The Pediatric Infectious Disease Journal • Volume 31, Number 9, September 2012 www.pidj.com |
e163
O
RIGINAL
S
TUDIES
Background: The aim of the study was to investigate whether the 7-valent
pneumococcal conjugate vaccine (PCV7) alters common risk factors of nasopharyngeal carriage by Streptococcus pneumoniae in children.
Methods: From July 2005 through December 2010, we performed a
cross-sectional study investigating risk factors associated with pneumococcal car-riage in children. Parents of participating children completed questionnaires including whether or not the children received PCV7 vaccination.
Results: Among 9705 children, 20.2% of them received at least 1 dose of
the PCV7 vaccine. Multivariate logistic regression models identified older age, having 1 sibling in a family, history of acute otitis media and house-hold exposure to smoking as independent risk factors for pneumococcal carriage in the unvaccinated group, but not associated with pneumococcal carriage in the vaccinated group. The number of siblings ≥2 in a family, history of upper respiratory tract infection and child-care attendance were strong factors associated with pneumococcal carriage in children, regard-less of vaccination. In vaccinated group, breast-feeding was associated with increased nonvaccine type pneumococcal carriage, mainly in children with upper respiratory tract infection.
Conclusions: PCV7 decreased the association between pneumococcal
carriage and older age, 1 sibling in a family, history of acute otitis media and household exposure to smoking, but increased the association between pneumococcal carriage and breast-feeding.
Key Words: pneumococcal conjugate vaccine, risk factor, nasopharyngeal
carriage
(Pediatr Infect Dis J 2012;31: e163–e168)
S
treptococcus pneumoniae is a leading extracellular Gram-posi-tive pathogen that commonly colonizes the nasopharynx of chil-dren. Such carriage acts as a reservoir where adaptability provided through recombination enables S. pneumoniae to overcome variousenvironmental challenges and continues to transmit in the com-munity.1 By residing on the mucosal surface of the upper
respira-tory tract, pneumococci can cause respirarespira-tory tract infections such as sinusitis, otitis media and pneumonia. If pneumococci spread beyond this niche into the blood or meninges, invasive disease of bacteremia, sepsis and meningitis occurs. A reduction of pneumo-coccal nasopharyngeal carriage would result in a decrease in res-piratory infection and invasive disease caused by S. pneumoniae.
The mechanism of natural immunity to pneumococcal colo-nization has been studied. Malley et al2 showed that passive transfer
of serotype-specific antibodies protected against nasopharyngeal pneumococcal colonization in infant rats. However, colonization was shown to generate minimal anticapsular antibodies in a human carriage study,3 and no correlation between naturally acquired IgG
and carriage protection has been found.3–5 In recent years, Toll-like
receptor 2, CD4+ T cells and IL-17A have been shown to mediate
antibody-independent clearance of pneumococci in the airway.6–8
Pneumococcal conjugate vaccine has been shown to reduce vaccine serotype (VT) and cross-reacting serotype pneumococcal nasopharyngeal carriage among vaccinated children by preventing new acquisition9 and by a consequent reduction in the
transmis-sion of S. pneumoniae from vaccinated children to unvaccinated children and adults.10 Induction of serum serotype–specific
pneu-mococcal anticapsular antibodies to reduce acquisition of certain S. pneumoniae serotypes has been demonstrated among recipients of pneumococcal conjugate vaccine.4,11 Since October 2005, the
7-valent pneumococcal conjugate vaccine (PCV7) (Prevnar; Pfizer, New York, NY) has been available on the private market in Tai-wan, but not included in the national immunization program, with a vaccination schedules of 2, 4, 6 and 13 months, and catch-up vaccination for children aged up to 5 years. To expand our insights into the ability of PCV7 to influence pneumococcal carriage in the community, we conducted a prospective, cross-sectional study to evaluate risk factors for pneumococcal carriage between vaccinated and unvaccinated children.
MATERIALS AND METHODS
Study Population and Data Collection
From July 2005 through December 2010, we enrolled chil-dren aged between 2 months and 5 years who were brought to pri-mary care clinic because of acute illness or for regular vaccination at 3 tertiary teaching hospitals: Chang-Gung Memorial Hospital, Taoyuan (in northern Taiwan); Veterans General Hospital, Tai-chung (in central Taiwan); and Chang Gung Memorial Hospital, Kaohsiung (in southern Taiwan). Children with immunological, neoplastic, renal, cardiac or hematological disease; bronchopulmo-nary dysplasia or Down’s syndrome were excluded from the study. Part of the study results (from 2005 to 2008) which did not discuss whether PCV7 influence the risk factor for pneumococcal carriage has been published.12
Copyright © 2012 by Lippincott Williams & Wilkins ISSN: 0891-3668/12/3109-e163
DOI: 10.1097/INF.0b013e31825cb9f9
The Impact of the Heptavalent Pneumococcal Conjugate
Vaccine on Risk Factors for Streptococcus pneumoniae
Carriage in Children
Yu-Chia Hsieh, MD, PhD,* Cheng-Hsun Chiu, MD, PhD,* Kuang-Yi Chang, MD, PhD†‡,
Yhu-Chering Huang, MD,* PhD, Chih-Jung Chen, MD, PhD,* Chen-Yen Kuo, MD,* Po-Yen Chen, MD,§
Kao-Pin Hwang, MD,|| and Tzou-Yien Lin, MD*
Accepted for publication April 27, 2012.
From the *Department of Pediatrics, Chang Gung Children’s Hospital, Chang Gung Memorial Hospital, Chang Gung University, College of Medicine, Taoyuan, Taiwan; †Division of Biostatistics, Graduate Institute of Epi-demiology and Preventive Medicine, National Taiwan University, Taipei, Taiwan; ‡Department of Anesthesiology, Taipei Veterans General Hospital and National Yang-Ming University School of Medicine, Taipei, Taiwan; §Department of Pediatrics, Taichung Veterans General Hospital, Taichung, Taiwan; and ||Department of Pediatrics, School of Medicine, Children’s Hos-pital, China Medical University & HosHos-pital, Taichung, Taiwan.
Supported by Wyeth Pharmaceuticals Inc., a subsidiary of Pfizer Inc, Taiwan. The authors have no other funding or conflicts of interest to disclose. Address for correspondence: Tzou-Yien Lin, MD, 5 Fu-Hsin Street, Kwei-Shan
Hsiang, Division of Pediatric Infectious Diseases, Department of Pediatrics, Chang Gung Children’s Hospital, Taoyuan County, Taiwan. E-mail: pidlin@ adm.cgmh.org.tw
The Pediatric Infectious Disease Journal
31
9
© 2012 Lippincott Williams & Wilkins
0891-3668
INF
INF202787
Hsieh et al
Streptococcus pneumoniae Carriage
September
0
0
Trained investigators obtained informed consent from the parents of participating children and conducted interviews using a standard questionnaire. Parents of participating children were asked about demographic information, attendance of group child care, numbers of siblings, smoking by family members and dura-tion of breast-feeding. Parents of participating children also con-sented to a medical record review for information on PCV7 vac-cination history, history of acute otitis media (AOM), history of upper respiratory tract infection (URI) in the previous 2 weeks, antibiotic use in the previous 2 weeks and symptoms and diagnoses at the time of the visit. Analyses of PCV7 vaccination divided the participants into those who received at least 1 dose and those who did not receive any dose. Respiratory tract infection included viral URI, bronchitis, pharyngitis and sinusitis. All study procedures were approved by the Institutional Review Board of the Chang Gung Memorial Hospital.
Bacteria Sampling and Serotyping (Including
Serotype 6C and 6D Identification)
Trained study personnel collected the nasopharyngeal swabs from children by using a sterile swab (Transwab; Copan Italia, Brescia, Italy; Medical Wire & Equipment Co., Corsham, Wiltshire, England ), which was introduced into the nostrils and was advanced until resistance was found. The swabs were inocu-lated into Amies transport medium (Copan) and were pinocu-lated within 4 hours of sampling on blood agar plates with 5% sheep’s blood to isolate S. pneumoniae.
Pneumococcal isolates was identified by standard meth-ods. In brief, identification of S. pneumoniae was based on colony morphology and conventional methods of determination (optochin susceptibility and bile solubility assays). One S. pneumoniae colony per plate was then subcultured, harvested and kept frozen at −70°C for further testing. Pneumococcal serotyping was
deter-mined by the capsular swelling method (Quellung reaction) using antisera from the Statens Serum institut (Copenhagen, Denmark) at Chang Gung Children’s Hospital. All isolates determined to be
6A by Quellung reaction were selected for testing to resolve sero-types 6A and 6C with the use of polymerase chain reaction–tested serotyping methods.13 All isolates determined to be 6B by
Quel-lung reaction were selected for testing to resolve serotypes 6B and 6D with the use of polymerase chain reaction–tested serotyping methods.13
Statistical Analysis
Children were divided into PCV7-vaccinated and unvac-cinated groups. Statistical analyses were performed separately for the 2 groups. Differences between groups of categorical data were tested by the χ2 test. Multivariate logistic regression models were
then used to examine the effects of significant factors in the χ2 test
on S. pneumoniae colonization. Odds ratios with 95% confidence intervals were computed to assess the association between S. pneu-moniae colonization and factors included in the univariate and multivariate logistic regression models. The χ2 test or Fisher’s exact
test was used to evaluate differences in serotype prevalence rates. P value <0.05 was considered to be statistically significant. All sta-tistical analyses were performed using SPSS software, version 15.0 (SPSS Inc., Chicago, IL).
RESULTS
Between July 2005 and December 2010, we enrolled a total of 9708 children, 3 of which were excluded because they did not complete the questionnaire form. In total, 9705 specimen were collected, of which 1225 (12.6%) were colonized with S. pneumoniae. Characteristics of the 9705 children are shown in
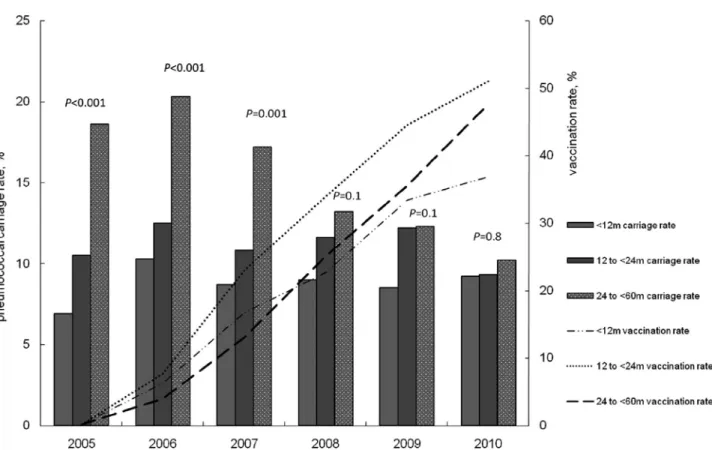
Table 1. The PCV7 vaccination rate among participants increased from 0.2% in 2005 to 45.5% in 2010 (Fig. 1). During the study period, VT carriage rate among all participating children by year significantly decreased (P < 0.001) and serotype 15B (P = 0.007) and 19A (P = 0.001) carriage rates by year significantly increased (Fig. 1). The overall pneumococcal carriage rate was lower in vaccinated group compared with that in unvaccinated group (8.9% versus 13.6%; P < 0.001). In 2005, all types of pneumococcal carriage rates among each age group were dif-ferent (P < 0.001) (Fig. 2). As the vaccination rate in each age group gradually increased over the study period, there was no significant variation in pneumococcal carriage rates among 3 age groups in 2010 (P = 0.8). One thousand nine hundred fifty-eight participants (20.2%) in this study received at least 1 dose of the PCV7 vaccine. In unvaccinated group, overall pneumo-coccal carriage rates were progressively lower only in the 24- to <60-month-old age group over the study period (18.6% in 2005 to 13.4% in 2010; P = 0.001).
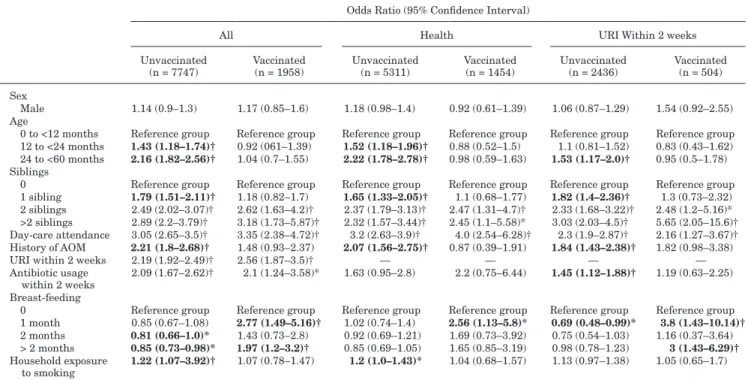
With the univariate analysis, older age, having 1 sibling, his-tory of AOM and household exposure to smoking were associated with an increased risk for pneumococcal colonization in the unvac-cinated group, but not in the vacunvac-cinated group (Table 2). Number of siblings ≥2 in a family, day-care attendance, URI within 2 weeks and antibiotic usage within 2 weeks were significantly associated with pneumococcal carriage in both group. Of note, breast-feeding decreased the risk of pneumococcal carriage in the unvaccinated group, but increased the risk of pneumococcal carriage in the vac-cinated group. In multivariate analysis, all factors except for anti-biotic usage within 2 weeks had similar effects on pneumococcal colonization between unvaccinated and vaccinated group (Table 3).
TABLE 1. Characteristics of the 9705 Children at the
Time of Enrollment N (%) Age 0 to <12 months 2733 (28.2) 12 to <24 months 2795 (28.8) 24 to <60 months 4177 (43) Sex distribution Male 5276 (54.4) Site North 3281 (33.8) Central 3144 (32.4) South 3280 (33.8) Siblings 0 3322 (34.2) 1 sibling 4298 (48.4) 2 siblings 1193 (12.3) >2 siblings 492 (5.1) Day-care attendance 1781 (18.3) History of AOM 894 (9.2) URI within 2 weeks 2940 (30.3) Antibiotic usage within 2 weeks 571 (5.9) Breast-feeding
0 2705 (27.9)
1 month 960 (9.9) 2 months 1398 (14.4) >2 month 4642 (47.8) Household exposure to smoking 4546 (46.8) PCV7 vaccination ≥1 dose 1958 (20.2) 2005 3 (0.2) 2006 150 (5.7) 2007 197 (17.1) 2008 404 (26.9) 2009 568 (37.5) 2010 636 (45.5)
FIGURE 1. Serotype carriage rates and PCV7 vaccination rates (received at least 1 dose of PCV7) in children below the age of
60 months among the study population by year.
FIGURE 2. Pneumococcal carriage rates and PCV7 vaccination rates (received at least 1 dose of PCV7) in 3 age groups among
TABLE 2. Univariate Analysis of Factors for Pneumococcal Carriage Between Unvaccinated and Vaccinated
Children
Odds Ratio (95% Confidence Interval)
All Health URI Within 2 weeks Unvaccinated
(n = 7747) Vaccinated (n = 1958) Unvaccinated (n = 5311) Vaccinated (n = 1454) Unvaccinated (n = 2436) Vaccinated (n = 504) Sex
Male 1.14 (0.9–1.3) 1.17 (0.85–1.6) 1.18 (0.98–1.4) 0.92 (0.61–1.39) 1.06 (0.87–1.29) 1.54 (0.92–2.55) Age
0 to <12 months Reference group Reference group Reference group Reference group Reference group Reference group 12 to <24 months 1.43 (1.18–1.74)† 0.92 (061–1.39) 1.52 (1.18–1.96)† 0.88 (0.52–1.5) 1.1 (0.81–1.52) 0.83 (0.43–1.62) 24 to <60 months 2.16 (1.82–2.56)† 1.04 (0.7–1.55) 2.22 (1.78–2.78)† 0.98 (0.59–1.63) 1.53 (1.17–2.0)† 0.95 (0.5–1.78) Siblings
0 Reference group Reference group Reference group Reference group Reference group Reference group 1 sibling 1.79 (1.51–2.11)† 1.18 (0.82–1.7) 1.65 (1.33–2.05)† 1.1 (0.68–1.77) 1.82 (1.4–2.36)† 1.3 (0.73–2.32) 2 siblings 2.49 (2.02–3.07)† 2.62 (1.63–4.2)† 2.37 (1.79–3.13)† 2.47 (1.31–4.7)† 2.33 (1.68–3.22)† 2.48 (1.2–5.16)* >2 siblings 2.89 (2.2–3.79)† 3.18 (1.73–5.87)† 2.32 (1.57–3.44)† 2.45 (1.1–5.58)* 3.03 (2.03–4.5)† 5.65 (2.05–15.6)† Day-care attendance 3.05 (2.65–3.5)† 3.35 (2.38–4.72)† 3.2 (2.63–3.9)† 4.0 (2.54–6.28)† 2.3 (1.9–2.87)† 2.16 (1.27–3.67)† History of AOM 2.21 (1.8–2.68)† 1.48 (0.93–2.37) 2.07 (1.56–2.75)† 0.87 (0.39–1.91) 1.84 (1.43–2.38)† 1.82 (0.98–3.38) URI within 2 weeks 2.19 (1.92–2.49)† 2.56 (1.87–3.5)† — — — — Antibiotic usage
within 2 weeks 2.09 (1.67–2.62)† 2.1 (1.24–3.58)* 1.63 (0.95–2.8) 2.2 (0.75–6.44) 1.45 (1.12–1.88)† 1.19 (0.63–2.25) Breast-feeding
0 Reference group Reference group Reference group Reference group Reference group Reference group 1 month 0.85 (0.67–1.08) 2.77 (1.49–5.16)† 1.02 (0.74–1.4) 2.56 (1.13–5.8)* 0.69 (0.48–0.99)* 3.8 (1.43–10.14)†
2 months 0.81 (0.66–1.0)* 1.43 (0.73–2.8) 0.92 (0.69–1.21) 1.69 (0.73–3.92) 0.75 (0.54–1.03) 1.16 (0.37–3.64) > 2 months 0.85 (0.73–0.98)* 1.97 (1.2–3.2)† 0.85 (0.69–1.05) 1.65 (0.85–3.19) 0.98 (0.78–1.23) 3 (1.43–6.29)†
Household exposure
to smoking 1.22 (1.07–3.92)† 1.07 (0.78–1.47) 1.2 (1.0–1.43)* 1.04 (0.68–1.57) 1.13 (0.97–1.38) 1.05 (0.65–1.7)
Significant odds ratios and 95% confidence interval only in 1 group (vaccinated or unvaccinated) are shown in boldface. *P ≤ 0.05.
†P ≤ 0.005.
TABLE 3. Multivariate Analysis of Risk Factors for Pneumococcal Carriage Between Unvaccinated and Vaccinated
Children
Odds Ratio (95% Confidence Interval)
All Healthy URI Within 2 Weeks Unvaccinated
(n = 7747) Vaccinated (n = 1958) Unvaccinated (n = 5311) Vaccinated (n = 1454) Unvaccinated (n = 2436) Vaccinated (n = 504) Age
0 to <12 months Reference group Reference group Reference group Reference group Reference group Reference group 12 to <24 months 1.29 (1.06–1.57)* 0.84 (0.55–1.29) 1.43 (1.11–1.84)* 0.84 (0.49–1.46) 1.07 (0.78–1.48) 0.77 (0.38–1.55) 24 to <60 months 1.04 (0.85–1.27) 0.48 (0.3–0.79)† 1.18 (0.9–1.54) 0.4 (0.21–0.78)* 0.84 (0.61–1.15) 0.57 (0.27–1.21)
Siblings
0 Reference group Reference group Reference group Reference group Reference group Reference group 1 sibling 1.62 (1.36–1.92)† 1.27 (0.87–1.85) 1.51 (1.21–1.89)† 1.2 (0.73–1.98) 1.74 (1.33–2.28)† 1.3 (0.72–2.37) 2 siblings 2.27 (1.83–2.81)† 2.71 (1.65–4.45)† 2.2 (1.65–2.93)† 2.88 (1.49–5.57)† 2.36 (1.69–3.28)† 2.45 (1.14–5.3)* >2 siblings 2.68 (2.02–3.55)† 3.31 (1.75–6.27)† 2.22 (1.48–3.31)† 2.42 (1.02–5.71)* 3.2 (2.12–4.82)† 6.51 (2.3–18.9)† Day-care attendance 2.77 (2.32–3.3)† 4.47 (2.85–7.0)† 3.01 (2.37–3.83)† 6.94 (3.77–12.8)† 2.56 (1.97–3.33)† 2.61 (1.32–5.2)* History of AOM 1.43 (1.17–1.75)† 1.13 (0.68–1.9) 1.38 (1.02–1.86)* 0.77 (0.34–1.78) 1.5 (1.14–1.97)† 1.58 (0.78–3.19) URI within 2 weeks 1.74 (1.51–2.01)† 2.34 (1.66–3.3)† — — — — Antibiotic usage
within 2 weeks 1.18(0.92–1.51) 1.25 (0.69–2.26) 1.51 (0.86–2.64) 2.07 (0.68–6.29) 1.15 (0.87–1.52) 1.12 (0.55–2.27) Breast-feeding
0 Reference group Reference group Reference group Reference group Reference group Reference group 1 month 0.91 (0.71–1.16) 2.89 (1.51–5.5)† 1.09 (0.79–1.5) 2.33 (1.0–5.43)* 0.73 (0.51–1.06) 4.1 (1.49–11.3)*
2 months 0.93 (0.75–1.15) 1.61 (0.81–3.21) 1.07 (0.81–1.43) 1.81 (0.76–4.28) 0.77 (0.56–1.08) 1.21 (0.37–4.0) >2 months 1.02 (0.87–1.19) 2.06 (1.24–3.42)† 0.99 (0.8–1.23) 1.59 (0.8–3.13) 1.1 (0.85–1.35) 2.95 (1.37–6.4)*
Household exposure
to smoking 1.18 (1.03–1.35)* 1.08 (0.77–1.5) 1.16 (0.97–1.39) 0.94 (0.6–1.47) 1.19 (0.97–1.47) 1.27 (0.75–2.14)
Significant odds ratios and 95% confidence interval only in 1 group (vaccinated or unvaccinated) are shown in boldface. *P ≤ 0.05.
The protective effect of breast-feeding on pneumococcal coloniza-tion in the unvaccinated group was not seen, but remained associ-ated with increased pneumococcal colonization in the vaccinassoci-ated group. All participants were divided into healthy children and children having URI within 2 weeks for analysis as S. pneumoniae colonization among these 2 populations may be different (Tables 2 and 3). With multivariate analysis, PCV7 decreased the association between pneumococcal carriage and having 1 sibling and history of AOM in children, regardless of URI (Table 3). Breast-feeding increased a higher risk of pneumococcal carriage mainly in vac-cinated children with URI (Table 3). Further analysis on VT and nonvaccine serotype (NVT) carriage showed that breast-feeding significantly increased NVT pneumococcal carriage in vaccinated children with URI (P < 0.05) (data not shown). However, the over-all pneumococcal carriage rate was lower in vaccinated group com-pared with that in unvaccinated group among children with URI (15.5 % versus 20.3 %; P = 0.01).
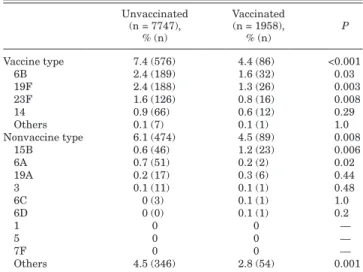
Children having received the PCV7 vaccine had a significantly lower rate of carrying VT (P < 0.001) and NVT (P = 0.008) pneumo-cocci than the unvaccinated children (Table 4). Only 4 isolates were identified as serotype 6C. One isolate was identified as serotype 6D. The vaccinated group had a higher rate of carrying serotype 15B (P = 0.006) and a lower rate of carrying serotype 6A (P = 0.02). The vaccinated group did not have a higher rate of carrying serotype 19A, 3, 6C and 6D. Among the children exposed to household smoking, those who received the PCV7 vaccine had a significantly lower rate of VT pneumococcal carriage (P < 0.001) (Table 5).
DISCUSSION
The primary finding of this study was that PCV7 vacci-nation decreased the association between pneumococcal carriage and older age, 1 sibling in a family, history of AOM and house-hold exposure. In the United States where PCV7 has been used routinely since 2000, the presence of a sibling, history of URI and child-care attendance remain common predictors of pneumococ-cal carriage.14 Similarly, number of siblings ≥2, history of URI
and child-care attendance were strong factors associated with pneumococcal carriage in children, regardless of vaccination in our study.
Age and the presence of siblings influence the carriage of S. pneumoniae in children.14 In the current study, we found that PCV7
vaccination decreases the risk for pneumococcal carriage that is associated with older age, and the presence of a sibling. S. pneu-moniae is one of the most common pathogens that cause AOM, and there is a direct relationship between the frequency of colonization and episodes of AOM.15 Studies have shown that PCV7
immuniza-tion reduces the rate of nasopharyngeal carriage of vaccine-type S. pneumoniae, reduces the incidence of AOM and alters the micro-biology of AOM.16,17 In the current study, we observed that the
his-tory of AOM was a risk factor for S. pneumoniae carriage among unvaccinated children, whereas the history of AOM was not associ-ated with S. pneumoniae carriage among vaccinassoci-ated children. The reason for this disparity is likely that PCV7 vaccination reduced nasopharyngeal colonization of S. pneumoniae and decreased the occurrence of AOM among the vaccinated children. This finding-implicated S. pneumoniae is an important pathogen causing AOM in children in Taiwan.
Cigarette smoking impairs mucociliary clearance and induces inflammation of the respiratory epithelium predisposing individuals to bacterial adherence and colonization.18–20 Studies
have shown that children exposed to household smoking have a higher rate of S. pneumoniae carriage.21–23 Likewise, our study
observed that household exposure to smoking was significantly associated with pneumococcal carriage among unvaccinated children. Among vaccinated children, exposure to smoking was not associated with a higher rate of pneumococcal carriage than children not exposed to household smoking. For the first time, we have demonstrated that PCV7 vaccination can decrease the influence of exposure to smoking for S. pneumoniae carriage among children, which may further reduce pneumococcal trans-mission and pneumococcal disease in adults as a result of smok-ing exposure.
Breast-feeding has been shown to be protective against respiratory tract infection and invasive pneumococcal disease.24,25
However, the association between breast-feeding and pneumococ-cal carriage was inconclusive.26–28 In this study, we found
breast-feeding significantly increased the risk of NVT pneumococcal carriage, mainly in vaccinated children with URI. Pneumococcal colonization is higher during URI than during health.29 In
vacci-nated children with URI, NVT pneumococci should increase to occupy the niche. The reason for the association between breast-feeding and NVT pneumococcal carriage is not clear. Further well-designed, longitudinal studies to control socioeconomic fac-tors, survey adult carriage and investigate the interaction between S. pneumoniae and other bacterial species that commonly colonize the nasopharynx among the vaccinated group with breast-feeding can be carried out.
TABLE 4. Serotype Distribution Between
Unvaccinated and Vaccinated Children
Unvaccinated (n = 7747), % (n) Vaccinated (n = 1958), % (n) P Vaccine type 7.4 (576) 4.4 (86) <0.001 6B 2.4 (189) 1.6 (32) 0.03 19F 2.4 (188) 1.3 (26) 0.003 23F 1.6 (126) 0.8 (16) 0.008 14 0.9 (66) 0.6 (12) 0.29 Others 0.1 (7) 0.1 (1) 1.0 Nonvaccine type 6.1 (474) 4.5 (89) 0.008 15B 0.6 (46) 1.2 (23) 0.006 6A 0.7 (51) 0.2 (2) 0.02 19A 0.2 (17) 0.3 (6) 0.44 3 0.1 (11) 0.1 (1) 0.48 6C 0 (3) 0.1 (1) 1.0 6D 0 (0) 0.1 (1) 0.2 1 0 0 — 5 0 0 — 7F 0 0 — Others 4.5 (346) 2.8 (54) 0.001
TABLE 5. Serotype Distribution Between
Unvaccinated and Vaccinated Children Exposed to Household Smoking
Smoking Exposure Unvaccinated (n = 3737), % (n) Vaccinated (n = 809), % (n) P Vaccine type 8.1 (301) 4.4 (24) <0.001 19F 2.7 (102) 0.9 (5) 0.001 23F 1.8 (68) 0.7 (4) 0.03 6B 2.4 (88) 1.6 (9) 0.08 14 1.0 (38) 1.1 (6) 1.0 Others 0.1 (5) 0 (0) 0.6 Nonvaccine type 6.7 (251) 5.4 (44) 0.21 15B 0.7 (28) 1.5 (12) 0.06 6A 0.7 (25) 0.2 (2) 0.21 19A 0.2 (9) 0.5 (4) 0.27 Others 5.1 (189) 3.2 (26) 0.03
During the study period, the evolution of vaccine-type sero-types since vaccination does not appear as substantially reduc-tion compared with the evolureduc-tion of PCV7 vaccinareduc-tion rate: 6% in 2005, then 10%, then back to 5–6% in 2009–2010. The results may be due to low PCV7 vaccination rate. However, an increase in the colonization rates with serotypes 15B (initial) and 19 A (later) was observed. Currently, there are another 2 vaccines available in the market: PCV10 and PCV13. In the study, the rates of serotypes covered by PCV7 and PCV10 were the same because serotypes 1, 5 and 7F were not found. Serotypes 1, 5 and 7F are not common in nasopharyngeal isolates, as well as invasive isolates in Taiwan.30
Serotypes 6A, 19A and 3 not covered by PCV7, but covered by PCV13 accounted for 4.3%, 1.9% and 1% of total pneumococcal isolates, respectively. Continued surveillance is warranted. Chil-dren with selected risk factors that could significantly benefit from vaccination of pneumococcal conjugate vaccine to decrease pneu-mococcal carriage should aggressively receive the new generation pneumococcal conjugate vaccine with broader serotype coverage to reduce pneumococcal spread and evolution.
REFERENCES
1. Hiller NL, Ahmed A, Powell E, et al. Generation of genic diversity among
Streptococcus pneumoniae strains via horizontal gene transfer during a
chronic polyclonal pediatric infection. PLoS Pathog. 2010;6:e1001108. 2. Malley R, Stack AM, Ferretti ML, et al. Anticapsular polysaccharide
anti-bodies and nasopharyngeal colonization with Streptococcus pneumoniae in infant rats. J Infect Dis. 1998;178:878–882.
3. McCool TL, Cate TR, Moy G, et al. The immune response to pneumococcal proteins during experimental human carriage. J Exp Med. 2002;195:359–365. 4. Millar EV, O’Brien KL, Bronsdon MA, et al. Anticapsular serum antibody
concentration and protection against pneumococcal colonization among children vaccinated with 7-valent pneumococcal conjugate vaccine. Clin
Infect Dis. 2007;44:1173–1179.
5. McCool TL, Weiser JN. Limited role of antibody in clearance of
Strep-tococcus pneumoniae in a murine model of colonization. Infect Immun.
2004;72:5807–5813.
6. Malley R, Trzcinski K, Srivastava A, et al. CD4+ T cells mediate antibody-independent acquired immunity to pneumococcal colonization. Proc Natl
Acad Sci U S A. 2005;102:4848–4853.
7. Lu YJ, Gross J, Bogaert D, et al. Interleukin-17A mediates acquired immu-nity to pneumococcal colonization. PLoS Pathog. 2008;4:e1000159. 8. Zhang Z, Clarke TB, Weiser JN. Cellular effectors mediating
Th17-dependent clearance of pneumococcal colonization in mice. J Clin Invest. 2009;119:1899–1909.
9. Dagan R, Givon-Lavi N, Zamir O, et al. Reduction of nasopharyngeal car-riage of Streptococcus pneumoniae after administration of a 9-valent pneu-mococcal conjugate vaccine to toddlers attending day care centers. J Infect
Dis. 2002;185:927–936.
10. O’Brien KL, Millar EV, Zell ER, et al. Effect of pneumococcal conju-gate vaccine on nasopharyngeal colonization among immunized and unimmunized children in a community-randomized trial. J Infect Dis. 2007;196:1211–1220.
11. Dagan R, Givon-Lavi N, Fraser D, et al. Serum serotype-specific pneumo-coccal anticapsular immunoglobulin g concentrations after immunization with a 9-valent conjugate pneumococcal vaccine correlate with nasopharyn-geal acquisition of pneumococcus. J Infect Dis. 2005;192:367–376.
12. Kuo CY, Hwang KP, Hsieh YC, et al. Nasopharyngeal carriage of
Strepto-coccus pneumoniae in Taiwan before and after the introduction of a
conju-gate vaccine. Vaccine. 2011;29:5171–5177.
13. Jin P, Kong F, Xiao M, et al. First report of putative Streptococcus
pneumo-niae serotype 6D among nasopharyngeal isolates from Fijian children. J Infect Dis. 2009;200:1375–1380.
14. Huang SS, Hinrichsen VL, Stevenson AE, et al. Continued impact of pneu-mococcal conjugate vaccine on carriage in young children. Pediatrics. 2009;124:e1–11.
15. Harabuchi Y, Faden H, Yamanaka N, et al. Nasopharyngeal colonization with nontypeable Haemophilus influenzae and recurrent otitis media. Ton-awanda/Williamsville Pediatrics. J Infect Dis. 1994;170:862–866. 16. Casey JR, Pichichero ME. Changes in frequency and pathogens causing
acute otitis media in 1995-2003. Pediatr Infect Dis J. 2004;23:824–828. 17. Block SL, Hedrick J, Harrison CJ, et al. Community-wide
vaccina-tion with the heptavalent pneumococcal conjugate significantly alters the microbiology of acute otitis media. Pediatr Infect Dis J. 2004;23: 829–833.
18. Stanley PJ, Wilson R, Greenstone MA, et al. Effect of cigarette smok-ing on nasal mucociliary clearance and ciliary beat frequency. Thorax. 1986;41:519–523.
19. El Ahmer OR, Essery SD, Saadi AT, et al. The effect of cigarette smoke on adherence of respiratory pathogens to buccal epithelial cells. FEMS
Immu-nol Med Microbiol. 1999;23:27–36.
20. Willemse BW, ten Hacken NH, Rutgers B, et al. Association of current smoking with airway inflammation in chronic obstructive pulmonary dis-ease and asymptomatic smokers. Respir Res. 2005;6:38.
21. Sung RY, Ling JM, Fung SM, et al. Carriage of Haemophilus influenzae and
Streptococcus pneumoniae in healthy Chinese and Vietnamese children in
Hong Kong. Acta Paediatr. 1995;84:1262–1267.
22. Greenberg D, Givon-Lavi N, Broides A, et al. The contribution of smoking and exposure to tobacco smoke to Streptococcus pneumoniae and Haemo-philus influenzae carriage in children and their mothers. Clin Infect Dis. 2006;42:897–903.
23. Cardozo DM, Nascimento-Carvalho CM, Andrade AL, et al. Prevalence and risk factors for nasopharyngeal carriage of Streptococcus pneumoniae among adolescents. J Med Microbiol. 2008;57(pt 2):185–189.
24. Chantry CJ, Howard CR, Auinger P. Full breastfeeding duration and asso-ciated decrease in respiratory tract infection in US children. Pediatrics. 2006;117:425–432.
25. Levine OS, Farley M, Harrison LH, et al. Risk factors for invasive pneumo-coccal disease in children: a population-based case-control study in North America. Pediatrics. 1999;103:E28.
26. Duffy LC, Faden H, Wasielewski R, et al. Exclusive breastfeeding protects against bacterial colonization and day care exposure to otitis media.
Pediat-rics. 1997;100:E7.
27. Kaleida PH, Nativio DG, Chao HP, et al. Prevalence of bacterial respiratory pathogens in the nasopharynx in breast-fed versus formula-fed infants. J
Clin Microbiol. 1993;31:2674–2678.
28. Labout JA, Duijts L, Arends LR, et al. Factors associated with pneumo-coccal carriage in healthy Dutch infants: the generation R study. J Pediatr. 2008;153:771–776.
29. Syrjänen RK, Kilpi TM, Kaijalainen TH, et al. Nasopharyngeal carriage of
Streptococcus pneumoniae in Finnish children younger than 2 years old. J Infect Dis. 2001;184:451–459.
30. Kim SH, Song JH, Chung DR, et al.; ANSORP Study Group. Changing trends in antimicrobial resistance and serotypes of Streptococcus
pneu-moniae isolates in Asian countries: an Asian Network for Surveillance of
Resistant Pathogens (ANSORP) study. Antimicrob Agents Chemother. 2012;56:1418–1426.