研究報告
台 灣 昆 蟲 2 1 : 4 7 - 6 4 ( 2 0 0 1 ) F o r m o s a n E n t o m o l . 2 1 : 4 7 - 6 4 ( 2 0 0 1 )Introduction
The family Tephritidae includes 4,257 species in 471 genera (Thompson, 1998). Approximately 1,500 species are probably
fruit associated, and an estimate of 600 species in which larvae probably mine the leaves, stems, or roots of their host plants (White and Elson-Harris, 1992). Many fruit flies in the subfamily Ceratitidinae are
Species Diversity and Seasonal Fluctuation of Fruit Flies (Diptera:
Tephritidae) in Bamboo Stands in Taipei
Horng-Yih Chang* Plant Protection Department, Bureau of Animal and Plant Health Inspection and Quarantine, Council of Agriculture, 9 Fl., 51 Chung
Ching S. Rd., Sec. 2, Taipei 100, Taiwan, R.O.C.
Tung-Ching Hsu and Wen-Jer Wu Department of Entomology, National Taiwan University, Taipei 106, Taiwan, R.O.C.
ABSTRACT
Fruit flies were regularly trapped by using four different-colored sticky traps in four bamboo cultivation areas in the vicinity of Taipei from January 1996 to May 1999. In total, 136,160 fruit flies of 62 species were captured. Among them, five species belonged to the Ceratitidinae, eight to the Phytalmiinae, 33 to the Trypetinae, 11 to the Dacinae, and five to the Tephritinae. Carpophthoracidia matsumotoi Shiraki was new to Taiwan. Seasonal population fluctuations of 27 fruit fly species were observed. Their composition among the bamboo stands varied from six predominant species and represented 89.97% to 96.87% of the individuals captured. Five preponderant species, i.e. Acrotaeniostola sexvittata Hendel, Acroceratitis plumosa Hendel, Gastrozona fasciventris (Macquart), Euphranta chrysopila Hendel, and Ptilona persimilis Hendel, were always associated with bamboo. Fly populations varied greatly among and within the study areas; however, their peak population densities coincided with the bamboo shoot production season, and the availability of bamboo shoots could be considered the most important environmental factor affecting population fluctuations of bamboo shoot fruit flies. The survey showed that yellow was the most attractive color to most of the fruit flies, followed in order by green, blue, and white. However, blue and white sticky traps captured most of the Euphranta species. Results of this study support colored traps can be used as a tool for monitoring non-frugivorus fruit flies, and their effectiveness can be enhanced if they are used in combinations of yellow or green traps with white or blue traps.
Key words: Tephritidae, bamboo, species diversity, seasonal fluctuation,
important pests of fruit crops, particularly in Africa. However, in most of the Asian Gastrozonini, a tribe of the Ceratitidinae, the host plants are grass, particularly bamboo (Hardy, 1988; Permkam, 1995; Hancock, 1999). Hancock and Drew (1999) reviewed the bamboo-shoot breeding Gastrozonini of Asia and reported that 86 species were recognized in 17 genera as follows: Acroceratitis, Acrotaeniostola, Carpophthorella, Chaetellipsis, Chelyophora, Cyrtostola, Dietheria, Enicoptera, Galbi-fascia, Gastrozona, Paragastrozona, Par-axarnuta, Phaeospila, Phaeospilodes, Spi-locosmia, Taeniostola, and Xanthorrachis. Several genera of Acanthonevrini are also associated with bamboo, namely, Acanth-onevra, Felderimyia, Pseudacrotoxa, Ptilona, Rioxa, Themara, and Tritaeniopteron (Hardy, 1988; Hancock and Drew, 1994; Permkam, 1995).
Totally 159 species of fruit flies belonging to five subfamilies have thus far been recorded in Taiwan (Shiraki, 1933, 1968; Munro, 1935; Chen, 1948; Hardy, 1977; Tseng and Chu, 1983; Tseng et al., 1992a, b, c; Norrbom, 1994; Han, 1996; Wang, 1996; Hancock and Drew, 1999; Sueyoshi, 1999). Among them, 15 species belong to the Ceratitidinae, ten to the Phytalmiinae, and 50 to the Trypetinae. In Taiwan, Acroceratitis plumosa Hendel and Gastrozona fasciventris (Macquart) (as Gastrozona macquarti Hendel) were the earliest species recorded as bamboo pests
(Issiki et al., 1928). Thereafter,
Acanthonevra formosana Enderlein,
Euphranta chrysopila Hendel, Taeniostola connecta Hendel, Ptilona confinis (Walker) (as Ptilona nigriventris Bezzi), and Ptilona persimilis Hendel were recorded as being associated with bamboo (Shiraki, 1933). Yen et al. (1979) reported nine fruit fly species under seven genera that were associated with bamboo, in which Acanthonevra speciosa (Hendel) and Acrotaeniostola sexvittata Hendel were recorded as bamboo feeders.
Bamboo is an important resource in
many Asian communities, particularly with the use of bamboo shoots as a vegetable. In Taiwan, 40 species, three varieties, and three cultivars of bamboo have so far been recorded, in which the following species are widely cultivated throughout the island: Bambusa dolichoclada Hayata, Bambusa olahami Munro, Bambusa stenostachya Hackel, Dendrocalamus latiflorus Munro,
Phyllostachys makinoi Hayata, and
Phyllostachys pubescens Mazel (Lin, 1978). All of these bamboo species were recorded as hosts of Acroceratitis plumosa and Gastrozona fasciventris (Shiraki, 1933). The importance of tephritid flies as pests of bamboo has been highlighted, and losses from these and other pests in Thailand are often severe, with damage reaching 100% (Hancock and Drew, 1999). However, because the larvae of Acroceratitis plumosa and Gastrozona fasciventris only feed on cut bamboo or bamboo shoots as a decomposer, they are not economically important and have not received much attention.
Beginning in 1996, we initiated a long-term field survey of seasonal fluctuations of fruit fly species in bamboo stands and adjacent areas in Taipei, Taiwan. We herein report the results of (1) the species composition of fruit flies in bamboo stands, (2) the seasonal abundance of fruit flies, and (3) the response of both male and female fruit flies to colored sticky traps. Since little is known about the ecology of bamboo shoot fruit flies, we review and compare our results with species of frugivorous fruit flies, such as Anastrepha and Bactrocera.
Materials and Methods
This study was conducted from January 1996 to May 1999 in and around four bamboo cultivation areas near Taipei City at elevations of 85 to 320 m. Three bamboo stands were selected from each of the three areas; Tachichiao, Changsansyh, and Sancherng, as well as two from Nankang. In these stands, planted bamboo
consisted of green bamboo, Bambusa oldhami; edible green bamboo, Bambusa edulis (Odashima); Makino’s bamboo, Phyllostachys makinoi; and some patches of long-shoot bamboo, Bambusa dolichoclada. The bamboo shoot harvest seasons in Taipei are as follows: green bamboo and edible green bamboo from May to October, with two production peaks one from mid-June to mid-July and the other one in late August; and Makino’s bamboo from April to May. Vegetation adjacent to those selected bamboo stands varied. In general, bamboo stands were surrounded by patches of native vegetation. Several fruit fly host plants, such as guava, Psidium guajava L., papaya, Carica papaya L., tankan, Citrus tankan Hayata, wen-tan pomelo, Citrus grandis (L.), grapefruit, Citrus paradisi Macfady, sponge gourd, Luffa aeptiaca Mill., bitter gourd, Luffa charantia L., angled luffa, Luffa acutangula (L.), and bottle gourd, Luffa siceraria (Mol.) were found along roadsides or in small patches in close proximity to the study stands.
Yellow, blue, green, and white sticky traps (adhesive paper, 21.5 ×21.5 cm) from the Kao-kung Co., Taiwan, were used in different phases of this survey. Yellow traps were used from January 1996, blue traps from May 1996, green traps from June 1996, and white traps from August 1996. All of the traps were rolled up, suspended vertically, and fixed by staplers on bamboo stems, or tree branches or trunks about 100-200 cm above the ground. About 20 traps of each color were used for each survey, depending on the size of the bamboo stand. Traps were distributed in bamboo stands and their bordering areas in patches. The distance between any two patches was more than 10 m. Traps were replaced every 2 weeks. The captured flies were counted in the laboratory. At each count, the number and the sex of captured fruit flies were recorded. After each count, some of the specimens were removed from the sticky paper and pinned for taxonomic use.
We measured tephritid species
diversity in each study area by computing the Simpson-Yule indices (Southwood, 1978). Trap captures were expressed as flies per trap every 2 weeks. Data were subjected to analysis of variance (ANOVA); mean differences of different color traps were tested by the least significant difference (LSD) test. The preferences of males and females for a specific color trap were tested by paired t-test. All statistical analyses were conducted using SAS (SAS Institute, 1990) with P < 0.05 as the significant criterion.
Results
Species diversity
From January 1996 to May 1999, a total of 136,160 flies was captured and classified according to the classification system of Thompson (1998), Korneyev (1999), and Hancock and Drew (1999). The captured flies were composed of 107,635 (79.1%) Ceratitidinae; 14,440 (10.6%) Dacinae, 7,599 (5.6%) Phytalmiinae, 6,426 (4.7%) Trypetinae, and 60 (< 0.01%) Tephritinae. The percentages of the preponderant species in decending order were 46.7% Acrotaeniostola sexvittata, 18.9% Acroceratitis plumosa, 13.3% Gastrozona fasciventris, 8.4% Bactrocera tau, 4.8% Ptilona persimilis, and 3.5% Euphranta chrysopila.
Totally of 62 species of fruit flies from 31 genera were recorded. Among them, five species belonged to the Ceratitidinae, 11 to the Dacinae, eight to the Phytalmiinae, 33 to the Trypetinae, and five to the Tephritinae (Table 1). The trypetines were in two tribes: Adramini (eight species), and Trypetini (25 species). In the survey, Carpophthoracidia matsumotoi was a new record for Taiwan.
Table 2 shows the proportion of the total catches of 27 fruit fly species in the four study areas from September 1996 to August 1998. Although the species composition varied among study areas, the total number of preponderant species of
Acrotaeniostola sexvittata, Acroceratitis
plumosa, Gastrozona fasciventris,
Bactrocera tau, Euphranta chrysopila, and Ptilona persimilis accounted for 96.87%, 96.07%, 93.54%, and 89.97% of all individuals captured in Tachichiao, Changsansyh, Sancherng, and Nankang, respectively. Moreover, Euphranta sexsignata was a more common species in Nankang. The pattern of species dominance represented a basic pattern of niche utilization. For example, five of the predominant species, except Bactrocera tau, are known as bamboo
shoot feeders. Interestingly, the proportion of Bactrocera tau also reached 12.79% in Sancherng. We believe this relative abundance was attributable to patches of their host plants, sponge gourd and bottle gourd, being planted in the vicinity of the study areas.
Simpson-Yule indeces for each study area are list in Table 2. The highest and lowest diversity was found in Nankang and Changsansyh, respectively.
Seasonal abundance of fruit flies
Table 1. Fruit fly species captured by colored traps from January 1996 to May 1999
C e r a t i t i d i n a e
G a s t r o z o n i n i
Acroceratitis plumose Hendel, Acrotaeniostola flavoscutellata Shiraki, Acrotaeniostola sexvittata Hendel, G a s t r o z o n a fasciventris Macquart, Spilocosmia p u n c t a t a ( Shiraki).
P h y t a l m i i n a e
A c a n t h o n e v r i n i
A c a n t h o n e v r a f o r m o s a n a Enderlein, Acanthonevra speciosa ( Hendel), Acanthonevra unicolor ( Shiraki), Phorelliosoma hexachaeta Hendel, Ptilona confinis (Walker), Ptilona persimilis Hendel, Tritaeniopteron excellens Hendel.
Blepharoneurini
H e x a p t i l o n a p a l p a t a Hendel
T r y p e t i n a e
A d a m i i n i
Coelotrypes sp., E u p h r a n t a apicalis Hendel, Euphranta chrysopila Hendel, Euphranta j u c u n d a Hendel, E u p h r a n t a lemniscata Enderlein, Euphranta sexsignata Hendel, E u p h r a n t a sp. A., E u p h r a n t a sp. B.
T r y p e t i n i
Acidiella f o m o s a n a ( Shiraki), Acidiella longipennis Hendel, Acidiella persimilis ( Hendel), Acidiella sonani
Shiraki, Acidiostigma sp., A n o m o i a a p p r o x i m a t a ( Hendel), A n o m o i a f o r m o s a n a (Shiraki),
Carpophthoracidia matsumotoi Shiraki*, Chaetostoma sp., Chenacidiella purpureisetae (Chen), Feshyia musaensis ( Shiraki), Fusciludia sp., H e m i l e a praestans Bezzi, H e m i l e a sp., M a c h a o m y i a c a u d a t a Hendel, Philophylla fossata ( Fabricius), Philophylla superflucta ( Enderlein), Philophylla sp. A, Philophylla sp. B, Trypeta luteonota Shiraki, Trypeta sp. A, Trypeta sp. B, Vidalia bidens Hendel, Vidalia sp.
D a c i n a e
D a c i n i
Bactrocera cilifer ( Hendel), Bactrocera cucurbitae ( Coquillett), Bactrocera d i a p h o r a ( Hendel), Bactrocera dorsalis ( Hendel), Bactrocera ferruginea ( Fabricius), Bactrocera parvula ( Hendel), Bactrocera s y n n e p h e s
(H e n d e l ) , Bactrocera scutellata ( Hendel), Bactrocera tau (Walker), Callentra f o r m o s a n a Tseng & Chu,
Callentra sp.
T e p h r i t i n a e
Dioxyna sororcula ( Wiedemann), E l a p h r o m y i a sp., Sphenella sinensis Schiner, Sphathulina a c r o l e u c a
Schiner, R h a b d o c h a e t a asteria Hendel, R h a b d o c h a e t a f o r m o s a n a S h i r a k i . * New record for Taiwan.
Among the 62 species of fruit flies trapped in this survey, the following 13 species were the major ones: Acroceratitis
sexvittata, Acroceratitis plumosa,
Gastrozona fasciventris, Bactrocera tau, Euphranta chrysopila, Ptilona persimilis, Euphranta sexsignata, Bactrocera dorsalis,
Ptilona confinis, Carpophthoracidia
matsumotoi, Bactrocera cucurbitae,
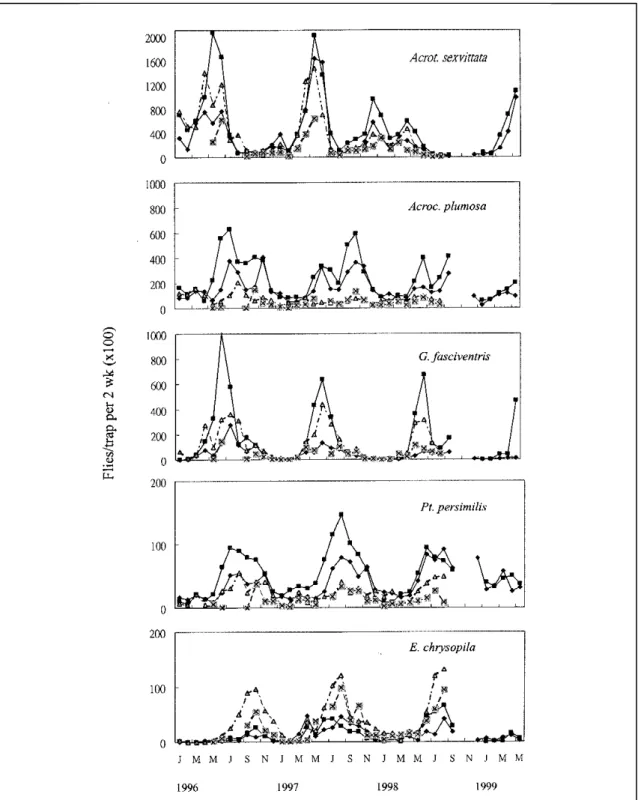
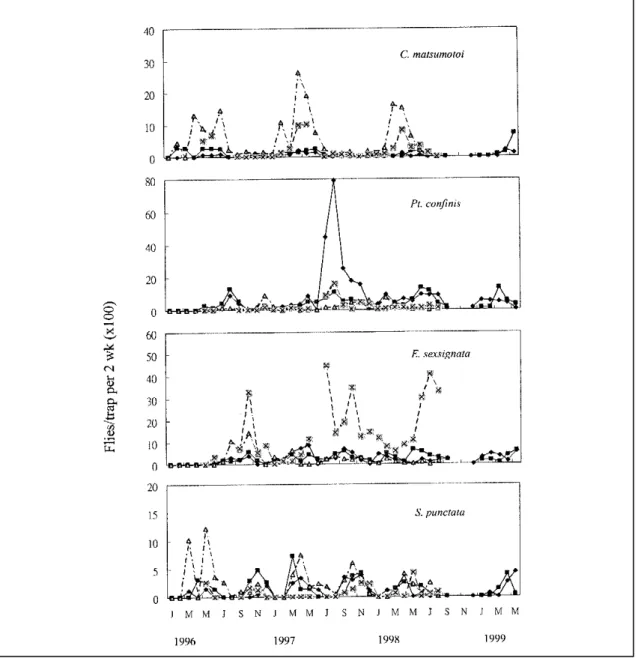
Bactrocera synnephes, and Spilocosmia punctata. Their seasonal fluctuations in the four areas during the period of 41 months of the study are shown in Figures 1-3. Fluctuations in the number of captured
fruit flies varied greatly among years, within stands, and among stands or areas. The duration during which the highest and lowest population levels were detected were almost the same in the four study areas. The mean catches per trap were 10.26, 7.09, 6.27, and 3.47 in Tachichiao, Sancherng, Changsansyh, and Nankang, respectively. The fruit fly population was significantly higher in Tachichiao, being almost three times higher than that in Nankang. The highest number of Acroceratitis sexvittata was recorded from April to June, and the lowest in February and from July to
Table 2. Fruit fly species diversity in four bamboo stands in Taipei from September 1996 to August 1998 Percent of total captured
F l y s p e c i e s Tachichiao S a n c h e r m g C h a n g s a n s y h N a n k a n g A c r o t a e n i o s t o l a s e x v i t t a t a 4 3 . 2 2 5 4 2 . 7 0 1 4 9 . 5 9 9 4 1 . 7 2 3 Acroceratitis p l u m o s a 2 4 . 6 8 1 2 3 . 6 5 9 1 0 . 7 0 2 1 4 . 0 3 5 Gastrozona fasciventris 1 3 . 9 1 8 5.375 1 9 . 4 5 7 1 3 . 1 9 2 Bactrocera tau 6.820 1 3 . 3 6 0 3.722 7.629 Euphranta chrysopila 2.200 2.453 8.366 9.572 Ptilona persimilis 6.036 5.996 4.219 3.825 Euphranta sexsignata 0.331 0.379 0.373 4.976 Bactrocera dorsalis 1.350 2.203 0.741 1.892 Ptilona confinis 0.478 1.600 0.429 0.637 Carpophthoracidia m a t s u m o t o i 0.057 0.054 0.902 0.679 Bactrocera cucurbitae 0.355 1.036 0.124 0.298 Bactrocera synnephes 0.191 0.421 0.361 0.442 Spilocosmia p u n c t a t a 0.158 0.189 0.321 0.247 Tritaeniopteron excellens 0.000 0.000 0.260 0.072 Philophylla f o s s a t a 0.055 0.064 0.056 0.144 Euphranta apicalis 0.000 0.175 0.068 0.031 Acidiella persimilis 0.019 0.046 0.012 0.062 Acanthonevra speciosa 0.036 0.075 0.088 0.031 Coelotrypes sp. 0.011 0.014 0.004 0.082 Bactrocera scutellata 0.030 0.071 0.024 0.031 Acanthonevra f o r m o s a n a 0.019 0.025 0.028 0.082 A n o m o i a f o r m o s a n a 0.000 0.004 0.056 0.021 Euphranta j u c u n d a 0.008 0.036 0.004 0.010 Bactrocera f e r r u g i n e a 0.008 0.029 0.004 0.010 Fusciludia sp. 0.003 0.014 0.032 0.000 Acanthonevra unicolor 0.011 0.007 0.000 0.000 Phorelliosoma hexachaeta 0.000 0.014 0.020 0.000 Total flies captured 3 7 , 5 1 7 2 6 , 8 0 9 2 2 , 0 1 8 1 1 , 1 3 8
November. However, its population peak in 1998 was relatively flat. Analysis of the annual fluctuations showed that the mean catch was significantly higher in 1996 and 1997 with an average of 4.52 and 4.46 flies per trap, respectively, but with an average of only 1.66 flies per trap in 1998 (Fig. 1). A pooled average across the four areas indicated that the highest capture was 14.4, Acroceratitis plumosa population reached its peak from July to November. Its population fluctu-ations were synchronized with the harvest season of bamboo shoots (Fig. 1). Its population density in 1996 averaged 1.73 flies per trap, which was higher than those for all other years, with the highest average number 3.68 flies per trap in July 1996.
A large number of Gastrozona
fasciventris were caught from April to August. However, from December to February, only a few individuals were captured. The annual population of Gastrozona fasciventris reached its peak in June from 1996 to 1998 (Fig. 1). The average population density was the highest in 1996, with an average of 1.31 flies per trap. The highest average number of this species was 4.0 flies per trap from June to July of 1996 and in July 1997, coinciding with the bamboo shoot production season. The maximum capture of Ptilona persimilis occurred from June to November (Fig. 1). The highest average number was 0.75 flies per trap in August 1997. Its annual population density averaged 0.3 flies per trap in 1996 and 0.4 flies per trap in 1998. The population of Euphranta chrysopila maintained a high level from June to November with the highest number at 0.83 flies per trap in August 1998 (Fig. 1). In contrast to the higher population density of the former four predominant species found in Tachichiao and Sancherng, Euphranta chrysopila population density was higher in Changsansyh and Nankang. Seasonal population fluctuations of Bactrocera in the four study areas are shown in Fig. 2. The highest number of
Bactrocera tau reached 10.58 flies per trap in Sancherng in January 1996. The average monthly population in the four areas peaked in January at 6.25 flies per trap. The annual population peaked from October to January 1997. We observed that this peak coincided with the sponge gourd production season in the Taipei area. It is interesting to note that, although the population density of Bactrocera cucurbitae was much lower than Bactrocera tau in this study, the seasonal fluctuations of both species were similar. The highest average number of Bactrocera cucurbitae was 0.57 flies per trap in January 1996. The population of Bactrocera dorsalis peaked from August to January, and very small numbers of this species were captured in the remaining months of the year. We found mature guava and citrus fruits available in the vicinity of bamboo stands from August to January. The highest numbers of Bactrocera synnephes caught were recorded from February to July; however, its population was at a low level from March to May in 1997. The highest average number of this fly was 0.14 flies per trap in June 1997 in Changsansyh.
F i g u r e 3 s h o w s t h e s e a s o n a l f l u c t u a t i o n s o f C a r p o p h t h o r a c i d i a matsumotoi, Ptilona confinis, Euphranta sexsignata, and Spilocosmia punctata in the study areas. The number of fruit flies trapped varied greatly with time and location in this study (Fig. 3). The highest numbers of Carpophthoracidia matsumotoi occurred from April to June. A total of 402 flies was captured, with most of them being trapped in Changsansyh. The highest average was 0.26 flies per trap in April 1997. Ptilona confinis peaked from July to November, with a total of 829 flies captured in the four study areas. The highest number was 0.79 flies per trap in August 1997 in Sancherng. Euphranta sexsignata was mostly trapped in Nankang, with a total of 873 flies captured in the four study areas. Its population peaked from July to October. 257 Spilocosmia punctata were caught in
Fig. 1 Seasonal fluctuatinons of five predominant fruit flies in four bamboo cultivation areas in Taipei from January 1996 to May 1999. : Sancherg; : Tachichiao; : Changsansyh;
Fig. 2 Seasonal fluctuatinons of Bactrocera fruit flies in four bamboo cultivation areas in Taipei from January 1996 to May 1999. : Sancherg; : Tachichiao; : Changsansyh; : Nankang.
the study areas, with the largest proportions captured from March to June and from October to December.
With respect to the remaining fruit flies, Table 3 presents the seasonal occurrences of 14 fruit flies per trap in May 1997. The Acroceratitis fly species captured in lesser numbers in the four study areas. In
Acanthonevrini, Acanthonevra formosana and Acanthonevra speciosa were trapped from April to May and the former had a peak from October to November. Only seven Acanthonevra unicolor individuals were captured from April to September. Phorelliosoma hexachaeta occurred only from December to the following April.
Fig. 3 Seasonal fluctuatinons of four predominant fruit flies in four bamboo cultivation areas in Taipei from January 1996 to May 1999. : Sancherg; : Tachichiao; : Changsansyh;
Tritaeniopteron excellens only occurred from April to August, and its peak was in May. In Dacini, Bactrocera ferruginea was mostly caught in August, and Bactrocera scutellata was trapped mainly from January to April and from July to September. Totally 116 Adramini fruit flies were captured in the study areas. Among them, the populations of Euphranta apicalis and Euphranta jucunda were higher from October to the following March, but the peak of Euphranta jucunda occurred in April. Coleotrypes sp. was caught mainly in January and June. In Trypetini, Acidiella persimilis and Anomoia formosana were trapped from November to April. A relatively high population of Philophylla fossata was maintained from August to the following March. Fusciludia sp. occurred only from November to March. If an average across all the study areas was calculated, a clear pattern showed that, on a regional basis, the population of the seven predominant species peaked from May to October. Among these species, the population of Acrotaeniostola sexvittata peaked in May, and then it was followed by
Gastrozona fasciventris in June, Ptilona persimilis and Euphranta sexsignata in July, Euphranta chrysopila and Ptilona confinis in August, and Acroceratitis plumosa in October. Except for Acroceratitis plumosa in which males peaked 1 month earlier than its females, the males and females of the remaining species reached peaks at the same time. However, females outnumbered males during the months of high density. In Acroceratitis plumosa, females outnum-bered males throghout the year.
Response of fruit flies to colored traps
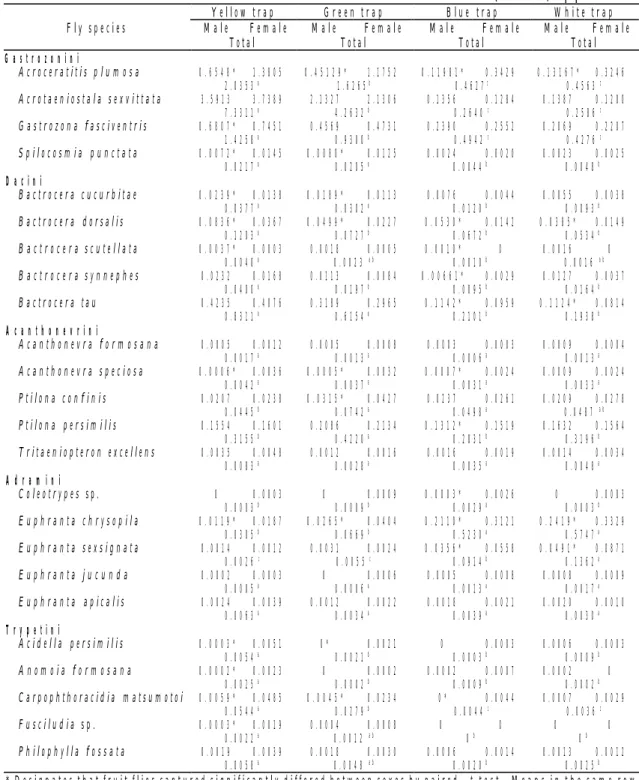
From 1997 to 1998, 89,841 fruit flies were caught in 13,649 traps, consisting of 3,629 yellow, 3,352 green, 3,382 blue, and 3,286 white traps. Among these fruit flies, 45.33% were male and 54.67% were female. The number of trapped fruit flies was strongly affected by trap color. The percentages of fruit flies caught by yellow, green, blue, and white color traps were 4 9 . 8 3 % , 3 0 . 8 4 % , 9 . 8 2 % a n d 9 . 5 0 % , respectively. Yellow traps captured
signi-Table 3. Monthly capture of 14 fruit fly species in four bamboo stands in Taipei 1996-1998.
Species Jan Feb Mar Apr M a y J u n e July A u g Sept Oct N o v Dec Total
A c a n t h o n e v r i n i Acanthonevra f o r m o s a n a 0 0 0 8 6 5 0 1 3 7 6 1 3 7 Acanthonevra speciosa 1 8 5 1 2 1 5 3 1 0 3 5 4 5 6 2 Acanthonevra unicolor 0 0 0 2 1 1 1 1 1 0 0 0 7 Phorelliosoma hexachaeta 8 0 0 1 0 0 0 0 0 0 0 5 1 4 Tritaeniopteron excellens 0 0 0 1 1 5 3 1 8 1 1 0 0 0 0 8 4 D a c i n i Bactrocera f e r r u g i n e a 1 2 0 1 0 0 2 1 5 1 1 3 1 2 7 Bactrocera scutellata 6 1 0 7 9 2 1 1 0 8 1 0 1 1 0 6 5 A d r a m i n i Coleotrypes sp. 5 1 2 0 2 3 0 1 0 0 2 2 1 8 Euphranta apicalis 1 0 8 1 0 1 2 4 2 0 1 1 2 1 4 1 9 8 3 Euphranta j u c u n d a 2 2 1 6 0 0 0 0 0 1 2 1 1 5 T r y p e t i n i A c i d i e l l a Persimilis 6 7 6 1 1 2 0 0 2 0 0 3 4 4 1 A n o m o i a F o r m o s a n a 5 2 5 3 0 1 0 0 0 0 0 1 1 7 Philophylla f o s s a t a 1 8 8 4 1 1 0 2 6 1 4 2 2 6 1 0 9 2 Fusciludia sp. 9 0 1 0 0 0 0 0 0 0 1 2 1 3
Table 4. Fruit flies captured by different-colored traps in four study areas 1997-1998
(Unit: flies/trap per 2 weeks) Yellow trap Green trap Blue trap White trap Male Female Male Female Male Female Male Female Fly species
Total Total Total Total
G a s t r o z o n i n i Acroceratitis p l u m o s a 0 . 6 5 4 8 * 1 . 3 8 0 5 0 . 4 5 1 2 9 * 1 . 1 7 5 2 0 . 1 1 9 8 1 * 0 . 3 4 2 9 0 . 1 3 1 6 7 * 0 . 3 2 4 6 2 . 0 3 5 3 a 1 . 6 2 6 5b 0 . 4 6 2 7c 0 . 4 5 6 3 c Acrotaeniostala sexvittata 3 . 5 9 1 3 3 . 7 3 8 9 2 . 1 3 2 7 2 . 1 3 0 6 0 . 1 3 5 6 0 . 1 2 8 4 0 . 1 3 8 7 0 . 1 2 0 0 7 . 3 3 1 1 a 4 . 2 6 3 2 b 0 . 2 6 4 0 c 0 . 2 5 8 6 c Gastrozona fasciventris 0 . 6 8 0 7 * 0 . 7 4 5 1 0 . 4 5 6 9 0 . 4 7 3 1 0 . 2 3 9 0 0 . 2 5 5 2 0 . 2 0 6 9 0 . 2 2 0 7 1 . 4 2 5 8 a 0 . 9 3 0 0 b 0 . 4 9 4 2 c 0 . 4 2 7 6 c Spilocosmia p u n c t a t a 0 . 0 0 7 2 * 0 . 0 1 4 5 0 . 0 0 8 0 * 0 . 0 1 2 5 0 . 0 0 2 4 0 . 0 0 2 0 0 . 0 0 2 3 0 . 0 0 2 5 0 . 0 2 1 7 a 0 . 0 2 0 5 a 0 . 0 0 4 4 b 0 . 0 0 4 8 b D a c i n i Bactrocera cucurbitae 0 . 0 2 3 9 * 0 . 0 1 3 8 0 . 0 1 8 9 * 0 . 0 1 1 3 0 . 0 0 7 6 0 . 0 0 4 4 0 . 0 0 5 5 0 . 0 0 3 8 0 . 0 3 7 7 a 0 . 0 3 0 2 a 0 . 0 1 2 0 b 0 . 0 0 9 3 b Bactrocera dorsalis 0 . 0 8 3 6 * 0 . 0 3 6 7 0 . 0 4 9 9 * 0 . 0 2 2 7 0 . 0 5 3 0 * 0 . 0 1 4 2 0 . 0 3 8 5 * 0 . 0 1 4 9 0 . 1 2 0 3 a 0 . 0 7 2 7 b 0 . 0 6 7 2 b 0 . 0 5 3 4 b B a c t r o c e r a scutellata 0 . 0 0 3 7 * 0 . 0 0 0 3 0 . 0 0 1 8 0 . 0 0 0 5 0 . 0 0 1 0 * 0 0 . 0 0 1 6 0 0 . 0 0 4 0 a 0 . 0 0 2 3 a b 0 . 0 0 1 0 b 0 . 0 0 1 6 a b B a c t r o c e r a synnephes 0 . 0 2 3 2 0 . 0 1 6 8 0 . 0 1 1 3 0 . 0 0 8 4 0 . 0 0 6 6 1 * 0 . 0 0 2 9 0 . 0 1 2 7 0 . 0 0 3 7 0 . 0 4 0 0 a 0 . 0 1 9 7 b 0 . 0 0 9 5 b 0 . 0 1 6 4 b Bactrocera tau 0 . 4 2 3 5 0 . 4 0 7 6 0 . 3 1 8 9 0 . 2 9 6 5 0 . 1 1 4 2 * 0 . 0 9 5 9 0 . 1 1 2 4 * 0 . 0 8 1 4 0 . 8 3 1 1 a 0 . 6 1 5 4 a 0 . 2 1 0 1 b 0 . 1 9 3 8 b A c a n t h o n e v r i n i Acanthonevra f o r m o s a n a 0 . 0 0 0 5 0 . 0 0 1 2 0 . 0 0 0 5 0 . 0 0 0 8 0 . 0 0 0 3 0 . 0 0 0 3 0 . 0 0 0 9 0 . 0 0 0 4 0 . 0 0 1 7 a 0 . 0 0 1 3 a 0 . 0 0 0 6 a 0 . 0 0 1 3 a Acanthonevra speciosa 0 . 0 0 0 6 * 0 . 0 0 3 6 0 . 0 0 0 5 * 0 . 0 0 3 2 0 . 0 0 0 7 * 0 . 0 0 2 4 0 . 0 0 0 9 0 . 0 0 2 4 0 . 0 0 4 2 a 0 . 0 0 3 7 a 0 . 0 0 3 1 a 0 . 0 0 3 3 a Ptilona confinis 0 . 0 2 0 7 0 . 0 2 3 8 0 . 0 3 1 5 * 0 . 0 4 2 7 0 . 0 2 3 7 0 . 0 2 6 1 0 . 0 2 0 9 0 . 0 2 7 8 0 . 0 4 4 5 b 0 . 0 7 4 2 a 0 . 0 4 9 8 a 0 . 0 4 8 7 a b Ptilona persimilis 0 . 1 5 5 4 0 . 1 6 0 1 0 . 2 0 8 6 0 . 2 1 3 4 0 . 1 3 1 2 * 0 . 1 5 1 9 0 . 1 6 3 2 0 . 1 5 6 4 0 . 3 1 5 5 b 0 . 4 2 2 0 a 0 . 2 8 3 1 b 0 . 3 1 9 6 b Tritaeniopteron excellens 0 . 0 0 3 5 0 . 0 0 4 8 0 . 0 0 1 2 0 . 0 0 1 6 0 . 0 0 1 6 0 . 0 0 1 9 0 . 0 0 1 4 0 . 0 0 3 4 0 . 0 0 8 3 a 0 . 0 0 2 8 a 0 . 0 0 3 5 a 0 . 0 0 4 8 a A d r a m i n i Coleotrypes sp. 0 0 . 0 0 0 3 0 0 . 0 0 0 9 0 . 0 0 0 3 * 0 . 0 0 2 6 0 0 . 0 0 0 3 0 . 0 0 0 3 b 0 . 0 0 0 9 b 0 . 0 0 2 9 a 0 . 0 0 0 3 b Euphranta chrysopila 0 . 0 1 1 9 * 0 . 0 1 8 7 0 . 0 2 6 5 * 0 . 0 4 0 4 0 . 2 1 1 0 * 0 . 3 1 2 1 0 . 2 4 1 9 * 0 . 3 3 2 9 0 . 0 3 0 5 b 0 . 0 6 6 9 b 0 . 5 2 3 0 a 0 . 5 7 4 7 a Euphranta sexsignata 0 . 0 0 1 4 0 . 0 0 1 2 0 . 0 0 3 1 0 . 0 0 2 4 0 . 0 3 5 6 * 0 . 0 5 5 8 0 . 0 4 9 1 * 0 . 0 8 7 1 0 . 0 0 2 6 c 0 . 0 0 5 5 c 0 . 0 9 1 4 b 0 . 1 3 6 2 a Euphranta j u c u n d a 0 . 0 0 0 2 0 . 0 0 0 3 0 0 . 0 0 0 6 0 . 0 0 0 5 0 . 0 0 0 8 0 . 0 0 0 8 0 . 0 0 0 9 0 . 0 0 0 5 a 0 . 0 0 0 6 a 0 . 0 0 1 3 a 0 . 0 0 1 7 a Euphranta apicalis 0 . 0 0 2 4 0 . 0 0 3 9 0 . 0 0 1 2 0 . 0 0 2 2 0 . 0 0 1 8 0 . 0 0 2 1 0 . 0 0 2 0 0 . 0 0 1 0 0 . 0 0 6 3 a 0 . 0 0 3 4 a 0 . 0 0 3 9 a 0 . 0 0 3 0 a T r y p e t i n i Acidella persimilis 0 . 0 0 0 3 * 0 . 0 0 5 1 0 * 0 . 0 0 2 1 0 0 . 0 0 0 3 0 . 0 0 0 6 0 . 0 0 0 3 0 . 0 0 5 4 a 0 . 0 0 2 1 b 0 . 0 0 0 3 b 0 . 0 0 0 9 b A n o m o i a f o r m o s a n a 0 . 0 0 0 2 * 0 . 0 0 2 3 0 0 . 0 0 0 2 0 . 0 0 0 2 0 . 0 0 0 7 0 . 0 0 0 2 0 0 . 0 0 2 5 a 0 . 0 0 0 2 b 0 . 0 0 0 9 b 0 . 0 0 0 2 b Carpophthoracidia matsumotoi 0 . 0 0 5 9 * 0 . 0 4 8 5 0 . 0 0 4 5 * 0 . 0 2 3 4 0 * 0 . 0 0 4 4 0 . 0 0 0 7 0 . 0 0 2 9 0 . 0 5 4 4 a 0 . 0 2 7 9 b 0 . 0 0 4 4 c 0 . 0 0 3 6 c Fusciludia sp. 0 . 0 0 0 3 * 0 . 0 0 1 9 0 . 0 0 0 4 0 . 0 0 0 8 0 0 0 0 0 . 0 0 2 2 a 0 . 0 0 1 2 a b 0 b 0 b Philophylla f o s s a t a 0 . 0 0 1 9 0 . 0 0 3 9 0 . 0 0 1 8 0 . 0 0 3 0 0 . 0 0 0 6 0 . 0 0 1 4 0 . 0 0 1 3 0 . 0 0 1 2 0 . 0 0 5 8 a 0 . 0 0 4 8 a b 0 . 0 0 2 0 b 0 . 0 0 2 5 b
* Designates that fruit flies captured significantly differed between sexes by paired t-test. Means in the same row followed by the same letter do not significantly differ from each other at the 5% level by LSD.
ficantly more Gastrozonini, Dacini, and most of the Trypetini fruit flies than did any of the other colored traps. This was followed in order by green, blue, and white traps (Table 4).
In Gastrozonini, most of Acroceratitis plumosa, Acrotaeniostola sexvittata, and Gastrozona fasciventris were attracted to yellow traps. However, yellow traps and green traps were equally attractive to Spilocosmia puctata (Table 4). There was no significant difference in the color preference between males and females; however, significantly more females of
Acroceratitis plumosa, Gastrozona
fasciventris, and Spilocosmia puctata were caught than males. The numbers of flies captured by blue and white traps were significantly less than those by yellow and green traps.
In Dacini, yellow traps caught signi-ficantly more Bactrocera dorsalis and Bactrocera synnephes than did other colored traps (Table 4). Bactrocera cucurbitae, Bactrocera scutellata, and Bactrocera tau responded more favorably to yellow and green traps. However, about 23.6% of Bactrocera dorsalis males were trapped on blue traps, and 23.6% of Bactrocera synnephes males were trapped on white traps, indicating that more male flies were attracted to the objects with high light intensity such as blue and white traps. Significantly more males than females of Bactrocera cucurbitae, Bactrocera dorsalis, and Bactrocera scutellta were caught on yellow traps.
In Acanthonevrini, Acanthonevra
formosana, Acanthonevra speciosa, and Tritaeniopteron excellens were equally attracted by the four different-colored traps (Table 4). Although more Ptilona persimilis were caught on green traps, green, blue, and white traps were equally attractive to Ptilona confinis. More females than males of Ptilona confinis were caught on green traps. In Adramini, Coleotrypes sp. was most attracted to blue traps, Euphranta chrysopila to blue and white traps, and
Euphranta sexsignata to white traps. Moreover, significantly more females than males of these flies were trapped (Table 4). The numbers of these flies captured on yellow and green traps were significantly less than those on blue and white traps. In contrast, Euphranta apicals and Euphranta jucunda were equally attracted to the four different-colored traps.
In Trypetini, all five predominant trypetines were most attracted to yellow traps, followed by green, blue, and white traps (Table 4). More females than males of Acidiella persimilis, Anomoia formosana,
Carpophthoracidia matsumotoi, and
Fusciludia sp. were caught by yellow traps.
Discussion
Of the five subfamilies of Tephritidae caught by colored traps in bamboo cultivation areas in Taipei, Acrotaeniostola sexvittata, Acroceratitis plumosa, and Gastrozona fasciventris of Gastrozonini, Ptilona persimilis, Ptilona confinis, and Acanthonevra formosana of Acanthonevrini, and Euphranta chrysopila and Euphranta sexsignata of Adramini were the major species associated with bamboo (Table 2). Although 62 tephritid species were present in the study areas, six species accounted for more than 85% of all flies captured on traps (Table 2). Of these, Acrotaeniostola sexvittata, Acroceratitis plumosa, and Gostrozona fasciventris of Gastrozonini were by far the most preponderant species in bamboo stands in Taipei. This finding is consistent with studies conducted in Costa Rica, Brazil, and Mexico on Anastrepha, showing that a few fly species were captured in a consistently high proportion irrespective of the number of species present in the orchard (Aluja, 1994; Aluja et al., 1996).
Annal analysis of fly populations shows that the fluctuations of most species of the Gastrozonini and some of the Adramini, whose larvae are known as bamboo shoot feeders, coincided with bamboo shoot
harvest seasons (Figs. 1 and 3). Our study showed that population densities of the 13 preponderant species varied sharply from bamboo stands in the four bamboo cultivation areas (Figs. 1-3). We believe that this results from the differences in the habitats surrounding the bamboo stands and their management. In bamboo stands
surrounded by diverse eco- or
agroecosystems, fly populations were greater and more diverse. In addition, management practices in the bamboo stands also greatly influenced the number of captured flies. We observed that pruning in the bamboo stands, mainly from January to March, significantly affected trap catches, because such practice decreased the bamboo canopy for shelters or resting. However, populations of bamboo shoot flies did not distinctively decrease, because the decaying cut bamboo placed in the vicinity of the bamboo stands also provided food for the larval development of these flies.
Species diversity varied in the four study areas. According to the Simpson-Yule index, there was a 1.32-fold difference in species diversity between Nankang and Changsansyh; however, the indices in Tachichiao and Sancherng were very similar (Table 2). Aluja (1994) stated that the degree of species dominance of Anastrepha was influenced by the ecological background and elevational gradients. Although the vertical distribution of green bamboo and Makino’s bamboo were extend to 1,000 and 1,500 m above sea level, respectively, the elevation of the survey areas in our study ranged only from 85 to 180 m above sea level in Sancherng and Tachichiao, and 150 to 320 m above sea level in Changsansyh and Nankang. Our data suggest that the mean population density of fruit flies was higher in lower-elevation areas; however, the species diversity was higher in Nankang where the fruit fly population was the lowest.
The abundance of the host of larvae is one of the major factors regulating Bactrocera and Anastrepha populations
(Fletcher, 1987; Jiron and Hedstrom, 1991). Chen et al. (1996) reported that the Bactrocera dorsalis population density was highly correlated with the yield indicator of both guava and seasonal fruits with a 1- to 2-month time lag between them. In this study, we found that bamboo shoots may be a major factor regulating Gastrozonini populations. The odor of susceptible bamboo stems or shoots after cuting served as a specific stimulus to attract both males and females of Acroceratitis plumosa and Gastrozona fasciventris, but with a higher proportion of females. Therefore, a cut bamboo stem or shoot appears to play a role in host recognition in these flies. Hancock and Drew (1999) reported that all Asian Ceratitidine genera with species known to breed in bamboo shoots are attracted to cut bamboo shoots in the field. Since no species with a known non-bamboo host was attracted to cut bamboo, this habit appears to be a reliable indicator of host preference. The Gastrozonini fruit fly populations were lowest at the end of winter. This may be due to the slowdown of breeding or egg laying of some species, which would not mature in the absence of hosts. However, the presence of hosts in early spring appears to strongly induce females to start heavily laying eggs. Interestingly, despite the large number of fruit fly species captured on the traps, none of these species presents a potential threat to bamboo plants. We observed that during the period when large numbers of Acroceratitis plumosa and Gastrozona fasciventris were captured on traps, infestation of cut bamboo or bamboo shoots by these two species was high. However, there was no threat to bamboo shoots after harvest because bamboo shoots are regularly harvested in the early morning in Taiwan.
Although Bactrocera and trypetines were also found to be associated with bamboo stands, their seasanal fluctuation patterns were not synchronized with the phenology of bamboo shoot production. We also observed that none of them was
attracted to cut bamboo shoots in the field. Hwang et al. (1997) in a 3-year survey of Bactrocera dorsalis reported an annual decrease of the population during winter, with a population peak from June to September. On the contrary, the highest numbers of Bactrocera dorsalis were recorded from September to February in 1995 and from September to December in 1996 in Taipei. Since the population fluctuation patterns of Bactrocera dorsalis in this study were similar to those reported by Hwang et al. (1997), we conclude that Bactrocera dorsalis and other Bactrocera species move into bamboo stands from nearby orchards where they forage for honeydew, rest, or find shelter from the late autumn to winter.
Tephritidae has been grouped into three categories based on their trophic strategies (Zwolfer, 1983). Category I includes polyphagous fruit-infesting species, and category II includes specific fruit-infesting species. Nonfrugivorous tephri-tines belong to category III. A previous review of the biology of nonfrugivorous tephritid fruit flies indicated that the majority of nonfrugivorous species studied are aggregative and display a variety of mating strategies, including resource defense and paternal assurance strategies (Headrick and Goeden, 1998). Since this review targeted only Tephritinae fruit flies, most of these fruit flies are known to use vegetative parts of a host or flower head as larval food, and many of them form galls in and on these plant structures.
Our studies showed that the seasonal occurrence of bamboo shoot fruit flies varied widely. For example, the population of Acrotaeniostola sexvittata peaked from April to June, and that of Gastrozona fastriventris from April to August, while very few of them were caught during the rest of the year. Relatively high proportions of Acroceratitis plumosa, Ptilona persimilis, and Euphranta chrysopila were maintained in June and from July to November. We conclude that a combination of selection
pressures from interspecific interactions, such as a type of synchronization with the abundance or development of host plants, occurred in these nonfrugivorous tephritids in nature. Moreover, the trophic strategies of bamboo shoot fruit flies are much more diverse than those of other category III tephritines, which prefer non-bamboo hosts. Although 24 species of Trypetini were trapped in the survey, none of them is so far considered a bamboo feeder. Most of these rare fruit fly species were captured on traps placed at the periphery of bamboo stands or at the top layer of the foliage canopy. This implies that most flies captured in bamboo stands were migrating from neighboring vegetation into the bamboo stands. During the study, several of the fruit fly species,
such as Phorelliosoma hexachaeta,
Fusciludia sp., Acidiella longipennis, and Acidiella sonani, occurred only in winter. Some of these species could be found in higher mountain areas at elevations over 1,500 m. Therefore, we believed that these species migrated from mountain areas to the study areas for overwintering.
Many reports have shown that yellow is the most attractive color to adults of many genera of frugivorous fruit flies, such as Anastrepha, Bactrocera, Ceratitis, and Rhagoletis, which are serious pests of fruit crops in temperate and tropical areas (Economopoulos, 1989; Katsoyannos, 1989; Robacker and Moreno, 1990). Our data on
Bactrocera showed similar results.
Moreover, many genera of Gastrozonini and
Trypetini, such as Acrotaeniostola,
Acroceratitis, Gastorozona,
Carpoph-thoracidia, Acidiella, and Anomoia, were also most attracted to yellow traps. However, green and yellow traps captured significantly more Spilocosmia punctata, Philophylla, and Fusciludia (Table 4). This result shows that the color preferences of these species are similar to those of Anastrepha ludens as reported by Robacker and Moreno (1990). Therefore, we conclude that the color preferences of nonfrugivorous fruit flies are the same as those of
frugivorous fruit flies. There was no significant difference in the catches of three species of Ancanthonevrini and two species of Adramini fruit flies by four different-colored traps. Since the catch of each of these flies was less than 85 flies in the study, we consider that additional studies are needed to clarify their real color preference in nature.
Chen (1997) observed that the yellow trap caught more Bactrocera dorsalis males than females when fewer guava fruits on the trees in winter. Our data showed a similar result. Significantly more males than females of Bactrocera dorsalis, Bactrocera cucurbitae, Bactrocera synnephes, and Bactrocera scutellta were caught in bamboo stands (Table 4). Vargas et al. (1991) reported that the high number of Bactrocera dorsalis captured on white spheres might also be an indicator of the attractiveness of certain objects such as fruits and flowers to
Bactrocera dorsalis under natural
conditions. In our study, the majority of individuals of the species, Euphranta chrysopila, Euphranta sexsignata, and Coleotrypes sp. of Adramini, were attracted to blue or white traps, indicating that these flies were more attracted to objects with high light intensity. Further research on the behavior of Euphranta spp. is needed to clarify if the color preference for their oviposition is the same as that of Ceratitis capitata.
It is evident that frugivorus fruit flies are attracted to colors. Results of this study support the usefulness of colored traps as a monitoring tool for non-frugivorus fruit flies. The effectiveness of these devices can be enhanced if they are used in combinations of yellow or green color traps with white or blue traps.
Acknowledgements
We gratefully acknowledge Chiou -Nan Chen, and Shwu-Bin Horng, Department of Entomology, National Taiwan University, Po-Yung Lai, Institute of Tropical
Agriculture, National Pingtung Univ. of Science and Technology, and Yi-Hsiung Tseng, Kaohsiung Branch, Bureau of Standards, Meteorology and Inspection, Ministry of Economic Affairs for their valuable suggestions on the interpretation of data and comments on an early version of this manuscript. The authors also thank Kun-Yaw Ho, Department of Plant Protection, Chiayi Experimental Station, Taiwan Agriculture Research Institute, and Shi-Wui Lo, Wen-I Chou, and Ya-Chun Hsu, Department of Entomology, National Taiwan Univ. for their assistance in the survey and data analysis. This research was supported by funds from the Council of Agriculture and Bureau of Animal and Plant Health Inspection and Quarantine, COA in 1995-1999.
References
Aluja, M. 1994. Bionomics and management
of Anastrepha. Annu. Rev. Entomol. 39: 155-178.
Aluja, M., H. H. Celedonio, P. Liedo, M. Cabera, F. Castillo, J. Guillen, and E. Rios. 1996. Seasonal population
fluctuations and ecological implications for management of Anastrepha fruit flies (Diptera: Tephritidae) in commer-cial mango orchards in southern Mexico. J. Econ. Entomol. 89: 654-667.
Chen, C. N., E. Y. Cheng, Y. B. Hwang, C. H. Kao, and W. Y. Su. 1996. Relationship
between the population density of Oriental fruit fly, Bactrocera dorsalis (Hendel), and its host fruit yield in Taiwan. Plant Prot. Bull. (Taiwan, R.O.C.) 38: 149-166 (in Chinese).
Chen, S. H. 1948. Notes on Chinese
Trypetinae. Sinensia 18: 69-123.
Chen, Y. J. 1997. Assessment of the
trapping efficacy of the yellow sticky board to the oriental fruit fly. M. S. thesis. Taipei: National Taiwan University. 65 pp (in Chinese).
Economopoulos, A. P. 1989. Control: use of
315-327. In: A. S. Robinson, and G. Hopper, eds. Fruit Flies: Their Biology, Natural Enemies and Control. World Crop Pests, 3 (B). Elsevier, Amsterdam.
Fletcher, B. S. 1987. The biology of dacine
fruit flies. Annu. Rev. Entomol. 32: 115-144.
Han, H. Y. 1996. A new Cornutrypeta species
from Taiwan with notes on its phylogenetic relationships (Diptera: Tephritidae). Insect Koreana 13: 113-119.
Hancock, D. L. 1999. Grass-breeding fruit
flies and their allies of Africa and Asia (Diptera: Tephritidae: Ceratitidinae). J. Nat. Hist. 33: 911-948.
Hancock, D. L., and R. A. I. Drew. 1994.
New species and records of Asian
Trypetinae (Diptera: Tephritidae).
Raffles Bull. Zool. 42: 555-591.
Hancock, D. L., and R. A. I. Drew. 1999.
Bamboo-shoot fruit flies of Asia (Diptera: Tephritidae: Ceratitidinae). J. Nat. Hist. 33: 633-775.
Hardy, D. E. 1977. Family Tephritidae. pp.
44-134. In: M. D. Delfinado, and D. E. Hardy, eds. A Catalogue of Diptera of the Oriental Region, 3. Univ. of Hawaii, Honolulu.
Hardy, D. E. 1988. Fruit flies of the subtribe
Gastrozonina of Indonesia, New Guinea and the Bismarck and Solomon Islands (Diptera, Tephritidae, Try-petinae, Acanthonevrini). Zool. Scripta 17: 77-121.
Headrick, D. H., and R. D. Goeden. 1998.
The biology of nonfrugivorous tephritid fruit flies. Annu. Rev. Entomol. 43: 217-241.
Hwang, Y. B., C. H. Kao, and E. Y. Cheng.
1997. The monitoring and control of the Oriental fruit fly in Taiwan. Plant Prot. Bull. (Taiwan, R.O.C.) 39: 125-136 (in Chinese).
Issiki, S., J. Sonan, and R. Takahashi, 1928.
Studies on bamboo trypetids I. Bull. Dept. Agric. Res. Inst. Formosa 61: 1-16 (in Japanese).
Jiron, L. F., and I. Hedstrom. 1991.
Population fluctuations of economic
species of Anastrepha (Diptera:
Tephritidae) related to mango fruiting phenology in Costa Rica. Florida Entomol. 74: 98-105.
Katsoyannos, B. I. 1989. Behaviour:
response to shape, size and color. pp. 307-324. In: A. S. Robinson, and G. Hopper, eds. Fruit Flies: Their Biology, Natural Enemies and Control. World Crop Pests, 3 (A). Elsevier, Amsterdam.
Korneyev, V. A. 1999. Phylogenetic
relationships among higher groups of Tephritidae. pp. 73-113. In: M. Aluja, and A. L. Norrbom, eds. Fruit Flies (Tephritidae): Phylogeny and Evolution of Behavior. CRC Press, New York, Washington, D. C.
Lin, W. C. 1978. Subfamily 6. Bambusoideae.
pp. 706-783. In: H. L. Li, T. S. Liu, T. C. Huang, T. Koyama, and C. E. DeVol, eds. Flora of Taiwan. V. Angiospermae. Epoch Publ., Taipei.
Munro, H. K. 1935. Observation and
comment on the Trypetidae of Formosa. Arb. Phys. Entomol. Berlin-Dahlem 2: 195-271.
Norrbom, A. L. 1994. New genera of
Tephritidae (Diptera) from Brazil and Dominican amber, with phylogenetic analysis of the tribe Ortalotrypetini. Insecta Mundi 8: 1-15.
Permkam, S. 1995. Bamboo shoot fruit flies
in southern Thailand. Songklanakarin J. Sci. Technol. 17: 229-238.
Robacker, D. C., and D. S. Moreno. 1990.
Effects of trap color, height, and placement around trees on capture of Mexican fruit flies (Diptera: Teph-ritidae). J. Econ. Entomol. 83: 412-419.
SAS Institute. 1990. User’s Guide, ver. 6.
SAS Institute, Cary, NC.
Shiraki, T. 1933. A systematic study of
Trypetidae in the Japanese Empire. Mem. Fac. Sci. Agric. Taihoku Imp. Univ. 8. 509 pp.
Shiraki, T. 1968. Fruit flies of Ryukyu
Islands. U.S. Natl. Mus. Bull. 263: 1-104.
Southwood, T. R. E. 1978. Ecological
Methods, 2nd ed. Chapman & Hall, London. 524 pp.
Sueyoshi, M. 1999. Immature stages of
three Oriental species of the genus Rhabdochaeta de Meijere (Diptera: Tephritidae), with brief biological notes. Entomol. Sci. 2: 217-230.
Thompson, F. C. 1998. Fruit Fly Expert
System and Systematic Information Database. MYIA 9. Leiden, Nether-lands. 534 pp.
Tseng, Y. H., and Y. I. Chu. 1983. A new
fruit fly Callentra formosana from Taiwan (Tephritidae, Diptera). Chinese J. Entomol. 3: 119-122.
Tseng, Y. H., C. C. Chen, and Y. I. Chu.
1992a. The fruit flies, genus Dacus Fabricius of Taiwan (Diptera: Teph-ritidae). J. Taiwan Mus. 45: 15-91.
Tseng, Y. H., Y. I. Chu, and C. C. Chen.
1992b. A new genus Cervarita of fruit fly from Taiwan (Diptera: Tephritidae: Trypetinae). Chinese J. Entomol. 12: 17-20.
Tseng, Y. H., Y. I. Chu, and C. C. Chen.
1992c. A new genus Neomyoleja of fruit fly from Taiwan (Diptera: Tephritidae: Trypetinae). Plant Prot. Bull. (Taiwan, R.O.C.) 34: 171-174.
Vargas, R. I., J. D. Stark, R. J Prokopy, and T. A. Green. 1991. Response of Oriental
fruit fly (Diptera: Tephritidae) and associated parasitoids (Hymenoptera: Braconidae) to different color spheres. J. Econ. Entomol. 84: 1503-1507.
Wang, X. J. 1996. The fruit flies (Diptera:
Tephritidae) of the East Asian region. Acta Zootaxon. Sinica 21, Supplement. 338 pp.
White, I. M., and M. A. Elson-Harris. 1992.
Fruit Flies of Economic Significance: Their Identification and Bionomics. CAB International, Wallingford, UK. 601 pp.
Yen, D. F., Y. H. Tseng, and S. S. Wu. 1979.
Family Tephritidae of Taiwan (2). The Fruit Flies Found Associated withBamboo in Taiwan. Bureau of Com-modity Inspection and Quarantine, Tainan. 40 pp (in Chinese).
Zwolfer, H. 1983. Life systems and
strategies of resource exploitation in tephritids. pp. 16-30. In: E. Cavalloro, ed. Fruit Flies of Economic Importance. Proc. CEC/ IOBC Intl. Symp. Rotter-dam, Balkema.
Received Nov. 16, 2000 Accepted. Dec. 23, 2000