Single Crystal Structure Determination and Molecular Modeling of Ethyl
4-{[4-(dodecanoyloxy)phenyl]diazenyl}benzoate; Various Mesogenic
Behaviors from Different Lengths of Alkyl Chains
Long-Li Laia* ( ), Feng-Ya Sua( ), Kai-Chao Zhana( ),Yi-Lyang Caia( ), Yi-Hung Liub( ) and Yu Wangb( ) a
Department of Applied Chemistry, National Chi Nan University, Puli 545, Taiwan, R.O.C. b
Instrumentation Center and Department of Chemistry, National Taiwan University, Taipei 106, Taiwan, R.O.C.
The molecular stacking and conformation of ethyl 4-{[4-(dodecanoyloxy)phenyl]diazenyl}benzoate was established on the basis of crystallographic data and further compared with those of 4-{[4-(decanoyl-oxy)phenyl]diazenyl}benzoate and 4-{[4-(octanoyl4-{[4-(decanoyl-oxy)phenyl]diazenyl}benzoate. The variation of mesogenic behaviors resulting from the different lengths of alkyl chains was also studied.
Key words: Liquid crystal; Azo dye; Single crystal structure determination and molecular simu-lation.
INTRODUCTION
Supramolecular aggregation by molecular self-as-sembly is an important issue in the filed of structural chem-istry.1In addition to electrostatic interaction, non-covalent forces also play a significant role in determining the struc-tural stacking and properties of molecular assemblies.2The interaction between molecules containing functionalities is found to be critical for molecular packing in crystalliza-tion3and for the varying mesogenic behaviour of the mole-cules.4Azo dyes 1a and 1b have previously been prepared5 and their liquid crystalline phases also studied in this labo-ratory: We found that the various conformations of the mol-ecules resulted in various molecular stackings in the solid state and different mesogenic phases were obtained in this way, namely, 1a with a C7H15chain showing nematic and SmA phases and 1b with a C9H19chain showing a SmB phase during the thermal process. As this observation is so unusual, we further prepared 1c and 1d and studied their mesogenic behaviours. Azo dye 1d with a C11H23chain was further studied by single crystal structure determination. The corresponding molecular stacking in the solid state was thus established, investigated and further compared with those of 1a and 1b. The conformation of 1d in the gas phase was also studied by molecular modeling and com-pared with those of 1a and 1b. Now we wish to report all
these results.
EXPERIMENTAL
Chemicals used were commercially available from ACROS. The mesogenic behaviour and phase transitions were characterized by polarizing optical microscopy (POM) and differential scanning calorimetry (Perkin-Elmer DSC 6). Powder X-ray diffraction (XRD) patterns were obtained from a Siemens D-5000 X-ray diffractometer equipped with a TTK 450 temperature controller and Cu radiation with the wavelength length,l = 1.5406 Å. Semi-empirical calculation was carried out by the Cache program using the AM1 model, which was provided by Fujitsu (version 4.1). Synthesis of Compounds 1c and 1d
Azo dyes 1c and 1d were prepared by a similar man-ner according to the reaction scheme, confirmed and char-acterized by spectroscopy1H-NMR (Varian 300 FT spec-trometer) and high resolution mass spectra (HRMS; VG70-250; EI, 70 eV). Compound 2 (0.54 g, 2 mmol), prepared according to a previous procedure,5was further treated with hexanoyl chloride (0.33 g, 2 mmol) in dichloro-methane (20 mL) with an excess of triethylamine to yield crude product 1c, which was further purified by chroma-* Corresponding author. Tel: +886-49-2910960 ext. 4975; Fax: +886-49-2917956; E-mail: lilai@ncnu.edu.tw
tography (Al2O3; II); it amounted to 0.48 g (65.5%) after normal work-up.1H-NMR (CDCl3):d 0.90 (t, 3H, J = 6.9 Hz, CH3), 1.33-1.43 (m, 7H, 2CH2+CH3), 1.77 (quint, 2H, CH2), 2.58 (t, 2H, J = 7.2 Hz,CH2), 4.40 (quart, 2H, CH2), 7.25 (d, 2H, J = 8.7 Hz, Ar-H), 7.92 (d, 2H, J = 8.7 Hz, Ar-H), 7.97 (d, 2H, J = 8.7 Hz, Ar-H), 8.17 (d, 2H, J = 8.7 Hz, Ar-H). HRMS for C21H24N2O4: 368.1736. Found: 368.1738. Compound 1d (0.62 g. 69.3%) was prepared similarly. 1H-NMR (CDCl3):d 0.85 (t, 3H, J = 6.9 Hz, CH3), 1.25-1.44 (m, 19H, 8CH2+CH3), 1.76 (quint, 2H, CH2), 2.58 (t, 2H, J = 7.2 Hz, CH2), 4.41 (quart, 2H, CH2), 7.25 (d, 2H, J = 8.7 Hz, Ar-H), 7.91 (d, 2H, J = 8.7 Hz, Ar-H), 7.96 (d, 2H, J = 8.7 Hz, Ar-H), 8.17 (d, 2H, J = 8.7Hz, Ar-H). HRMS for C27H36N2O4: 452.2675. Found: 452.2678.
X-ray Crystallography Analysis
Crystals of compound 1d were grown from dichloro-methane/hexane (1/1) at room temperature. A single or-ange crystal of suitable quality was mounted on a glass fi-ber and used for measurement of precise cell constants and intensity data collection. Diffraction measurement was made on a Nonius Kappa CCD diffractometer with graph-ite-monochromated Mo-Karadiation (l = 0.71073 Å), op-erated at 295(2) K over theq range of 1.06-25.00°. No sig-nificant decay was observed during the data collection. 2850 reflections were observed with I³ 2s(I) among the 11331 unique reflections, and 7434 reflections were used in the refinement. Data were processed on a PC using the SHELXTL software package.6 The structure of 1d was solved using direct methods and refined by full-matrix least square on an F2value. All non-hydrogen atoms were re-fined anisotropically. The positions of hydrogen atoms were identified by calculation, and their contributions in
structure factors were included. The final indices were R1 = 0.1043, wR2 = 0.2572 with goodness-of-fit on F2= 1.059. Other data are described as follows: formula C27H36N4O2; formula weight 452.27; crystal size 0.20 × 0.15 × 0.10 mm3; unit cell a = 5.9860(7), b = 7.6040(9), c = 57.628(7), a = 91.461(2), b = 92.582(3), g = 92.404(3); crystal system triclinic; space group P-1; volume 2617.1(5) Å3; cell units (Z) 4; density (calcd.) 1.154 g cm-3; linear absorption coef-ficient 0.077 (m/cm-1
). Crystallographic data for the com-pound 1d have been deposited with the Cambridge Crystal-lographic Data Center as supplementary publication No. CCDC-267498. Copies of the data can be obtained free of charge on publication to the Director, CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (fax: +44-1223-336033, e-mail: deposit@ccdc.cam.ac.uk).
RESULTS AND DISCUSSION Synthesis and physical study
Reaction of phenol with diazonium salt 2 in basic conditions gave compound 3 in ~ 45% yield, and further treatment of compound 3 with the corresponding acyl chlo-rides yielded the desired azo dyes 1c and 1d in approx. 65% yields. It was found that this series of azo dyes were not chemically stable in acid media but thermally stable during the thermal process. Azo dye 1c, like 1a, showed nematic and SmA phases during the cooling process (Table 1). However, azo dye 1d, unlike 1b with the SmB phase, showed a SmA phase during the thermal process. The ne-matic phase was characterized by its schlieren texture; the SmA phase was characterized by a typical focal-conic tex-ture (Fig. 1). To confirm the nematic and SmA phases of azo dyes 1c and 1d, respectively, they were further studied by powder XRD. As there is no layer structure in the ne-matic phase during the cooling, no reflection at small angle was observed for azo dye 1c at 81.6-66.7°C. The nematic phase of azo dye 1a was confirmed by powder XRD as indi-cated previously.5aAlso, the d-spacing distance (the z-component of an extended molecular length) for compound 1d at 87°C was measured as 31.95 Å obtained by powder XRD during the cooling process; the extended molecular length of compound 1d was calculated to be 31.45 Å be-tween two ending hydrogens by the Cache program on the basis of molecular conformation from the crystallographic data. As liquid crystal molecules in the SmA range are di-rected along the z-axis, the d-spacing from the XRD study
HO N2 O OC2H5 N N BF4- + 2 HO R O Cl N N O O R 3 O OC2H5 O OC2H5 1a: R = C7H15 1b: R = C9H19 1c: R = C5H11 1d: R = C11H23 Reaction scheme
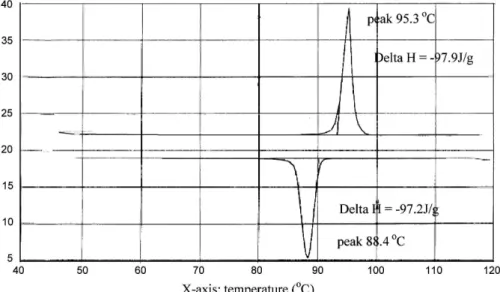
should be approximately equal to the fully extended length of the molecule; therefore, our experimental results are consistent with each other. Additionally, as it is so unusual that azo dye 1b possesses a SmB phase during the thermal process (Figs. 2a and 2b), reconfirmation of its mesogenic behaviour is necessary. Compared with azo dye 1d, the enthalpy change of azo dye 1b from liquid crystalline to
isotropic states is much higher. As shown in Table 1, the enthalpy change of 1d is 39.1 KJ/mole, but that of 1b is 229.2 KJ/mole. Azo dye 1b possesses a mosaic texture un-der an optical polarizing microscope in the SmB phase and was further studied by powder XRD (Fig. 3). At 88°C, two types of major reflection peaks are observed. One set of major reflection peaks are found at approx. 3.4° (stronger; 26.10 Å) and 13.4° (weaker; 6.61 Å), which result from the first-ordered and fourth-ordered reflection of the molecule 1b along the z direction, respectively. The other set of ma-jor reflection peaks at approx. 10.0° (stronger, first-ordered; 8.83 Å) and 20.1° (weaker, second-ordered; 4.42 Å) to-gether with other minor reflection peaks at approx. 10.8, 16.8, 22.6 and 23.5° should all result from the reflection of the molecule in hexagonal arrangement on the X-Y plane.
As indicated previously, the mesogenic behavior of liquid crystals may change according to the different lengths of the alkyl chain.4,5,7To understand the length of alkyl chain on the influence of molecular packing in the solid state in the presence of other functional groups, azo dye 1d was therefore studied by single crystal determination, and its molecular stacking was then established on the basis of the crystallographic data for comparison with those of azo
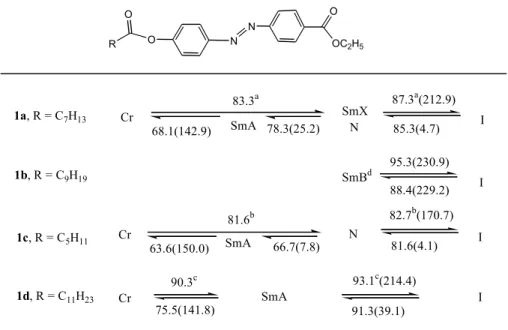
Table 1. Phase transition temperature and corresponding enthalpies (KJ/mole), in parentheses, of compounds 1a-1b
Abbreviations: Cr = crystalline, SmX = unidentified smectic phase, N = nematic, SmA = smectic A phase, SmB = smectic B phase, I = isotropic liquid.
a,b,c
peaks overlapped d
the SmB phase of compound 1b remained even at room temperature N N O O R O OC2H5 I 87.3a(212.9) 78.3(25.2) N 85.3(4.7) 1b, R = C9H19 83.3a I 95.3(230.9) 88.4(229.2) SmBd 1c, R = C5H11 SmX SmA 68.1(142.9) Cr 1a, R = C7H13 I 1d, R = C11H23 93.1c(214.4) 75.5(141.8) 91.3(39.1) Cr 90.3c SmA I 81.6b 66.7(7.8) 81.6(4.1) N SmA 63.6(150.0) Cr 82.7b(170.7)
Fig. 1. The texture of azo dye 1d at 85°C during the cooling process.
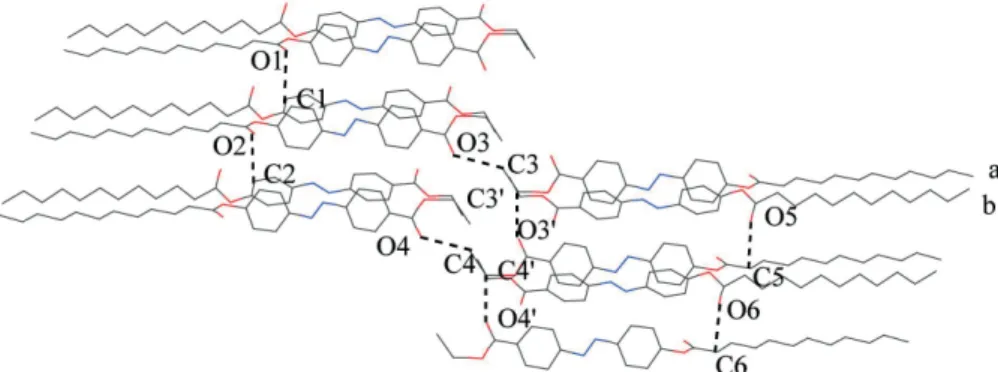
dyes 1a and 1b (Fig. 4). As the molecule 1d is disordered in the solid state, some atoms are omitted for clarity on estab-lishing the molecular stacking. Each molecule of 1d is reg-ularly arranged by a head-to-head or tail-to-tail pattern with its adjacent molecules in the same layer (e.g.
mole-cules a1 and a2 in the A layer) and found to be parallel to the neighboring molecules. The distances between corre-sponding atoms in the same layer (e.g. Na and Nb, Ca and Cb) are estimated to be approx. 5.98 Å, which are close to the corresponding distances (5.51 and 5.57 Å) found in azo
Fig. 2b. The texture of azo dye 1b at 55°C during the cooling process.
Fig. 3. The powder X-ray diffraction (XRD) pattern of compound 1b at 88°C.
Fig. 4. The molecular packing of compound 1d in the solid state. The hydrogen atoms are omitted for clarity. Fig. 2a. The DSC analysis of azo dye 1b.
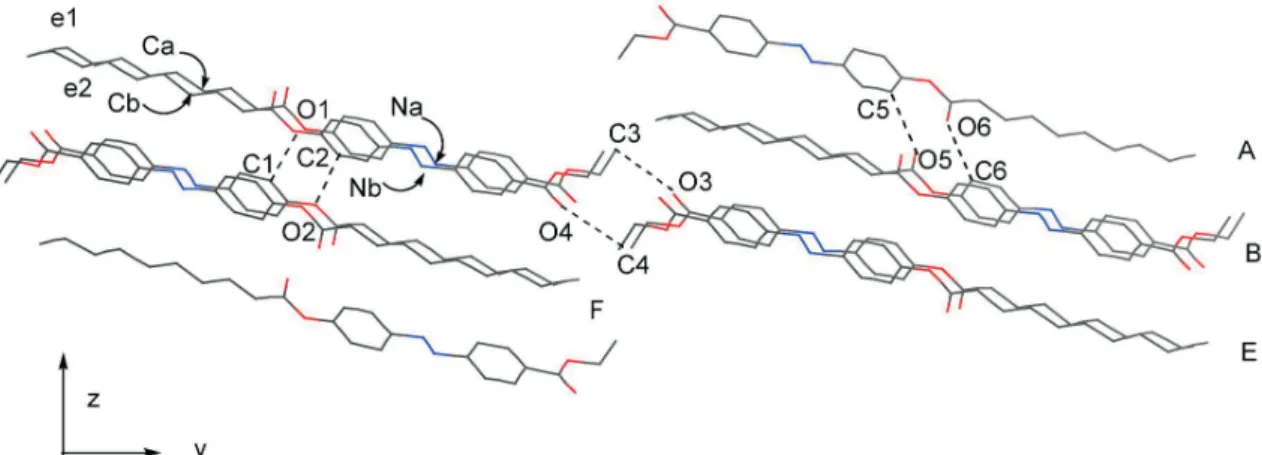
dyes 1a and 1b, respectively.5The molecules in different layers are also similarly arranged with the adjacent mole-cules in different layers (e.g. molemole-cules in the A and B lay-ers in Fig. 4), which are different from the arrangements in the molecular stackings of azo dyes 1a and 1b (Figs. 5 and 6). For 1a and 1b, the molecules in different layers are ap-proximately parallel to each other.5In other words, the
quadrupolar interaction between benzene moieties is not significant (Figs. 5, 5a, 6, 6a). However, for azo dye 1d, the quadrupolar interaction between benzene moieties, to some extent, influences the molecular stacking; the aryl-Hs in one molecule are directed to the plane containing benzene moiety in an adjacent molecule,5and the intercepting angle between the plane containing azobenzene moiety of
mole-Fig. 4a. The molecular packing of compound 1d in the solid state along the view of the z direction. The hydrogen atoms are omitted for clarity.
Fig. 5. The molecular packing of compound 1a in the solid state. The hydrogen atoms are omitted for clarity.
Fig. 5a. The molecular packing of compound 1a in the solid state along the view of the z direction. The hydrogen atoms are omitted for clarity.
cule a and the corresponding plane of molecule b is esti-mated to be about 40° based on crystallographic data (Fig. 4a).
It is believed that such an arrangement in the molecu-lar stacking should also partly arise from the weak H-bond interaction in the molecular stacking. In Fig. 4a, the dis-tances between O (CO2) and aromatic C or saturated C pairs in adjacent layers are similar: Both C1¼O1 and C2¼O2 distances are 3.40 Å and also both C5¼O5 and C6¼O6 distances are 3.32 Å. Consequently, the distances for H(at Cn)¼On (n = 1, 2) and H(at Cn)¼On (n = 5, 6) are short and which are calculated as 2.55 and 2.59 Å, re-spectively; these distances are shorter than the sum of the van der Waals radii of H and O atoms (Bondi radii: H 1.20, O 1.52).9a
The H-bond interaction between the terminal ethoxy-carboxylate moieties of molecules in the same or adjacent layers also contributes in the molecular stacking: The C3¼O3 and C4¼O4 distances are both 3.78 Å and the C3’¼O3’ and C4’¼O4’ distances are both 3.62 Å. Conse-quently, the distances for H (at Cn)¼On (n = 3, 4) and H (at
Cn’)¼On’ (n = 3, 4) are 3.00 and 2.83 Å, respectively, which are slightly longer than the sum of the van der Waals radii of H and O atoms. It is noteworthy that the aromatic or saturated C-H in this case acts as the H-bond donor for the formation of the intermolecular H-bond with an O atom in the liquid crystalline network. Though unusual, this has been observed in other systems.5,9b-9e
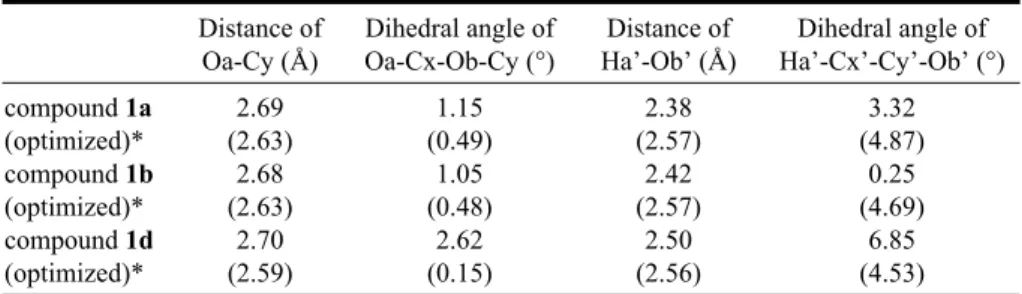
To understand the influence of the polarity of the CO2 group, quadrupolar interaction and intermolecular H-bond-ing interaction on the conformation of the azo dye mole-cules in the solid state, the corresponding conformations of 1a, 1b and 1d are established from crystallographic data, and the bond distances of Oa-Cy and Ha’-Ob’ together with the dihedral angles of Oa-Cx-Ob-Cy and Ha’-Cx’-Cy’-Ob’ for 1a, 1b and 1d are respectively calculated (Table 2 and Fig. 7): The dihedral angles of Ha’-Cx’-Cy’-Ob’ in com-pounds 1a, 1b and 1d are calculated as 3.32, 0.25 and 6.85°, respectively. The dihedral angles of Oa-Cx-Ob-Cy in compounds 1a, 1b and 1d, which are calculated as 1.15, 1.05 and 2.62°, also demonstrate a similar trend as those of Ha’-Cx’-Cy’-Ob’. It may thus be regarded that the mutual
Fig. 6. The molecular packing of compound 1b in the solid state. The hydrogen atoms are omitted for clarity.
Fig. 6a. The molecular packing of compound 1b in the solid state along the view of the z direction. The hydrogen atoms are omitted for clarity.
interaction in molecular stacking 1d between adjacent lay-ers (e.g. laylay-ers A and B in Figs. 4a, 5, 6) is strongest and the mutual interaction in molecular stacking 1b is weakest among azo dyes 1a, 1b and 1d. This result is also consistent with the intermolecular H-bond interaction in the
molecu-lar stackings of 1a, 1b and 1d (Table 3). The H-bond dis-tances of H(at Cn)¼On (n = 1, 2), H(at Cn)¼On (n = 5, 6) and H (at Cn’)¼On’ (n = 3, 4) between adjacent layers in 1d are calculated as 2.55, 2.59 and 2.83 Å, respectively. The H-bond distances of H (at Cn)¼On (n = 1,2) and H(at
Table 3. Some of the intermolecular H-bond distances for compounds 1a, 1b and 1d Distances (Å) of
H (at C1)-O1 and H (at C2)-O2
Distances (Å) of H (at C3)-O3 and
H (at C4)-O4
Distances (Å) of H (at C5)-O5 and
H (at C6)-O6
compound 1a 2.85 2.75 2.57
compound 1b 3.02 3.00 2.58
compound 1d 2.55 3.00 (2.83*) 2.59
* Distances (Å) of H (at C3’)-O3’ and H (at C4’)-O4’.
Fig. 7. The conformation of a single molecule for compounds 1a, 1b and 1d.
Table 2. Some of the distances of two atoms and dihedral angles for compounds 1a, 1b and 1d Distance of Oa-Cy (Å) Dihedral angle of Oa-Cx-Ob-Cy (°) Distance of Ha’-Ob’ (Å) Dihedral angle of Ha’-Cx’-Cy’-Ob’ (°) compound 1a (optimized)* 2.69 (2.63) 1.15 (0.49) 2.38 (2.57) 3.32 (4.87) compound 1b (optimized)* 2.68 (2.63) 1.05 (0.48) 2.42 (2.57) 0.25 (4.69) compound 1d (optimized)* 2.70 (2.59) 2.62 (0.15) 2.50 (2.56) 6.85 (4.53) * The optimization was completed on the basis of original conformation from crystallographic
Cn)¼On (n = 5, 6) between adjacent layers in 1a are calcu-lated as 2.85, and 2.57 Å, respectively; analogously, the corresponding H-bond distances in 1b are calculated as 3.02, and 2.58 Å, respectively.
The conformations of azo dyes 1a, 1b and 1d were further optimized by the CaChe program, using the AM1 model in the gas phases. After optimization, the bond dis-tances of Oa-Cy and Ha’-Ob’ together with the dihedral an-gles of Oa-Cx-Ob-Cy and Ha’-Cx’-Cy’-Ob’ for 1a, 1b and 1d are further calculated (Table 2 and Fig. 7). The corre-sponding bond distances and dihedral angles are not signif-icantly different. This indicates that in the gas phase, the conformation of molecules 1a, 1b and 1d might be quite similar because of the absence of the polar attraction and quadrupolar and intermolecular H-bonding interaction. However, the conformations of molecules 1a, 1b and 1d in the solid state are different because of these forces in the corresponding molecular stackings.
Additionally, it is worthwhile to discuss the H-bond interaction between different molecules in the same layer for 1a, 1b and 1d: The H-bond distances of H(at Cn)¼On (n = 3, 4) in 1a are calculated as 2.75 Å, and those of corre-sponding distances are both 3.00 Å for 1b and 1d. The stronger interaction between two ending functional groups in 1a may lead to the bending of the molecular stacking as shown in Fig. 5a and the resulting nematic phase is thus ob-served. It is known that liquid crystal with SmA phase pos-sesses a layer structure; additionally, if a regular hexagonal arrangement exists among the layer structure, then a SmB phase is expected. Based on the above observations, it is clear that the interactions between adjacent layers are stronger in 1d than those in 1b. This stronger interaction in some regional areas leads to the breaking of regular hexag-onal arrangement, and thus azo dye 1b shows a SmB phase but 1d shows a SmA phase during the thermal process.
In summary, we have clearly showed that azo dyes 1a, 1b and 1d, having the same core and different lengths of alkyl chains, possess various mesogenic behaviours and their corresponding molecular stackings are different based on crystallographic study.10Although we do not directly demonstrate the exact molecular arrangements of 1a, 1b and 1d in the liquid crystalline phases, we do show that dif-ferent lengths of alkyl chain obviously can lead to the vari-ous stackings of 1a, 1b and 1d in the solid state because of the different interaction of functional polarity, H-bond and
quadrupolarity of benzene moiety.
We thank National Chi Nan University and the Na-tional Science Council (NSC 93-2113-M-260-002) for fi-nancial support. The National Center of High-Performing Computing and the Institute of Chemistry, Academia Sinica, are also highly appreciated for providing the Beilstein data-base system and the most helpful library service, respec-tively.
Received November 23, 2005.
REFERENCES
1. (a) Lehn, J.-M. Supramolecular Chemistry; VCH: Weinheim, 1995. (b) Hamilton, A. D. Perpectives in Supramolecular Chemistry; John Wiley, 1996.
2. (a) Hunter, C. A. Angew. Chem. int. Ed. Engl. 1993, 32, 1584. (b) Hunter, C. A. Chem. Soc. Rev. 1994, 23, 101. 3. (a) Desiraju, G. R. Crystal Engineerring: The Design of
Or-ganic Solids; Elsevier: New York, 1989. (b) Desiraju, G. R. Chem. Commun. 1997, 1475.
4. (a) Lee, K. M.; Lee, C. M.; Lin, I. J. B. Angew. Chem. Int. Ed. Engl. 1997, 36, 1850. (b) Chang, J.; Moore, J. S. J. Am. Chem. Soc. 1994, 116, 2655.
5. (a) Lai, L. L.; Su, F. Y.; Hung, C. H. Liq. Cryst. 2004, 31, 773. (b) Lai, L. L.; Su, F. Y.; Lin, Y. J.; Ho, C. H.; Wang, E.; Hung, C. H.; Liu, Y. H.; Wang, Y. Helv. Chim. Acta 2002, 85, 1517.
6. Sheldrick, G. M. SHELXTL version 5.03, Siemens Analyti-cal X-ray Instruments Inc., Wisconsin: Madison, 1994. 7. (a) Lai, L. L.; Ho, C. H.; Lin, Y. J.; Wang, E.; Liu, Y. H.;
Wang, Y.; Lin, Y. C.; Cheng, K. L. Liq. Cryst. 2002, 29, 1477. (b) Petrov, V. F. Liq. Cryst. 2001, 28, 217.
8. (a) Haase, W.; Athanassopoulou, M. A. Structure and Bonding 1999, 44, 140 and references cited therein. (b) Ciunik, Z.; Desiraju, G. R. Chem. Commun. 2001, 703. 9. (a) Bondi, A. J. Phys. Chem. 1964, 68, 441. (b) Allen, F. H.;
Lommerse, J. M. P.; Hoy, V. J.; Howard, J. K. H.; Desiraju, G. R. Acta Cryst. 1996, B52, 734. (c) Thaimattam, R.; Xue, F.; Sarma, J. A. R. P.; Mak, T. C. W.; Desiraju, G. R. J. Amer. Chem. Soc. 2001, 123, 4432. (d) Muthuraman, M.; Masse, R.; Nicoud, J.-F.; Desiraju, G. R. Chem. Mater. 2001, 13, 1473.
10. Crystals of 1a, 1b and 1d for crystallographic studies were all grown from dichloromethane/hexane (1/1) at room tem-perature.