, Y. C., L. H. Su, and T. Y. Lin. 2004. Nasal carriage of methicillin-resistant Staphylococcus aureus in contacts of an adolescent with community-acquired disseminated disease. Pediatr. Infect. Dis. J. 23:919–922. 16. Huang, Y. C., L. H. Su, T. L. Wu, C. E. Liu, T. G. Young, P. Y. Chen, et al.
2004. Molecular epidemiology of clinical isolates of methicillin-resistant
Staphylococcus aureus in Taiwan. J. Clin. Microbiol. 42:307–310.
17. Huang, Y. C., L. H. Su, C. J. Chen, and T. Y. Lin. 2005. Nasal carriage of methicillin-resistant Staphylococcus aureus in school children without iden-tifiable risk factors in northern Taiwan. Pediatr. Infect. Dis. J. 24:276–278. 18. Huang, Y. C., L. H. Su, T. L. Wu, and T. Y. Lin. 2005. Molecular surveillance
of methicillin-resistant Staphylococcus aureus in neonatal intensive care units. Infect. Control Hosp. Epidemiol. 26:157–160.
19. Hussain, F. M., S. Boyle-Vavra, C. D. Bethel, and R. S. Daum. 2001. Com-munity-acquired methicillin-resistant Staphylococcus aureus colonization in healthy children attending an outpatient pediatric clinic. Pediatr. Infect. Dis. J. 20:763–767.
20. Kaplan, S. L., K. G. Hulten, B. E. Gonzalez, W. A. Hammerman, L.
Lam-berth, J. Versalovic, et al. 2005. Three-year surveillance of community-acquired Staphylococcus aureus infections in children. Clin. Infect. Dis. 40: 1785–1791.
21. Kuehnert, M. J., D. Kruszon-Moran, H. A. Hill, et al. 2006. Prevalence of
Staphylococcus aureus nasal colonization in the United States, 2001-2002.
J. Infect. Dis. 193:172–179.
22. Lina, G., Y. Piemont, F. Godail-Gamot, M. Bes, M. Peter, V. Gauduchon, F.
Vandenesch, and J. Etienne.1999. Involvement of Panton-Valentine leuko-cidin-producing Staphylococcus aureus in primary skin infections and pneu-monia. Clin. Infect. Dis. 29:1128–1132.
23. Lu, P. L., L. C. Chin, C. F. Peng, Y. H. Chiang, T. P. Chen, L. Ma, and L. K.
Siu.2005. Risk factors and molecular analysis of community methicillin-resistant Staphylococcus aureus carriage. J. Clin. Microbiol. 43:132–139. 24. Naimi, T. S., K. H. LeDell, K. Como-Sabetti, S. M. Borchardt, D. J. Boxrud,
J. Etienne, et al.2003. Comparison of community- and health care-associ-ated methicillin-resistant Staphylococcus aureus infection. JAMA 290:2976– 2984.
25. Nakamura, M. M., K. L. Rohling, M. Shashaty, H. Lu, Y. W. Tang, and
K. M. Edwards.2002. Prevalence of methicillin-resistant Staphylococcus
aureus nasal carriage in the community pediatric population. Pediatr. Infect.
Dis. J. 21:917–922.
26. Oliveira, D. C., and H. de Lencastre. 2002. Multiplex PCR strategy for rapid identification of structural types and variants of the mec element in methi-cillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 46: 2155–2161.
27. Shopsin, B., B. Mathema, J. Martinez, et al. 2000. Prevalence of methicillin-resistant and methicillin-susceptible Staphylococcus aureus in the commu-nity. J. Infect. Dis. 182:359–362.
28. Suggs, A. H., M. C. Maranan, S. Boyle-Vavra, and R. S. Daum. 1999. Methicillin-resistant and borderline methicillin-resistant asymptomatic
Staphylococcus aureus colonization in children without identified risk factors.
Pediatr. Infect. Dis. 18:410–414.
29. von Eiff, C., K. Becker, K. Machka, H. Stammer, and G. Peters. 2001. Nasal carriage as a source of Staphylococcus aureus bacteremia. N. Engl. J. Med.
344:11–16.
30. Wang, C. C., W. T. Lo, M. L. Chu, and L. K. Siu. 2004. Epidemiological typing of community-acquired methicillin-resistant Staphylococcus aureus isolates from children in Taiwan. Clin. Infect. Dis. 39:481–487.
31. Wu, K. C., H. H. Chiu, J. H. Wang, N. S. Lee, H. C. Lin, C. C. Hsieh, et al. 2002. Characteristics of community-acquired methicillin-resistant
Staphylo-coccus aureus in infants and children without known risk factors. J. Microb.
Immunol. Infect. 35:53–56.
TABLE 5. Reported prevalence rates of MRSA nasal colonization for healthy Taiwanese children between 2001 and 2006
Period Area of
Taiwan Subjects Age of subjects
No. of subjects
No. (%) with
MRSAb Reference
2001
Southern
Schoolchildren
2 yr to
⬃18 yr
987
33 (3.3)
Lu et al. (23)
2001-2002
Northern
Schoolchildren
3 yr to
⬃12 yr
262
5 (1.9)
Huang et al. (17)
2003
Northern
Schoolchildren, health care visits
⬍12 yr
640
48 (7.5)
aBoyle-Vavra et al. (1)
2005-2006
Northern
Children for health care visits
2 mo to
⬃5 yr
1,279
121 (9.5)
This study
Central
Children for health care visits
2 mo to
⬃5 yr
1,011
49 (4.8)
This study
Southern
Children for health care visits
2 mo to
⬃5 yr
756
51 (6.7)
This study
aIf restricted to those without risk factors, no. of colonized subjects would be 34 (5.3%).
bThe prevalence increased significantly in the northern (P⬍ 0.0001 by chi-square test) and southern (P ⬍ 0.001) regions of Taiwan during the period of 2001 to
2006.