行政院國家科學委員會專題研究計畫成果報告
Cytokines 與癲癇機制之研究
The involvement of cytokines in the epileptogenesis or in neur opr otection?
計畫編號:NSC 90-2320-B-039-025
執行期限:90 年 8 月 1 日至 91 年 7 月 31 日
主持人:張芳嘉 中國醫藥學院神經部
共同主持人:蔡崇豪 中國醫藥學院神經部
一、中文摘要
Cytokines, 例如: interleukin-1 (IL-1)、 interleukin-6、tumor necrosis factor (TNF), 已經廣泛被認為除了免疫系統能分泌外, 亦存在於中樞神經系統以調節中樞功能。 先前的研究發現抑制 corticotropin-releasing hormone (CRH) 作 用 後 所 增 加 的 睡 眠 (slow wave sleep; SWS) 主要是經由增加中 樞內生性 cytokine --- IL-1 所產生的作用。 而 IL-1 在此的作用為神經調節物質, 已知 在中樞神 經系統 可能 借由 NF-κB, nitric oxide (NO) 等訊息傳遞改變睡眠。以往的 文獻顯示癲癇發作後,無論是在人類或是 動物,中樞內生性 cytokine 的 mRNAs 表現 在中樞或周邊有增加的現象,而此增加的 現象可能與癲癇相關機制有關。 但至今對 cytokine 所 扮 演 的 角 色 究 竟 是 neuroprotection 或是 epileptogenesis 還不明 確。 因此本計畫藉由觀察睡眠行為的改變 來進一步釐清癲癇所誘發 cytokines 的作 用。我 們 以 amygdaloid kindling 誘發 temporal lobe seizures 的 動 物 模 式 來 決 定 kindling 所 誘 發 的 癲癇是否改變腦內 cytokine mRNAs 的表現,以及 sleep-wake activity 的 改 變 。 我 們 的 結 果 發 現 amygdaloid kindling 誘 發 Racine's stage 5 seizure 的 老 鼠 在 腦 內 hippocampus 及 cortex 的 IL-1α, -1β, 及 TNF-α mRNAs 的 表 現 在 12h:12h light:dark cycle 中 的 dark period 的 第 一 及 第 二 小 時 均 有 增 加 的 現 象 。 非 快 速 動 眼 期 睡 眠 (non-REM sleep)的 量 在 dark period 有 明 顯 的 增 加 , 而 此 增 加 的 睡 眠 能 被 腦 室 投 與 IL-1 receptor antagonist (IL-1ra)所 阻 斷。睡 眠 的 增 加 可 防 止 外 在 傷 害 的 產 生 及 增 進 能 量 的 恢 復 , 可 視 為 中 樞 保 護 機 制 的 一 指 標 。 因 此 我 們 的 結 果 顯 示 癲 癇 所 誘 發 中 樞 cytokines 的 增 加 可 能 是 protective 的 作 用 。 關鍵詞:癲 癇、睡眠、cytokine、amygdaloid kindling Abstr act
It is widely accepted that cytokines, such as interleukin-1 (IL-1), interleukin-6 (IL-6), tumor necrosis factor (TNF), etc, produced not only by cells of the immune system but also by cells of the central nervous system to modulate central function. Our previous observation indicates that the enhancement of endogenous cytokine, IL-1 in four discrete brain structures, acting physiologically as a neuromodulator, mediates the increase of slow wave sleep in response to the blockade of central corticotropin-releasing hormone (CRH) actions (2). IL-1 alters sleep-wake activity via
signal transduction cascades of NF-κB and/or nitric oxide (NO) (9). This proposal focuses
on the actions of cytokines in the regulation/modulation of epileptic activity, since we are aware of literature indicating that seizure enhances cytokines expression in the central nervous system (7,8,13,14,15,16) or in
the peripheral (12), and that the enhancement
of endogenous cytokines may be involved in seizure-associated process. However, it still remains controversial on how and whether cytokines exhibit neuroprotective or epileptogenetic actions after inducing electroencephalographic (EEG) seizures.
Sleep was used as an indication to
Zeon PDF Driver Trial
www.zeon.com.tw
evaluate cytokine’s neuroprotective or epileptogenetic actions, and amygdaloid kindling was applied to mimic temporal lobe seizure. Kindling stimulation was given at the beginning of the dark onset everyday for 4 weeks until reach Racine’s stage 5 seizure scale. Our result indicates that the mRNA expression of IL-1α, -1β, and TNF-α in hippocampus and cortex are increased at hour 1 and hour 2 after the dark onset. Time spent in slow wave sleep (SWS) during the 12-h dark period increases and it could be blocked by ICV administration of IL-1 receptor antagonist (IL-1 ra) in a dose-dependent manner. These results suggest that the increased central cytokines induced after kindling contribute to the alteration of sleep activity, and the incremental SWS implies the neuroprotection of cytokines.
Keywor ds: epilepsy, sleep, cytokine,
amygdaloid kindling
二、緣由與目的
The term cytokine refers to a group of regulatory proteins that are produced by a large number of cell types in response to a variety of stimuli, and include substances such as the interleukins (IL), tumor necrosis factor (TNF), and interferons (IFN). Cytokines are key mediators of many of the physiological responses to infection or trauma, and these responses are collectively referred to as the acute phase response. The role of cytokines in the complex physiological changes of the acute phase response has been extensively reviewed elsewhere (5,6). The behavioral changes
associated with the acute phase response include altered vigilance. Cytokines however have been reconsidered as a neurotransmitter and/or neuromodulator, since evidence indicates that the gene coding for theses cytokines and their accessory proteins are expressed by neurons, in addition to glial cells, in normal brain [see review (17)]. In
addition, the cytokine receptors are present on all neural cell types in response to its signal. A great deal of evidence suggests that cytokines involves in the regulation of sleep
behavior via signal transduction cascade of NF-kB and nitric oxide (NO) (9). Blockade
of IL-1 action by IL-1 receptor antagonist (IL-1 ra) (10) or neutralized IL-1 by antibody
(2,11) reduces spontaneous sleep. This
indicates that cytokines likely modulate in the brain behavior of a normal organism. Evidence also suggests that cytokines may involve in synaptic plasticity, neural transmission, and calcium signal [see review
(17)]. Thus, these observations strongly
suggest that cytokines perform neural functions in normal brain, in addition to their predominant role as inflammatory mediators.
Some observations indicate that EEG-seizure induces an increase in cytokine expression. For example, focal application of kainic acid or bicuculline induces EEG-seizure and enhances IL-1β immunoreactivity (16). In addition, an
audiogenic seizure induced by noninvasive sensory stimuli on the DBA/2J audiogenic seizure-susceptible mouse strain increases IL-1α expression in hypothalamus (8).
Furthermore, evidence indicates that cytokine production increases in peripheral (12) or in
brain (15) in human subject. These
observations suggest a role of cytokines in an adaptive mechanisms associated with generalized seizure activity, with implications for neuroprotection, neural dysfunction, or vulnerability associated with epileptic activity. However, it remains controversial on whether the enhancement of central cytokines induced by EEG-seizure involves in neuroprotection or epileptogenesis. Evidence supports cytokines implicated in neuroprotection such as transforming growth factor (TGF)-beta, a cytokine implicated in metabotropic glutamate receptor 3-mediated neuroprotection, was upregulated during the first three weeks after status epilepticus throughout the hippocampus (1). However,
observation that intrahippocampally injection of human recombinant IL-1β 10 min before kinate enhances the time spent in seizure (16)
indicates cytokines may contribute to the epileptogenesis. Furthermore, IL-1β has been reported to inhibit post-synaptic NMDA receptors (4), but other suggests that the
neurotoxicity induced by TNF-α or IL-1β is mediated by NMDA receptors to increase
Zeon PDF Driver Trial
www.zeon.com.tw
nitric oxide (NO) production (3). Therefore,
this proposal is trying to further identify the action of central cytokines induced by amygdaloid kindling.
三、結果與討論
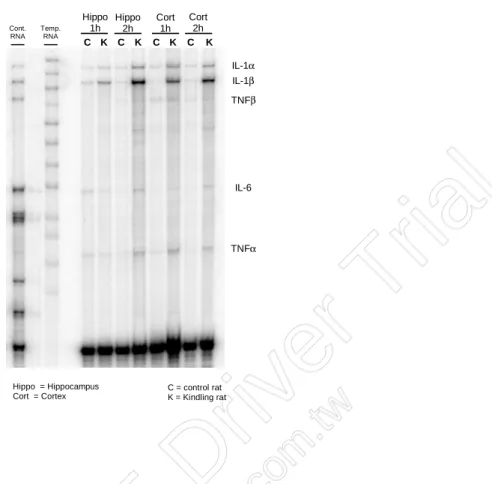
Racine’s stage 5 seizure appears after 3-to 4-week kindling stimulation given at 20-min before the dark onset of 12:12h light dark cycle everyday. Rats were sacrificed at 1 hour and 2 hours after the beginning of dark period, and series of cytokine mRNAs were detected by ribonuclease protection assay (RPA). Our result indicates increases of IL-1α, -β and TNF-α mRNAs in hippocampus and cortex at both hour 1 and hour 2 after dark onset (Figure 1). Expressions of IL-1α mRNAs (value represents optic density [O.D.] ± SEM) were increased from 0.78 ± 0.22 to 2.52 ± 0.4 (independent-samples T-test, p<0.05), and from 1.08 ± 0.17 to 2.95 ± 0.23 (independent-samples T-test, p<0.05) in hippocampus at hour 1 and hour 2, respectively. In cortex, IL-1α mRNA was increased from 1.02 ± 0.09 to 2.89 ± 0.32 (independent-samples T-test, p<0.05), and from 1.05 ± 0.12 to 2.95 ± 0.43 (independent-samples T-test, p<0.05) at hour 1 and 2, respectively. IL-1β mRNA was significantly altered in cortex at hour 1 and hour 2, OD increases from 2.05 ± 0.43 to 6.95 ± 2.52 (independent-samples T-test, p<0.05), and from 2.01 ± 0.21 to 7.97 ± 1.24 (independent-samples T-test, p<0.05), respectively. There was a strong tendency for an increase in IL-1β mRNA expression in hippocampus at hour 1 and 2. In contrast, IL-6 mRNA was variably detected in both brain areas. TNF-α mRNA expression was not consistently altered by kindling, although there was an increase tendency for TNF-α in hippocampus at hour 1 after kindling protocol (Table 1). These results suggest certain cytokines, such as IL-1α and -1β in the brain, especially in the areas of hippocampus and cortex, are altered during the dark period after kindling seizure developed.
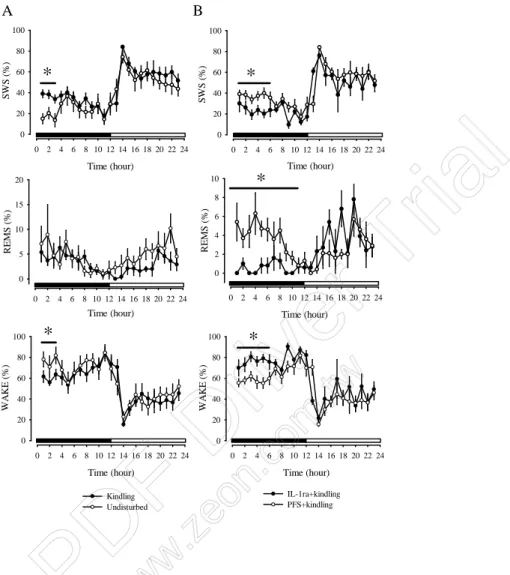
In addition, we found that SWS was increased during the 12-h dark period after
the kindling protocol; the time spent in SWS increases from 23.77 ± 2.18 % to 32.08 ± 1.60 % (ANOVA, p<0.05, Figure 2A) and the time spent in wakefulness decreases from 72.29 ± 2.48 % to 65.90 ± 1.87 % (ANOVA, p<0.05, Figure 2A). The total time spent in REM sleep was not altered by kindling protocol. ICV administration of IL-1 receptor antagonist (IL-1ra) dose-dependently blocks the sleep alteration induced by kindling (Figure 3). High dose (100 ng) of IL-1ra significantly reduces the total time spent in SWS from 32.08 ± 1.60 % to 21.59 ± 1.45 % (ANOVA, p<0.05, Figure 2B) and increases wakefulness from 65.90 ± 1.87 % to 77.86 ± 1.52 % (ANOVA, p<0.05, Figure 2B). REM sleep was also reduced by ICV IL-1ra. These results provide pharmacologically the involvement of increased cytokines, especially IL-1 in brain contributing to the kindling-induced sleep-wake alteration.
Sleep could be served as indication to evaluate the neuroprotective or epileptogenetic actions of cytokines induced after kindling protocol. It’s well known that SWS increases after infected by pathogen and the increase of SWS is essential for animal to recover from infectious state. The infection-induced increase of SWS could be mimicked by ICV administration of IL-1 and could be blockade by IL-1ra and/or suppressed by anti-IL-1β antibody. Increase of SWS avoids animals from the environmental harm and restores energy; in addition, the increase of IL-1 also elevates thermoregulatory set point and consequently increases body temperature and inhibits growth of pathogens. Although IL-1 action in peripheral primarily mediates inflammation, in the central nervous system IL-1 possesses a protective action during the recovery period after infection. Therefore, our results might suggest the protective action for IL-1 induced by kindling because of the contribution of IL-1 in SWS increase.
四、計畫成果自評
Our results suggest that amygdaloid kindling given at 20 min prior to the beginning of dark period induces increase of cytokines, such as IL-1α and –1β, at hour 1
Zeon PDF Driver Trial
www.zeon.com.tw
and hour 2 during the active period (dark period). The increase of IL-1 might contribute to the subsequent enhancement of SWS because of blockade of this incremental SWS by IL-1ra. Conclusively, present results provide the possibility of protective action of central cytokines induced after amygdaloid kindling protocol. However, a more detail of electrophysiological study needs to be conducted to further investigate the possible cellular mechanisms of central cytokines.
五、參考文獻
1. Aronica E., van Vliet EA, Mayboroda OA, Troost D., da Silva FH, Gorter JA, Upregulation of metabotropic glutamate receptor subtype mGluR3 and mGluR5 in reactive astrocytes in rat model of mesial temporal lobe. European J Neuroscience, 12: 2333-2344, 2000. 2. Chang FC, Opp, M, IL-1 is a mediator of
increases in slow-wave sleep induced by CRH receptor blockade. American J Physiology, 279: R793-R802, 2000 3. Chao CC, Hu S, Ehrlich L., Peterson PK,
Interleukin-1 and tumor necrosis factor-alpha synergistically mediate neurotoxicity: involvement of nitric oxide and of N-methyl-D-aspartate receptors. Barin, Behavior & Immunity, 9: 355-365, 1995.
4. Coogan A, O’Connor JJ, Inhibition of NMDA receptor-mediated synaptic transmission in rat dentate gyrus in vitro by IL-1 beta. Neuroreport, 8:2107-2110 5. Dinarello CA, Interleukin-1 and
interleukin-1 antagonism. Blood 77:1627-1652, 1991.
6. Dinarello CA, Biology of interleukin-1. FASEB J 2:108-115, 1988.
7. Donnelly S, Loscher C, Mills KH, Lynch MA, Glycerol-induced seizure: involvement of IL-1 beta and glutamate. Neuroreport 10:1821-1825, 1999.
8. Gahring LC, White HS, Skradski SL, Carlson NG, Rogers SW, Interleukin-1 alpha in the brain is induced by audiogenic seizure. Neurobiology of Disease 3:236-239, 1997.
9. Krueger JM, Obal JR, Fang JD, Kubota T, Taishi P, The role of cytokines in physiological sleep regulation. Annals of
the New York Academy of Sciences 933: 211-221, 2001.
10. Opp M, Krueger JM, Interleukin 1-receptor antagonist blocks interleukin 1-induced sleep and fever. American J Physiology 260:R453-R457, 1991.
11. Opp M, Krueger JM, Interleukin-1 antibodies reduce NREMS and attenuate NREMS rebound after sleep deprivation in the rabbit. Sleep Research 21:323, 1992.
12. Pacifici R, Paris L, Di Carlo S, Bacosi A, Pichini S, Zuccaro P, Cytokine production in blood mononuclear cells from epileptic patients. Epilepsa 36:384-387, 1995.
13. Peltola J, Nurme M, Miettinen A, Keranen T, Elevated levels of interleukin-6 may occur in cerebrospinal fluid from patients with recent epileptic seizure. Epilepsy Research 31:129-133, 1998.
14. Plata-Salaman CR, Ilyin SE, Turrin NP, Gayle D, Flynn MC, Romanovitch AE, Kelly ME, Bureau Y, Anisman H, McIntyre DC, Kindling modulates the IL-1 beta system, TNF-alpha, TGF-beta1, and neuropeptide mRNAs in specific brain regions. Brain Research. Molecular Brain Research 75:248-258, 2000.
15. Sheng JG, Boop FA, Mrak RE, Griffin WS, Increased neuronal beta-amyloid precusor protein expression in human temporal lobe epilepsy: association with interleukin-1 alpha immunoreactivity. J Neurochemistry 63:1872-1879, 1994 16. Vezzani A, Conti M, De Luigi A,
Ravizza T, Moneta D, Marchesi F, De Simoni MG, Interleukin-1 beta immunoreactivity and microglia are enhanced in the rat hippocampus by focal kainite application: functional evidence for enhancement of electrographic seizures. J Neuroscience 19:5054-5065, 1999.
17. Vitkovic L, Bockaert J, Jacque C, “Inflammatory” cytokines: neuromodulators in normal brain? J Neurochemistry 74:457-471, 2000.
Zeon PDF Driver Trial
www.zeon.com.tw
Figure 1. Alterations of cytokine mRNA expression during the dark period after amygdaloid kindling development.
C K C K C K C K Hippo 1h Cort 1h IL-1α IL-1β TNFβ IL-6 TNFα Hippo = Hippocampus Cort = Cortex C = control rat K = Kindling rat Cont. RNA Temp. RNA Hippo 2h Cort 2h
Table 1. Expression of cytokine mRNA in hippocampus and cortex during the dark period after amygdaloid kindling development.
Hippo 1 h Hippo 2 h Cort 1h Cort 2h
control kindling control kindling control kindling control kindling
IL-1α 0.78±0.22 (8/8) 2.52±0.40* (8/8) 1.08±0.17 (8/8) 2.95±0.23* (8/8) 1.02±0.09 (8/8) 2.89±0.32* (8/8) 1.05±0.12 (8/8) 2.95±0.43* (8/8) IL-1β 2.50±0.92 (8/8) 3.52±0.42 (8/8) 2.84±0.82 (8/8) 4.55±0.84 (8/8) 2.05±0.43 (7/7) 6.95±2.52* (7/7) 2.01±0.21 (7/7) 7.97±1.24* (7/7) IL-6 1.63±0.45 (3/8) 2.03±0.16 (3/8) 1.69 (1/8) 3.10±1.05 (4/8) Not detected Not detected Not detected 1.09 (1/8) TNF-α 1.60±0.60 (4/8) 3.01±0.71 (4/8) 2.01±0.70 (3/8) 3.10±0.95 (3/8) 3.25±0.42 (4/7) 4.07±0.80 (4/7) 3.02 (1/7) 4.03±0.51 (3/7)
Zeon PDF Driver Trial
www.zeon.com.tw
Zeon PDF Driver Trial
www.zeon.com.tw
Figure 2. The effects of IL-1 receptor antagonist (IL-1ra) on the alteration of sleep-wake activity induced by amygdaloid kindling.
Time (hour) 0 2 4 6 8 10 12 14 16 18 20 22 24 S W S (% ) 0 20 40 60 80 100 Time (hour) 0 2 4 6 8 10 12 14 16 18 20 22 24 S W S (% ) 0 20 40 60 80 100 Time (hour) 0 2 4 6 8 10 12 14 16 18 20 22 24 R E M S (% ) 0 5 10 15 20 Time (hour) 0 2 4 6 8 10 12 14 16 18 20 22 24 R E M S (% ) 0 2 4 6 8 10 IL-1ra+kindling PFS+kindling Time (hour) 0 2 4 6 8 10 12 14 16 18 20 22 24 W A KE (%) 0 20 40 60 80 100 Kindling Undisturbed Time (hour) 0 2 4 6 8 10 12 14 16 18 20 22 24 W A KE (%) 0 20 40 60 80 100 A B * * * * *
Figure 3. IL-1ra dose-dependently blocks Amygdaloid kindling-induced SWS enhancement during the dark period.
SW S (%) 0 10 20 30 40
*
# undisturbed kindling IL-1 ra 10 ng + kindling IL-1 ra 50 ng + kindling IL-1 ra 100 ng + kindlingZeon PDF Driver Trial
www.zeon.com.tw
Zeon PDF Driver Trial
www.zeon.com.tw
7