Cite this: RSC Advances, 2013, 3, 1514
Chemical vapor deposition of diamond on silicon
substrates coated with adamantane in glycol chemical
solutions
Received 11th October 2012, Accepted 20th November 2012 DOI: 10.1039/c2ra22486k www.rsc.org/advances
Yi-Chun Chen and Li Chang*
Diamonds were synthesized on adamantane-coated mirror-polished Si (100) substrates by microwave
plasma chemical vapor deposition (MPCVD) using a gas mixture of H2and CH4. Before MPCVD deposition,
Si substrates were dip-coated in chemical solutions of ethylene glycol and diethylene glycol with adamantane. With the coating of adamantane, the diamond nucleation can be enhanced with a density
over 1 6 108cm22and the deposited diamond films are shown to be of good crystallinity by scanning
electron microscopy and Raman spectrometry. The study demonstrates a simple and efficient way of diamond deposition on a smooth Si substrate.
Introduction
The synthesis of diamond has attracted great interest owing to its outstanding properties such as high hardness, wide band gap, chemical inertness, and high thermal conductivity.1–5 Microwave plasma chemical vapor deposition (MPCVD) is commonly used to grow high-quality diamond, and hydro-carbons of low molecular weight such as CH4, C2H2, and C2H4
are often used as carbon sources.6,7 However, diamond nucleation rarely occurs on smooth substrates without any pretreatment. Numerous pretreatment methods have been applied to enhance diamond nucleation.8,9 Although early
successful methods relied on mechanical scratching of the substrate to increase the nucleation density,10 more recent approaches involved with bias-enhanced nucleation method for diamond deposition to reduce the damage on the substrate surface.11–14 Previous studies had also used nanodiamond powder for ultrasonic seeding on substrates to grow diamond films. Although it can increase the nucleation density, it also scratches the substrate surface.15–17Adamantane (C10H16) can
be regarded as a small unit of nanodiamond covered with hydrogen. From the mechanical properties of adamantane, the softness of adamantane may not result in damage on the substrate surface. Recently we have used adamantane as precursor for diamond synthesis by coating a thick layer of adamantane on Si wafers.18However, it easily evaporates from
the substrate surface in vacuum and at high temperature.18To
reduce evaporation, a hexane solution with adamantane addition has been attempted for coating and/or pre-seeding on substrates of glasses and Pt on Si, which results in the
increase of the nucleation density.19 However, significant evaporation of adamantane may remain as a problem, which may result in non-uniform distribution of diamond deposi-tion. Also, the mechanism for diamond enhanced nucleation with adamantane is far from being understood. In this study, we demonstrate a novel dip coating method as pretreatment for enhancement of diamond nucleation on mirror-polished Si substrate by dissolution of adamantane in glycol-based chemical solutions which have higher viscosity than hexane to further reduce adamantane evaporation during diamond growth.
Experimental
The substrates used in the experiment were mirror-polished p-type (100) silicon wafers with dimensions of 1 cm 6 1 cm without any mechanical pretreatment. The substrates were ultrasonically cleaned with acetone for 15 min and dried with N2gas, followed by cleaning in 1% HF solution for 3 min to
remove the native oxide from the Si surface. Then, the substrates were dip-coated in a highly viscous glycol-based solution with adamantane powder (purity . 99%) at room temperature. Two glycol solvents were used. One was ethylene glycol (C2H6O2) and the other was diethylene glycol (C4H10O3).
The concentration of the solution was 1 g adamantane per 10 mL solvent. After mixing of adamantane with the solvent, the solution appeared became transparent. Adding a greater quantity of adamantane into the solution might not result in complete dissolution. The deposition of diamond on adaman-tane-coated Si was carried out in an ASTeX-type microwave plasma CVD reactor.31 The conventional CVD diamond deposition condition was adopted as following. The pressure
Department of Materials Science and Engineering, National Chiao Tung University, 1001 Tahsueh Road, Hsinchu, Taiwan 300, ROC. E-mail: lichang@cc.nctu.edu.tw; Fax: +886-3-5724727; Tel: +886-3-5731615
PAPER
Published on 22 November 2012. Downloaded by National Chiao Tung University on 28/04/2014 02:12:27.
View Article Online
substrate. We measured the thickness of the deposited adamantine by scale, it was approximately less than 1 mm. Then, MPCVD conditions for diamond growth on these glycol-only coated samples were the same as mentioned above. The surface morphology was examined in a field emission scanning electron microscope (SEM, JEOL JSM-6500F). The crystallinity of the diamond was evaluated with X-ray diffrac-tion (XRD) and Raman spectroscopy. XRD patterns were acquired from a Bruker D2 XRD system using Cu-Ka X-rays. Raman spectroscopy was performed on a LABRAM HR800 system, which allowed the 488 nm laser beam to be focused to 1 mm diameter for micro-mode operation.
Results and discussion
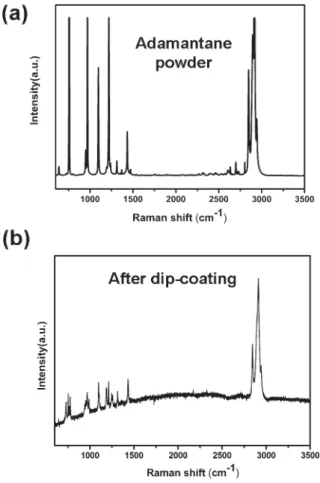
After dip coating of the silicon substrates in the solutions, Raman characterization was used to show that adamantane had been coated on Si. As shown in Fig. 1, the main characteristic peaks of adamantane in the Raman spectrum of the coated substrate are clearly observed in comparison with the spectrum obtained from pure adamantane powder. Compared with dry coating of adamantane on Si, adamantane evaporation on dip-coated substrates was found to be significantly reduced in air and MPCVD conditions. As a result, enhanced diamond nucleation on the substrate after MPCVD can be evidenced from SEM and Raman spectra as shown in Fig. 2 and 3. On the Si substrates dipped into both ethylene glycol and diethylene glycol solutions, the SEM images show that the deposited diamond crystallites are faceted with good crystallinity. The average size of well-faceted diamonds in ethylene glycol and diethylene glycol solutions are about 1.3–2.2 mm, and the growth rate is approximately 1–2 mm h21. However, the diamond density on the substrate dip-coated in diethylene glycol is 3.4 6 108 cm22and it is 1.4 6 108cm22on the substrate dip-coated in ethylene glycol, as shown in Fig. 2(a) and 2(c), respectively. The cause of the difference in density may be due to the sticking ability of the solvent with substrate on which adamantane evaporation may retard. It is well known that diethylene glycol has a higher viscosity than ethylene glycol.20 Therefore, it is likely that there exists larger amount of adamantane on Si which may act as an embryo for diamond nucleation during MPCVD.
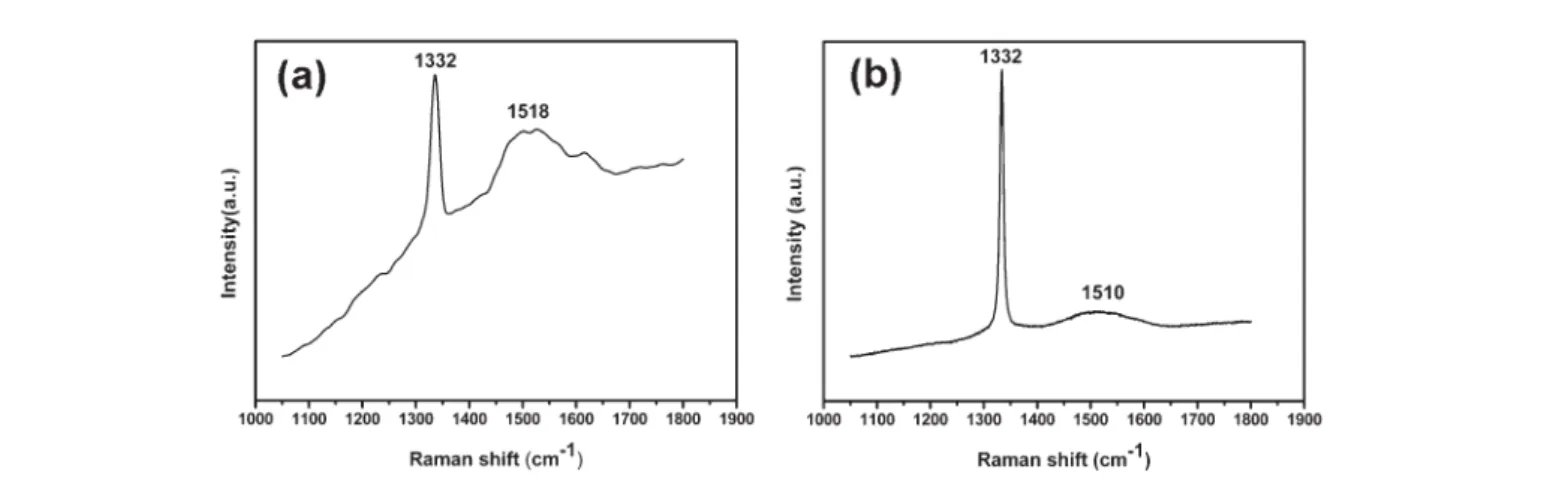
Raman spectra of the diamond films on Si are shown in Fig. 3 (a) and (b) for dip coating in ethylene glycol and diethylene glycol solutions, respectively. In both spectra, the diamond characteristic peak at 1332 cm21is sharp, while the G band at abouty1500–1580 cm21has a low intensity. The
full width at half-maximum of the diamond peak is 19.2 cm21 and 5.7 cm21, respectively, for ethylene and diethylene glycol cases, indicating that dip coating in diethylene glycol solution can obtain better quality of diamonds. The speculated reason for better quality diamond from diethylene glycol solution is that it contains more oxygen which is known to be able to improve the CVD diamond film quality by etching the sp2
carbon species.21–24Also, XRD patterns of the samples from
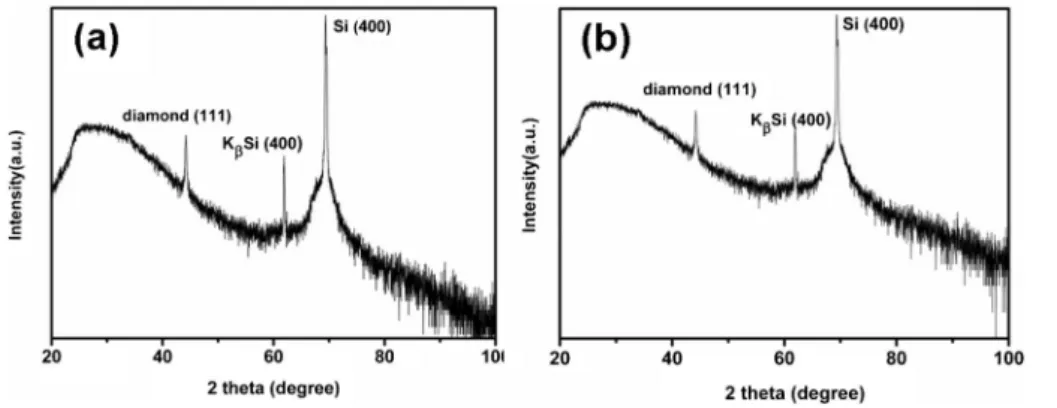
the ethylene glycol and diethylene glycol solutions show the diamond (111) peak at 2h = 43.9u in addition to Si peaks (Fig. 4), confirming that diamond has been deposited.
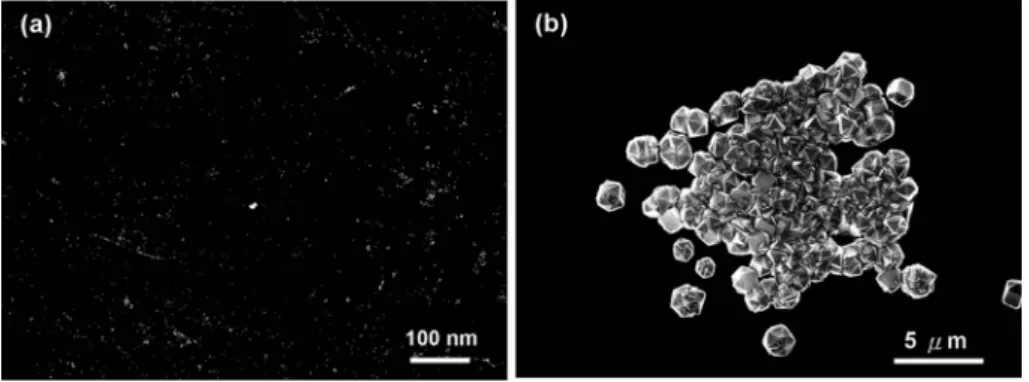
To distinguish the role of adamantane from glycol on diamond nucleation in synthesis, we have examined the surface morphology of the substrate dip-coated in the pure solvent without any addition of adamantane after diamond deposition in the same MPCVD conditions. Fig. 5 shows that the diamond density on the substrate is very low (y105cm22),
implying that the solvent itself plays a negligible role on diamond nucleation, and adamantane in solution is essential for diamond nucleation on Si. Also, we have evaluated the
Fig. 1 Raman spectra of (a) pure adamantane powder and (b) adamantane on Si substrate after dip-coating from the solution.
effect of the solvents on adamantane adhesion with Si by comparing with direct dry-coating of adamantane powder on 1 cm 6 1 cm Si substrate (y1 mm thickness) instead of using the solution method. As shown in Fig. 6, the diamond density on the dry-coated sample is 106cm22much less than on the dip-coated samples. Only on some local areas of 10–20 mm size a high density of diamond particles in continuous distribution may be observed in Fig. 6(b). Since adamantane has a high vapor pressure and a low melting point, it can easily evaporate even at room temperature. Also, vacuum pumping can remove a large amount of adamantane powders as they have weak adhesion with Si. As a result, it is difficult to obtain uniform distribution of adamantane on Si in the case of dry coating. To
increase the diamond density toy1 6 108 cm22, it may be
needed to use a 2 mm thick adamantane layer for dry coating as our previous report shows.25
According to previous work,18it is shown that diamond on Si can be synthesized using a hot-plate method to directly evaporate adamantane powder onto Si to form a thick layer for nucleation. However, the growth rate and the diamond density is less than those obtained in this work. It is because adamantane easily evaporates from the substrate surface at high temperature. Thus, less adamantane can be left on the substrate for nucleation. Also, compared with diamond deposition on Pt/SiO2/Si substrate using adamantane/hexane
solution shown in another previous work,19the uniformity and
Fig. 2 SEM of diamond on Si for the case of dipping in (a) ethylene glycol and adamantane solution and (c) diethylene glycol and adamantane solution. (b) and (d) high magnification of (a) and (c).
Fig. 3 Raman spectra of diamond on Si for the case of dipping in (a) adamantane in ethylene glycol and (b) adamantane in diethylene glycol.
the nucleation density of diamond are improved by using glycols, because the viscosity of hexane is less than glycol and the vapor pressure of hexane is higher than glycol as well. Thus, with glycol of higher viscosity, there might be more adamantane left on the substrate which can then act as nuclei for diamond growth. In this work, it is clear that a high viscosity solution is more effective on increasing the nuclea-tion density. Although the exact mechanism for the enhance-ment of diamond nucleation by dip coating has not been clearly understood yet, it is worthwhile to discuss the possible ways for diamond nucleation and growth from the seeding of adamantane by dip-coating on the substrate in solution. Because both ethylene glycol and diethylene glycol have no polarization, they are immiscible in water. Similarly, adaman-tane has high solubility in the glycol solutions as the adamantane molecule is covered with hydrogen atoms.26–28
Also, the OH-bond of the glycol can form relatively strong bonding with adamantane and Si.29,30 Besides, glycol and diethylene glycol have much higher surface tension,33hence, after evaporation of the solvent, adamantane may adhere with Si and act as an embryo or nucleus for diamond formation before it evaporates or transforms during deposition by hydrogen abstraction similar to the mechanism for diamond growth. When the CH4/H2plasma is applied to the substrate,
the remaining adamantane might be considered as a seed for
diamond nucleation.15 In addition to nucleation enhance-ment, another advantage is the improved uniformity of diamond nucleation by the solution method which may be due to the uniform distribution of adamantane on the substrate, in comparison with the bias-enhanced nucleation method which usually results in relatively non-uniform distribution due to the interaction of the electric field with the plasma.32Then, we may obtain a diamond film in a large area without complicated pretreatment. Also, the adhesion of adamantane with Si by glycol solution may help exploring the nucleation mechanism in future further investigations.
Conclusions
In summary, we demonstrate a simple and effective way to enhance diamond nucleation on mirror-polished Si by dip coating of Si substrate into a chemical solution of ethylene glycol and diethylene glycol with adamantane for diamond deposition. The results also show that adamantane is essential for diamond enhanced nucleation in CVD deposition in comparison with diamond deposition on Si treated with glycol without adamantane addition. Both the improved diamond quality and nucleation density can be obtained by dip coating the Si substrate into the adamantane/diethylene glycol solu-tion with higher viscosity and lower vapor pressure.
Fig. 4 XRD patterns of diamond grown on Si dip-coated with adamantane in (a) ethylene glycol and (b) diethylene glycol.
Fig. 5 SEM morphology of the substrate dip-coated from the solvent without mixing with adamantane in (a) low magnification and (b) high magnification.
Acknowledgements
The authors would like to thank the National Science Council of the Republic of China, Taiwan, for financially supporting this research under contract 98-2221-E-009-042-MY3.
References
1 S. M. Mominuzzaman, K. M. Krishna, T. Soga, T. Jimbo and M. Umeno, Jpn. J. Appl. Phys., 1999, 38, 658.
2 G. Dearnaley and J. H. Arps, Surf. Coat. Technol., 2005, 200, 2518.
3 J. K. Yan and L. Chang, Nanotechnology, 2006, 17, 5544. 4 M. S. You, F. C. N. Hong, Y. R. Jeng and S. M. Huang,
Diamond Relat. Mater., 2009, 18, 155.
5 H. G. Chen and L. Chang, Diamond Relat. Mater., 2009, 18, 141.
6 S. H. Wang, V. M. Swope, J. E. Butler, T. Feygelson and G. M. Swain, Diamond Relat. Mater., 2009, 18, 669.
7 Z. Yin, Z. Akkerman, B. X. Yang and F. W. Smith, Diamond Relat. Mater., 1997, 6, 153.
8 J. G. Buijnsters, L. Va´zquez, G. W. G. van Dreumel, J. J. Ter. Meulen, W. J. P. Van Enckevort and J. P. Celis, J. Appl. Phys., 2010, 108, 103514.
9 A. Fernandes, A. Neves, R. F. Silva and M. H. Nazar, Diamond Relat. Mater., 1997, 6, 769.
10 K. Mitsuda, Y. Kojima, T. Yoshida and K. Akashi, J. Mater. Sci., 1987, 22, 1557.
11 S. Yugo, T. Kanai, T. Kimura and T. Muto, Appl. Phys. Lett., 1991, 58, 1036.
12 X. Jiang and C. P. Klages, Diamond Relat. Mater., 1993, 2, 1112.
13 X. Jiang, C. P. Klages, R. Zachai, M. Hartweg and H. Fusser, Appl. Phys. Lett., 1993, 62, 3438.
14 B. R. Stoner and J. T. Glass, Appl. Phys. Lett., 1992, 60, 698.
15 V. Maria´n, I. Tibor, K. Alexander, V. Marian, Hrusˇka. Karel and M. Miroslav, Cent. Eur. J. Phys., 2012, 10, 218. 16 A. W. Oliver, D. Olivier, D. Michael, H. Ken, O¯. Eiji and
T. Makoto, Chem. Phys. Lett., 2007, 445, 255. 17 A. W. Oliver, Diamond Relat. Mater., 2011, 20, 621. 18 R. N. Tiwari, J. N. Tiwari and L. Chang, Chem. Eng. J., 2010,
158, 641.
19 R. N. Tiwari, J. N. Tiwari, L. Chang and M. Yoshimura, J. Phys. Chem. C, 2011, 115, 16063.
20 T. M. Aminabhavi and B. Gopalakrishna, J. Chem. Eng. Data, 1995, 40, 856.
21 C. J. Tang, A. J. Neves, S. Pereira, A. J. S. Fernandes, J. Gra´cio and M. C. Carmo, Diamond Relat. Mater., 2008, 17, 72.
22 C. J. Tang, A. J. Neves, A. J. S. Fernandes, J. Gra´cio and M. C. Carmo, J. Phys.: Condens. Matter, 2007, 19, 386236. 23 C. J. Tang, L. P. Gul, J. Gra´cio and J. L. Ribeiro, Phys. Status
Solidi A, 2009, 206, 2816.
24 Y. Michinori, T. Tokuyuki and I. Toshimichi, J. Cryst. Growth, 2005, 285, 130.
25 R. N. Tiwari and L. Chang, Appl. Phys. Express, 2010, 3, 045501.
26 J. L. West and J. A. Hubbell, Biomaterials, 1995, 16, 1153. 27 J. J. Sahlin and N. A. Peppas, J. Biomater. Sci., Polym. Ed.,
1997, 8, 421.
28 D. H. Kim, A. L. Klibanov and D. Needham, Langmuir, 2000, 16, 2808.
29 N. A. Alcantar, E. S. Aydil and J. N. Israelachvili, J. Biomed. Mater. Res., 2000, 51, 343.
30 J. A. Theil, D. V. Tsu, M. W. Watkins, S. S. Kim and G. Lucovsky, J. Vac. Sci. Technol., A, 2009, 8, 1374.
31 K. Kobashi, Diamond films : chemical vapor deposition for oriented and heteroepitaxial growth, 2005, ch3, 17–22. 32 D. Das and R. N. Singh, Int. Mater. Rev., 2007, 52, 29. 33 I. M. Smallwood, Handbook of organic solvent properties,
1996, 3, 105–109.
Fig. 6 SEM morphology of diamond deposits on the dry-coated substrate with adamantane (without dip-coating from the solvent) in (a) low magnification and (b) high magnification.