(51), re12. [DOI: 10.1126/scisignal.151re12]
1
Science Signaling
Ming-Daw Tsai (23 December 2008)
This information is current as of 11 June 2009.
The following resources related to this article are available online at http://stke.sciencemag.org.
Erratum
http://stke.sciencemag.org/cgi/reprint/sigtrans;2/58/er2 An erratum has been published for this article:
Article Tools
http://stke.sciencemag.org/cgi/content/full/sigtrans;1/51/re12
Visit the online version of this article to access the personalization and article tools:
Related Content
http://stke.sciencemag.org/cgi/content/abstract/sigtrans;1/25/ec230 http://stke.sciencemag.org/cgi/content/abstract/sigtrans;2/63/pc6
's sites: Science
The editors suggest related resources on
References
http://stke.sciencemag.org/cgi/content/full/sigtrans;1/51/re12#BIBL 1 article(s) hosted by HighWire Press; see:
cited by
This article has been
http://stke.sciencemag.org/cgi/content/full/sigtrans;1/51/re12#otherarticles This article cites 135 articles, 60 of which can be accessed for free:
Glossary
http://stke.sciencemag.org/glossary/
Look up definitions for abbreviations and terms found in this article:
Permissions
http://www.sciencemag.org/about/permissions.dtl Obtain information about reproducing this article:
(ISSN 1937-9145) is published weekly, except the last week in December, by the American Association Science Signaling
on June 11, 2009
stke.sciencemag.org
Introduction
The forkhead-associated (FHA) domain, a protein-phosphoprotein interaction motif with high specificity for phosphothreonine (pThr) residues, has been identified in more than 2000 proteins (from the Pfam database) in prokaryotes and eukaryotes since its dis-covery in forkhead family transcription fac-tors in 1995 (1). It is present in many regu-latory proteins, kinases, phosphatases, and transcription factors (1) (Fig. 1A). It is now clear that FHA domains play important roles in human diseases, particularly in rela-tion to DNA damage responses and cancers, and in biological processes such as cell growth, signal transduction, and cell cycle regulation (Fig. 2). Specific roles for FHA domains in checkpoint pathways have been explored with regard to various kinases in different species (Fig. 1B). Some examples of FHA domain–containing proteins include the human tumor suppressor checkpoint kinase 2 (Chk2), checkpoint with forkhead
and ring finger domains (CHFR) (2), TIFA [tumor necrosis factor receptor (TNFR)– associated factor (TRAF)–interacting pro-tein with a forkhead-associated domain] (3), and Nijmegen breakage syndrome 1 (NBS1, also known as nibrin) (4).
The FHA domain is unique among signal transduction domains in two aspects. First, it is the only presently known protein domain that specif ically recognizes pThr; other domains such as WW and 14-3-3 recognize both pThr and pSer residues. Second, the FHA domain shows very diverse ligand specificity; dif-ferent FHA domains recognize the pTXXD motif, the pTXXI/L motif, and TQ clusters (singly and multiply phospho-rylated) (5). The Ki67-FHA even recog-nizes an extended binding surface, but not short phosphopeptides. Here, we discuss FHA domains in terms of their structure, binding specificity, and biological func-tion, and we highlight multiple binding modes, possible alternative binding sites, and their importance in providing diver-sity to their functions in regulating mul-tiple signaling pathways. Although the FHA domain has been known for only a relatively short period of time, a great deal of information has already been acquired, particularly since it was last reviewed in 2002 by Durocher and Jackson (6) and in 2003 by Heierhorst and colleagues (7).
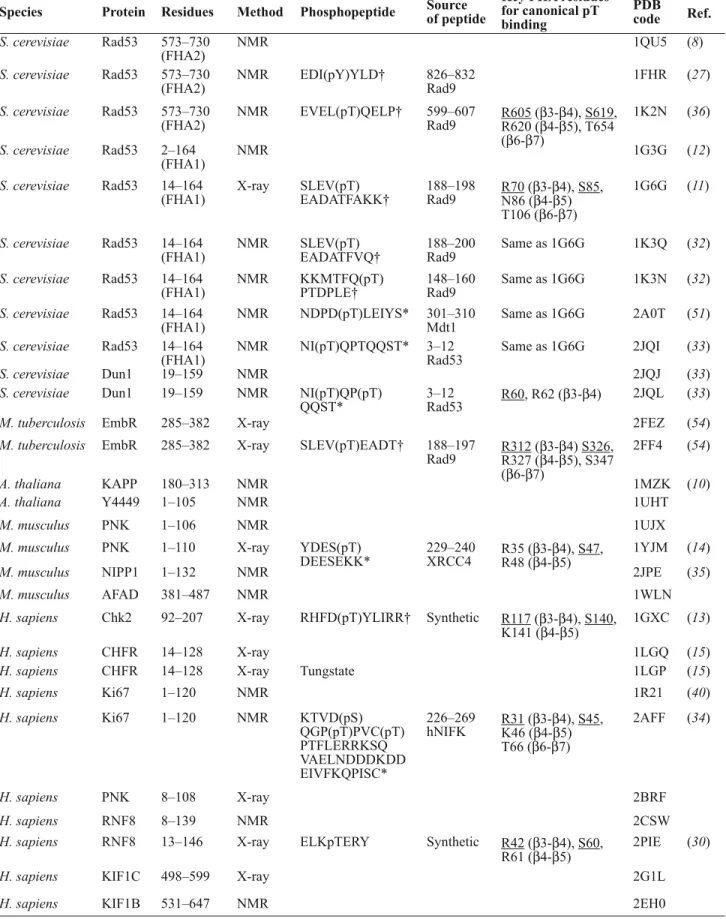
Structures of Free FHA Domains To date, the structures of 16 FHA domains have been determined either in their free forms, in complexes with ligand, or both, by nuclear magnetic resonance (NMR) and x-ray crystallography studies (http://www. rcsb.org) (Table 1). The first reported struc-ture is the C-terminal FHA domain of the protein Rad53 identified from radiosensitive mutants of budding yeast, Rad53-FHA2, which was solved by NMR (8) (Fig. 3). Con-sistent with proteolytic digestion studies (9), all structures clearly show that the FHA domain is considerably larger than the 55 to 75 amino acid residues—the core FHA domain homology region—predicted by sequence analyses (1). The minimal structure of an FHA domain encompasses the essential 11 β strands and spans approximately from 95 residues (in the case of Ki67-FHA) to 121 residues (in the case of Rad53-FHA2). Although some N-terminal or C-terminal flanking sequences are often needed to obtain soluble and stable proteins, an FHA domain construct is still generally under 150 residues, which makes it well suited for structural determination by solution NMR studies.
The FHA domain is typically character-ized by the following features (Fig. 3): (i) It is rich in β strands; there are 11 more or less well-defined β strands numbered from β1 to β11. (ii) It consists of two large β sheets. One sheet contains six antiparallel strands (β2, β1, β11, β10, β7, and β8); the other sheet con-tains five mixed β strands (β4, β3, β5, β6, and β9), of which β4 is the shortest and is parallel to β3. (iii) It contains a twisted β sandwich; this is the core architecture of the tertiary fold formed by burying hydrophobic side chains between the two large β sheets. (iv) It has structural modularity; the first and last β strands intimately interact with each other as part of one piece of the β sandwich, and the N terminus and C terminus meet at the end of the domain opposite to the pThr-binding site (Fig. 3). In conclusion, despite sharing low sequence homology, all known FHA domains adopt a strikingly similar fold, a structural fea-ture that could be attributed to the preserva-tion of hydrophobicity in the β strands (10). A structure-based sequence alignment of FHA domains is shown in Fig. 4A.
Major structural differences between dif-ferent FHA domains occur in the loops and turns that connect the β strands, which can either vary substantially in length, contain a small α-helical insertion, or both. This is exemplif ied by comparisons between Rad53-FHA1 and Rad53-FHA2, with the former having a short helical insertion
Structure and Function of the
Phosphothreonine-Specific FHA Domain
1Biophysics Program, Ohio State University,
Columbus, OH 43210, USA. 2Campus
Chem-ical Instrument Center, Ohio State University, Columbus, OH 43210, USA. 3Genomics
Research Center, Academia Sinica, Taipei 115, Taiwan. 4Institute of Biochemical
Sci-ence, National Taiwan University, Taipei 10617, Taiwan. 5Institute of Biological
Chem-istry, Academia Sinica, Taipei 115, Taiwan. *Corresponding author. E-mail: mdtsai@ gate.sinica.edu.tw
Anjali Mahajan,1Chunhua Yuan,2Hyun Lee,1,3Eric S.-W. Chen,3,4,5 Pei-Yu Wu,5Ming-Daw Tsai1,2,3,4,5*
Published 23 December 2008; Volume 1 Issue 51, re12; Revised 17 February 2009
The forkhead-associated (FHA) domain is the only known phosphoprotein-binding domain that specifically recognizes phosphothreonine (pThr) residues, distinguishing them from phosphoserine (pSer) residues. In contrast to its very strict specificity toward pThr, the FHA domain recognizes very diverse patterns in the residues surrounding the pThr residue. For example, the FHA domain of Ki67, a protein associated with cellular proliferation, binds to an extended target sur-face involving residues remote from the pThr, whereas the FHA domain of Dun1, a DNA damage–response kinase, specifically recognizes a doubly phosphoryl-ated Thr-Gln (TQ) cluster by virtue of its possessing two pThr-binding sites. The FHA domain exists in various proteins with diverse functions and is particularly prevalent among proteins involved in the DNA damage response. Despite a very short history, a number of unique structural and functional properties of the FHA domain have been uncovered. This review highlights the diversity of biological functions of the FHA domain–containing proteins and the structural bases for the novel binding specificities and multiple binding modes of FHA domains.
B I O C H E M I S T R Y
on June 11, 2009
stke.sciencemag.org
between the β2 and β3 strands (11, 12), whereas the latter has a much longer loop between the β5 and β6 strands (8). However, some structures determined so far show that the conserved residues located at the binding loops are positioned quite similarly in three-dimensional space. For example, despite a helical insertion in the loop between the β4 and β5 strands, the conserved Gly116, Arg117,
Ser140, and His143 residues of the FHA
domain of Chk2 can be spatially overlaid with the corresponding residues in Rad53-FHA1 (13). Moreover, regardless of the exact sequence, the conserved SXXH motif (S85NKH88 in FHA1) or its variant, such as
S47RNQ50in mouse polynucleotide kinase
(PNK)-FHA (14), forms a protruding U-turn shape preceding the β5 strand.
In the mitotic checkpoint protein CHFR, the FHA domain forms a segment-swapped dimer, in which a C-terminal segment of one molecule occupies the position of the cor responding segment of the other molecule (15). It remains to be established
whether this swapping has any biological implications, such as in protein oligomer-ization. However, human Chk2 and the Sac-charomyces cerevisiae kinase Rad53 can self-dimerize and oligomerize, and their FHA domains are indispensable for these phosphorylation-dependent events (16–18).
It is noteworthy that the structural topology of the FHA domain is similar to that of the MH2 (mothers against decapenta-plegic homolog 2) domain of the tumor sup-pressor Smad (11, 19). However, although they have an identical core β-sandwich, the MH2 domain contains several helical inser-tions and has a different topology of its loops compared with that of the FHA domain (8, 11). Huse et al. proposed that the functions of these domains may also be conserved (20). A type I transforming growth factor–β (TGF-β) receptor–derived phosphopeptide interacts with MH2 in a region that coincides with the phosphopeptide-interaction region of FHA domains (20).
Combinatorial Library Approach and Ligand Specificity
The combinatorial peptide library approach was introduced in the early 1990s to study the ligand specificity of the Src homology 2 (SH2) domain (21) and has since been s u c c e s s f u l l y applied to many p r o t e i n - p r o t e i n interaction domains, including the phos-photyrosine-binding (PTB) domain, WW, and 14-3-3 domains, which recognize short pThr-, pTyr-, or pSer-containing motifs (22). When the structure of the FHA domain was solved (8), its ligand specificity was not clear, despite some pioneering biological studies showing its phosphoprotein-binding role (23–26). Three types of com-binatorial libraries—pThr, pSer, and pTyr—with two or three residues on each side randomized (for example, XXXp-TXXX) were thus used to elucidate ligand specificity. Two laboratories, using
different screening and analytical methods, independently arrived at the conclusion that Rad53-FHA1 specifically recognizes pTXXD (11, 12). The specif icity of Rad53-FHA2 was less certain, and both pYXL (27) and pTXXL (11, 12) have been suggested as potential recognition motifs. Overall, the results of library screening and biological studies of several FHA domains led to the suggestion of the “pThr+3 rule” for FHA domains, with the pThr and the pThr+3 residues being the primary and secondary recognition sites, respectively, and the preferred +3 residues falling into two major categories: Asp and Ile or Leu (11, 28). There are some exceptions. For example, the FHA domain of kinase-asso-ciated protein phosphatase (KAPP) prefers Ser or Ala (11, 29), whereas the RING-finger protein 8 (RNF8)–FHA domain dis-plays a strong preference for Tyr and Phe at the pThr+3 position (30). The results from combinatorial library screenings are sum-marized in Table 2.
The structures of several complexes between FHA domains and phosphopep-tides obtained from library screening have been reported (Table 1). An example is the crystal structure of human Chk2-FHA in complex with the synthetic peptide “HFD(pT)YLI,” which contains Ile at the pThr+3 position (Fig. 5, A and B) (13). In a separate study, the pT68XXL motif from
Chk2 was also tested in vitro and shown, through NMR studies, to bind to Chk2-FHA with modest aff inity (Kd of ~12.3
μM) (31).
The pThr-binding region in an FHA domain typically includes residues in the loops and turns between the following pairs of β strands: β3-β4, β4-β5, β6-β7, and β10-β11 (Fig. 5A). Overall, the bound peptide typically exists in an extended conformation around the pThr residue, and its binding induces little global conforma-tional change in the FHA domain. The latter is also self-evident in two-dimen-sional 1H-15N heteronuclear
single-quantum coherence (HSQC) experiments, which show relatively small changes in chemical shifts in the FHA domain upon peptide binding (10, 27, 31–36), for
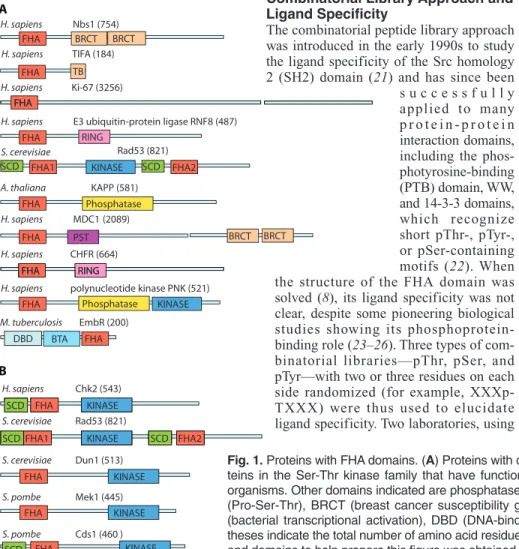
FHA BRCT H. sapiens Nbs1 (754) H. sapiens TIFA (184) BRCT H. sapiens Ki-67 (3256) FHA TB FHA FHA
H. sapiens E3 ubiquitin-protein ligase RNF8 (487) RING
FHA1 KINASE FHA2
S. cerevisiae Rad53 (821) KAPP (581) SCD SCD FHA Phosphatase A. thaliana H. sapiens MDC1 (2089) FHA FHA RING H. sapiens CHFR (664) BRCT BRCT PST FHA RING
H. sapiens polynucleotide kinase PNK (521)
FHA Phosphatase KINASE
FHA BTA DBD M. tuberculosis EmbR (200) A B FHA H. sapiens Chk2 (543) FHA KINASE SCD
FHA1 KINASE FHA2
S. cerevisiae Rad53 (821) SCD SCD S. cerevisiae Dun1 (513) FHA KINASE S. pombe Mek1 (445) S. pombe Cds1 (460 ) FHA KINASE FHA KINASE SCD
Fig. 1. Proteins with FHA domains. (A) Proteins with diverse functions in different organisms. (B)
Pro-teins in the Ser-Thr kinase family that have functions specific to checkpoint pathways in different organisms. Other domains indicated are phosphatase, RING (ring finger), SCD (SQ-TQ cluster), PST (Pro-Ser-Thr), BRCT (breast cancer susceptibility gene–1 C-terminal), TB (TRAF6 binding), BTA (bacterial transcriptional activation), DBD (DNA-binding), and kinase domains. Numbers in paren-theses indicate the total number of amino acid residues in each protein. [Information about sequences and domains to help prepare this figure was obtained from http://www.sanger.ac.uk/software/pfam/.]
on June 11, 2009
stke.sciencemag.org
example, Dun1 [DNA-damage un-inducible (Dun) mutant 1] (Fig. 5C). How-ever, NMR analyses also indicated that there may be important changes in back-bone dynamics, as evidenced by resonance broadening (34) and motion freezing (32, 33) of the residues at the binding site. A detailed dynamics study of the KAPP-FHA domain has revealed a net increase in backbone rigidity upon phosphopeptide binding (37).
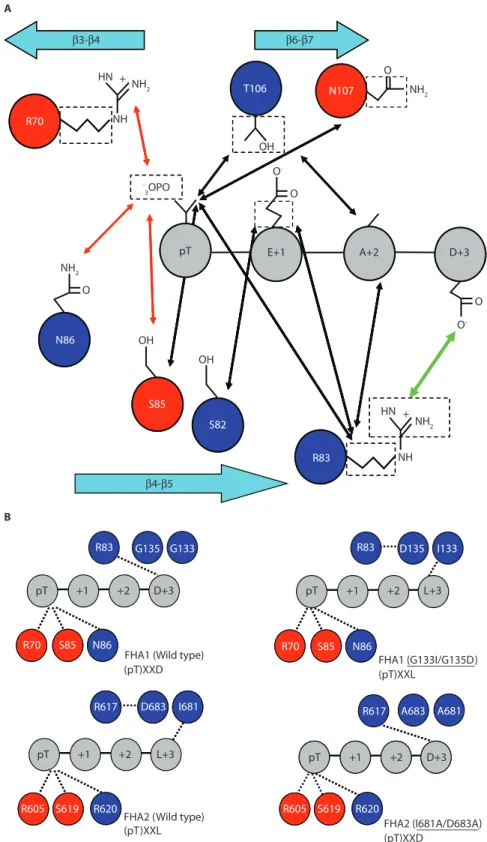
The mode of pThr recognition is highly conserved (Fig. 6A). To facilitate the comparison of key pThr-binding residues in different FHA domains, we have listed the residue number and the loop (for example, β3-β4 stands for the loop between the two strands) of these residues of some of the FHA domains in Table 1. The phosphate group is anchored through salt bridges, hydrogen bonds, or both, by conserved Arg (Arg70 in FHA1) and Ser
(Ser85 in FHA1) residues and
noncon-served residues, such as the one immedi-ately following the Ser; for example, Asn86 in FHA1, Lys46 in Ki67 (antigen
identified by monoclonal antibody Ki-67)
(38), Arg48 in mouse PNK, and Arg61 in
RNF8 (11, 14, 32, 34) (Fig. 4A). In NMR studies, the amide proton of this con-served Ser residue in six different FHA domains—from Rad53 (FHA1 and 2), Chk2, Ki67, Dun1 (39), and nuclear inhibitor of protein phosphatase 1 (NIPP1) (8, 12, 31, 40)—shows a charac-teristic downfield shift to around 11.5 to 12.5 parts per million upon binding of the pThr-peptide (Fig. 5C), in further support of microstr uctural similarity at the binding site. The methyl group of the pThr residue makes contacts with a number of FHA residues, for example, Ser82, Arg83, Thr106, and Asn107 in FHA1
(11, 32), which could explain how the FHA domain is prevented from binding to pSer residues.
The specif icity toward the pThr+3 residue is not conserved (Table 2). Whereas Rad53-FHA1 displays a clear preference for an Asp residue because of the pres-ence of Arg83in the β4-β5 loop,
Rad53-FHA2 preferentially binds to Ile or Leu, even though it
appears to have an Arg (Arg617) structurally
equivalent to that of Rad53-FHA1. As revealed in the FHA2 structure, the latter is engaged in an intramolecular salt bridge with Asp683, which makes its guanidino
g roup unavailable for inter molecular recognition (6, 27, 36). In light of this structural information, the binding speci-ficities of these two domains can be altered by rational site-directed mutagenesis (41), as we have illustrated (Fig. 6B).
Although the library approach and the structural determination of FHA domain complexes with short phosphopeptides derived from consensus motifs have pro-vided valuable information in early studies of FHA domains, these methods have had their shortcomings. First, the practical
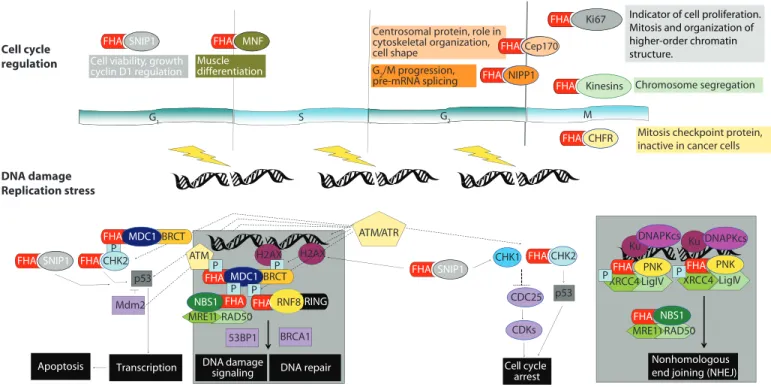
P LigIV PNK Cell cycle arrest Cell cycle regulation DNA damage Replication stress G1 S G2 M ATM/ATR CHK1 CHK2 CHK2 FHA FHA p53 Mdm2 Transcription Chfr FHA FHA MNF FHA FHA Chfr Chfr FHA CDC25 CDKs CHFR FHA Ki67 FHA Cep170 FHA NIPP1 FHA H2AX DNA repair Chromosome segregation MRE11 RAD50 NBS1 FHA H2AX P XRCC4 FHA Ku P Nonhomologous end joining (NHEJ) XRCC4 LigIV PNK FHA Ku P DNAPKcs DNAPKcs MRE11RAD50 NBS1 FHA Apoptosis
Mitosis checkpoint protein, inactive in cancer cells
MDC1 FHA
P
BRCA1 53BP1
Centrosomal protein, role in cytoskeletal organization, cell shape G2/M progression, pre-mRNA splicing Muscle differentiation G1 G2 FHA Kinesins FHA FHA SNIP1
Cell viability, growth cyclin D1 regulation FHA SNIP1 P BRCT RNF8RING FHA DNA damage signaling FHA SNIP1 MDC1 FHA BRCT p53 ATM P
Indicator of cell proliferation. Mitosis and organization of higher-order chromatin structure.
Fig. 2. Diagram of proposed functions of FHA domain–containing proteins. See main text for details.
Fig. 3. Basic structural features of the FHA domain. Stereoview of the FHA2 domain
of the S. cerevisiaekinase Rad53. The large β sheets are colored in green and blue, respectively, and the conserved residues highlighted in the sequence alignment are labeled. Note that in some studies the very short and parallel β4 strand was labeled as β3′ and the subsequent strands as β4-β10 instead of β5-β11.
on June 11, 2009
stke.sciencemag.org
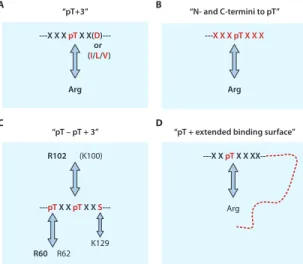
number of random sites is five, or six at most (for example, XXXpTXXX, where X is a randomized amino acid), which limits the ability to test the roles of residues remote from the pThr site. Second, the selection of random residues may be biased toward highly charged peptides because the electro-static term in the free energy calculations of protein-ligand recognition seems to con-tribute more to binding affinity (42, 43). In addition, a large number of hydrophobic peptides may not dissolve in the binding buffer, thus reducing the number of possible candidate peptides that can be screened (44). Thus, many laboratories have actively pur-sued functional studies to identify binding partners and binding sites, and a wealth of insightful information has been generated. Diversity of FHA-Ligand Interactions Based on Biological Approaches Biological studies from many laboratories showed that the ligand specificity of FHA domains is much more diverse than that suggested by the so-called “pThr+3 rule” generalized from library screenings. It was found that, in addition to the pThrXX(Asp or Ile or Leu) specificity described above, there are three additional types of recogni-tion specificities (Fig. 7).
FHA Domains with pThr+3 Specificities
FHA domains with specif icities for pTXXD or pTXX(I/L/V). The pTXXD or pTXX(I/L/V) specificities (Fig. 7A) identi-fied by library screening have been veri-f ied in a number overi-f biological studies (Table 2). For instance, S. cerevisiae Rad53-FHA1 and the Schizosaccha-romyces pombe checking DNA synthesis protein 1 (Cds1)–FHA domain both prefer a highly charged Asp residue at the pThr+3 position (11, 45, 46). On the other hand, FHA2 from S. cerevisiae Rad53 and FHA domains from human Chk2 and mediator of DNA-damage checkpoint–1 (MDC1) [also known as nuclear factor with an amino-terminal FHA domain and a tandem repeat of BRCT (breast cancer susceptibility gene–1 C-terminal) domains (NFBD1)] prefer a nonpolar residue at the pThr+3 position (Leu, Ile, or Val) (11, 18, 47, 48). The PP2C-type phosphatases Ptc2 and Ptc3 are required in DNA checkpoint inactivation because of their interaction with Rad53, but only as a result of double-strand breaks induced by HO endonucle-ases (49). The interaction of Ptc2 or Ptc3 with Rad53 is mediated by the FHA1
domain (7-163) through a phos-phorylation-dependent mecha-nism. Thr376of Ptc2 (within a
pTXXD motif) is the in vivo binding site for Rad53-FHA1 domain. Briefly, Ptc2 interacts with activated Rad53, dephos-phorylates it, and consequently leads to cell cycle resumption and recover y after DNA damage (45).
Single FHA domain with mul-tiple pThr+3 specificities. Bio-logical studies identified modi-f ier omodi-f damage tolerance–1 (Mdt1) as a binding partner of Rad53-FHA1, with Thr305as the
recognition site (50). However, the +3 residue from Thr305is a
hydrophobic Ile residue and not a charged Asp. How can the same FHA1 domain bind to two different phosphopeptides, one containing pTXXD and the other containing pTXXI? A detailed comparison of the binding inter-actions between FHA1 and the pTXXI peptide from Mdt1 with those of FHA1 and the pTXXD peptide from Rad9 (51) is shown in Fig. 8. The FHA1-pTXXI complex does indeed retain the features of FHA1-pTXXD in recognizing the pThr moiety, which is anchored by hydrogen bonding or salt bridges to Arg70,
Ser85, Asn86, and Thr106.
How-ever, although the Asp in the FHA1-pTXXD complex is involved in a strong charge-charge interaction with the Arg83
of FHA1, the Ile of the FHA1-pTXXI complex may contribute substantially to the binding aff inity by mediating hydro-phobic interactions with Gly135
and particularly Val136, which
are located in the β10-β11 loop. Furthermore, residues other than pThr and the +3 residue also provide fine-tuning of the binding interactions between the FHA domain and its target peptide (51). In summary, structural data indicate that an FHA domain is able to bind to pTXXD and to pTXX(I/L/V) peptides by adapt-ing to the different sequences of the phosphopeptide.
β1 β2
Dun1 30 EYTCLGHLVNLIPGKEQKVEITN...RN FHA1 29 GENIVCRVICTTG.QIPIRDLSADISQVLK..KK FHA2 573 GNGRFLTLKPLPDSIIQESLEIQQ...GVN Chk2 89 PTPAPWARLWALQDGFANLECVND... Cds1 31 NVFMKLVMTRMLDGKTEVIPLTTD...VHNG Ki67 1 MWPTRRLVTIKRSGVDGPHFPLSL...S RNF8 11 DRAGGRSWCLRRVGMSAGWLLLED...GC PNK 4 LGSRGRLWLQSPTGGPPPIFLPSDGQ... β3** * β4 β5 β8 β7 β6 * *
Dun1 55 VTTIGRSRSCDVIL....SEPDISTFHAEFHLLQM.. FHA1 65 VWTFGRNPACDYHLG...NISRLSNKHFQILLGE... FHA2 600 PFFIGRSEDCNCKI....EDNRLSRVHCFIFKKRHAV Chk2 112 NYWFGRDKSCEYCF.RTDKYRTYSKKHFRIFR... Cds1 59 FWRFGRHKSCEVVL....NGPRVSNFHFEIYQGH... Ki67 26 TCLFGRGIECDIRI....QLPVVSKQHCKIEIH.... RNF8 37 EVTVGRGFGVTYQL...PLMISRNHCVLKQNPEGQ
PNK 30 ALVLGRGPLTQVTD...RKCSRNQVELIADPES.
* * Dun1 86 ....DVDNFQRNLINVIDK.SRNGTFINGNRLVKK. FHA1 96 ...DGN...LLLNDI.STNGTWLNGQKVEKN. FHA2 633 GKSMYESPAQGLDDIWYCHTGTNVSYLNNNRMIQG. Chk2 149 ....EVGPKNSYIAYIEDH.SGNGTFVNTELVGKGK Cds1 89 ...RNDSDESENVVFLHDH.SSNGTFLNFERLAKNS Ki67 55 ...EQEAILHNFSSTNPTQVNGSVI.DEP RNF8 74 ...WTIMDNKSLNGVWLNRARLEPL. PNK 60 ...RTVAVKQL.GVNPSTVGVHELKPGL Dun1 116 DYILKN.GDRIVFGK...SCSFLFKYA.. FHA1 120 SNQLLSQGDEITVGVGVESD....ILSLVIFINDK FHA2 668 TKFLLQDGDEIKIIWDKNNK....FVIGFKVEIN. Chk2 180 RRPLNNNSEIALSLSRNKV...FVFFDLTVDD. Cds1 121 RTILS.NGDEIRIGL...GVPKDEISFLC Ki67 80 VRLKH.GDVITIID...RSFRYENE.. RNF8 96 RVYSIHQGDYIQLGVPLENKENAEYEYEVTEEDWE PNK 84 .SGSLSLGDVLYLVNGLYP...LTLRWEELS. SCD1 1 MENITQPTQQSTQATQ 16 SCD2 473 LGSQSYGDFSQISLSQSLSQ 490 Chk2 19 SQPHGSVTQSQGSSSQSQGI--SSQS--VSTQEL 71 Cds1 1 MEEPEEATQATQEAPLHVSQ 20 Rad9 390 TQIVNNPRTQE--DTQP--ETQIIVSCLSQGI--IKTQ 458 Sp Mrc1 572 SQK--NSQPSASQL--YSQP--STQPSQVDSLVDTQLD 648
649 STIPTQID 656
Sc Mrc1 90 TQ--FTQTQR--PTQL--SQA--GATQRIDSSGATSQTQ192 193 PIKSIEPQSQI--DTQM--DKTQAIGIPQATHQEQKTQ 273 MDC1 698 ATQCF--QSMEDEPTQAF--ATQPFCLRESEDSETQPF 768
10 13 8 13 16 23 6 16 27 9 30 22 10 10 37 10 A B α β9 β10 β11
Fig. 4. Sequence alignments. (A) FHA domains. The
residues colored in red highlight the 11 conserved β strands among FHA domains, while yellow highlights indicate conserved residues. Dun1, FHA1, and FHA2 are from S. cerevisiae, and Cds1 is from S. pombe. Chk2, RNF8, and Ki67 are human proteins. All FHA domain alignments are based on their structures except for that of Cds1-FHA, which is aligned on the basis of its sequence. (B) SCD domains. Proteins with
SCD domains that have been discussed in this review are presented. There is no evident homology among these proteins, but SQ and TQ residues are highlighted in red to illustrate their proximity to each other in a short stretch of protein. The numbers of amino acid residues omitted are shown between the SQ-TQ repeats. Sc, S. cerevisiae; Sp, S. pombe.
on June 11, 2009
stke.sciencemag.org
pThr+3 specificities identified from chemical versus biological approaches. An example of a discrepancy between chem-ical and biologchem-ical approaches was observed in Rad9, a suggested binding partner of Rad53-FHA1, which contains five possible ThrXXAsp motifs. Phospho-peptides corresponding to these five sites were synthesized and tested for their binding affinities for Rad53-FHA1. The peptide with the lowest Kd was
S188LEV(pT)EADATFVQ200 (0.36 μM),
which led to the suggestion that Thr192 of
Rad9 was the likely binding site and the deter mination of the str ucture of the Rad9:Rad53-FHA1 complex (Fig. 8A) (32). However, in a subsequent report, the most favorable binding site of Rad53-FHA1 to Rad9 in vivo was shown to be Thr390, which has a Val at the pThr+3
posi-tion (48). The discrepancy between the two approaches regarding the exact pThr site of Rad9 serves as one caution about the bio-logical relevance of the chemical library approach. However, there are many other cases where chemical and biological approaches identif ied the same ligand specificity (16, 18, 45, 49, 52, 53). FHA Domains That Recognize Residues Both N-Terminal and C-Terminal to pThr
At least two FHA domains interact sub-stantially with residues N-terminal to the pThr site (Fig. 7B). In murine PNK-FHA complexed with a peptide derived from its biological target XRCC4, only the residues N-ter minal to pThr, cradled between the β2-β3 and β4-β5 loops, are essential for binding (14). This unusual binding behavior may be due to several basic residues in the FHA domain inter-acting with the acidic residues N-terminal to the pThr of the target protein (Fig. 9A). Analysis of a complex containing the FHA domain of Mycobacterium tuberculosis EmbR and a low-aff inity phosphopeptide SLEV(pT)EADT (Kd =
185 μM) showed interactions similar to those observed in eukaryotic FHA-pep-tide complexes, with Asn348 interacting
with Glu and Asp at positions pThr+1 and pThr+3, respectively. In addition, a non-conserved Leu residue in this bacterial FHA domain interacts with a Leu at the pThr-3 position of the peptide (54) (Fig. 9B). However, the biological relevance of these interactions remains to be validated because the phosphopeptide used in the structure was from S. cerevisiae Rad9 and
not from the binding partner of EmbR in M. tuberculosis.
Dun1-FHA Contains Two pThr Sites SCDs (SQ-TQ cluster domains) are abun-dant in various proteins, and there are two such domains in the Rad53 kinase of S. cerevisiae, which precede each of the two FHA domains. The Rad53-SCD1 domain consists of four Thr-Gln (TQ) sites at posi-tions 5, 8, 12, and 15 (Fig. 4B) and is important for Rad53 dimerization and acti-vation (55, 56). Furthermore, Stern and co-workers demonstrated that the Dun1-FHA domain interacts with the phosphorylated SCD1 of Rad53, which leads to activation of Dun1 (56). It is an intriguing question how such a phosphorylation cluster orches-trates the sequential activation of two sepa-rate kinases in a temporally ordered manner. Our own study indicates that Rad53-FHA1 recognizes singly phospho-rylated SCD1, whereas Dun1-FHA specifi-cally binds to SCD1 with dual phosphory-lation at Thr5and Thr8(Fig. 9, C and D)
(33). The structure of the dual pThr com-plex indicates that the first pThr-binding site is formed mainly by the conserved Arg60residue and assisted by a
noncon-served Arg62located in the β3-β4 loop and
that the second pThr-binding site consists of the mostly nonconserved Arg102residue
with possible assistance from Lys100
located in the β6-β7 loop. In addition, Ser11at the +6 position relative to the first
pThr also makes a considerable contribu-tion to the binding, as evidenced by strong nuclear Overhauser effects (NOE) between this residue and Lys129of Dun1-FHA in the
β10-β11 loop. In vivo studies also showed that phosphorylation of both Thr5and Thr8
on SCD1 is required for Dun1 to be fully activated and for the Dun1-dependent tran-scriptional DNA damage response, whereas single phosphorylation was suffi-cient for Rad53 to be fully activated and for Rad53-dependent cell survival. The existence of double phosphorylation of Thr5 and Thr8and its enhancement upon
DNA damage were further corroborated by mass spectrometry analyses (33).
The recognition of dual pThr residues by Dun1-FHA (Fig. 7C) is unprecedented among FHA domains. The results also shed light on the biological functions of the cluster of phosphorylation sites, sug-gesting a potential mechanism of sequen-tial phosphorylation to direct a cascade of signaling events. Database searches have indicated that some other FHA domains
could also possess dual pThr-recognizing specificities (33).
Ki67-FHA Recognizes an Extended Binding Surface
The Ki67 antigen protein, which contains 3256 amino acid residues, includes an FHA domain near its N terminus. Ki67 is widely used as an indicator of growth in cell popula-tions because of its absence in resting cells (cells in G0phase) and its readily detectable
nuclear localization and association with condensed chromosomes during interphase and mitosis, respectively (57). The biological function of Ki67, however, is little known. In addition to its suggested role in cell cycle progression, its involvement in the organiza-tion of higher-order chromatin structures has also been postulated (58). Through yeast two-hybrid screening, Yoneda’s group identi-fied human kinesin–like protein 2 (Hklp2) and human nucleolar protein interacting with the FHA domain of pKi67 (hNIFK) as the binding partners of the Ki67-FHA domain (59, 60) and proposed that Hklp2 is likely phosphorylated during the mitotic phase, during which its interaction with Ki67-FHA is at its strongest.
After unsuccessful attempts to identify short phosphopeptides that bind to Ki67-FHA, we showed that the synthetic fragment consisting of residues 226 to 269 of hNIFK binds tightly to Ki67, provided that Thr234is
phosphorylated. In vitro kinase assays showed that Thr234 is phosphorylated by
glycogen synthase kinase 3 (GSK3), but only if Thr238is first phosphorylated by
cyclin-dependent kinase 1 (CDK1)–cyclin B (34, 40, 61). The structure of the Ki67-FHA complex with hNIFK (226-269) phosphory-lated at Ser230, Thr234, and Thr238was then
solved by NMR spectroscopy, which showed an extended binding surface for protein-phosphoprotein interactions (Fig. 7D). A particularly interesting feature is that the β strand of the peptide stacks with the β sheet of Ki67-FHA (Fig. 10). In addition, the interaction of a large number of hydrophobic residues with the peptide was also consider-ably different from what was observed with other FHA domain complexes (61). This structure represents the most extensive struc-tural information of a complex between an FHA domain and a phosphoprotein and clearly shows that the interaction goes beyond the short stretch surrounding the pThr site. This structure also provides a basis for the quantitative evaluation of specific interactions. For example, changing pThr234
to pSer234 led to a decrease in the binding
on June 11, 2009
stke.sciencemag.org
affinity of Ki67-FHA for hNIFK (226-269) by a factor of 70 (Kdincreased from 0.077 to
5.5 μM), whereas deleting the β strand from the peptide led to a decrease in the binding affinity by a factor of 180. On the other hand, individual substitutions of the –1, +1, +2, and +3 residues with Ala resulted in very small effects on binding affinity (a factor of <4), which is a clear indication that the “pThr+3 rule” does not apply to the Ki67-FHA domain. The residues surrounding pThr appear to regulate the phosphorylation of Thr234by GSK3 (34).
FHA Domains Mostly Use Loops for Ligand Binding and Specificity Control It is beyond the scope of this review to make detailed comparisons between FHA domains and other protein-protein
interac-tion domains. However, a cursory compar-ison of the FHA domain with three well-studied domains, 14-3-3 (specific for pSer and pThr) (62), SH2 (specific for pTyr) (63), and WW (specific for proline-rich sequences, or pThr-pSer containing trans-proline) (64) (Fig. 11), indicates that the FHA domain is unique in that its binding site mostly involves its loops. It is impor-tant to note that in addition to the β3-β4, β4-β5, and β6-β7 loops observed in early structural studies with synthetic peptides (11, 13, 32, 36), the involvement of the β10-β11 loop in pThr-peptide binding was clearly observed in later structural studies of Rad53-FHA1, Ki67-FHA, and Dun1-FHA complexed with their physiologically relevant targets. Briefly, intermolecular NOE assignments showed that Val136 in
Rad53-FHA1 (51), Ile91 in Ki67-FHA
(34), and Lys129 in Dun1-FHA (33)
inter-acted with peptide residues C-terminal to the pThr. Furthermore, it was also sug-gested, on the basis of peptide-induced chemical shift perturbation experiments, that the β8-β9 loop of the FHA domain of kinase-associated protein phosphatase (KAPP), which apparently is the longest among the structurally determined FHA domains, could be involved in binding (10). Thus, because the loops and turns connecting β strands are typically flexible in conformation, variable in length, and divergent in sequence, particularly those (for example, the β10-β11 loop) beyond the core FHA homology region, this might explain the diverse ligand-binding speci-f icities exhibited by various FHA Fig. 5. FHA-phosphopeptide complexes. (A) Crystal structure of the complex formed between Chk2-FHA and a pThr peptide
[RHFD(pT)YLIRR] [Protein DataBank (PDB) ID: 1GXC] (13). The side chains (heavy atoms only) of three conserved FHA domain residues are shown in ball-and-stick representation, whereas the peptide residues (heavy atoms only) are in stick representation. The carbon, nitrogen, oxygen, and phosphorus atoms are shown in gray, cyan, red, and blue, respectively. (B) Surface charge distribution of the same
complex. Positive, negative, and neutral potentials are represented in blue, red, and white, respectively. The peptide residues pThr and Ile (pThr+3) are highlighted in yellow; the remainder are in green. (C) Two-dimensional 1H-15N HSQC NMR spectra of Dun1-FHA in free
(black) and complex (red) forms. The peptide is the doubly phosphorylated Rad53 SCD1 peptide 3NI(pT)QP(pT)QQST12. Several
impor-tant residues that exhibit large chemical shift perturbations (for example, Arg62, Ser74, Lys129, and Ser130) or reappear in the complex form
[Thr75, Arg102, and Asn103(boxed)] are labeled. The top panel shows the changes in NHεof Arg.
on June 11, 2009
stke.sciencemag.org
domains, which in turn provides structural and functional versatility to FHA domain– containing proteins.
Biological Functions of FHA Domains
The diverse biological functions of FHA domains are highlighted in Fig. 2. Here, we briefly review several FHA domain–con-taining proteins. Because it is not possible to cover all the published literature, we place more emphasis on the systems that have also been studied for their structures and ligand specificities.
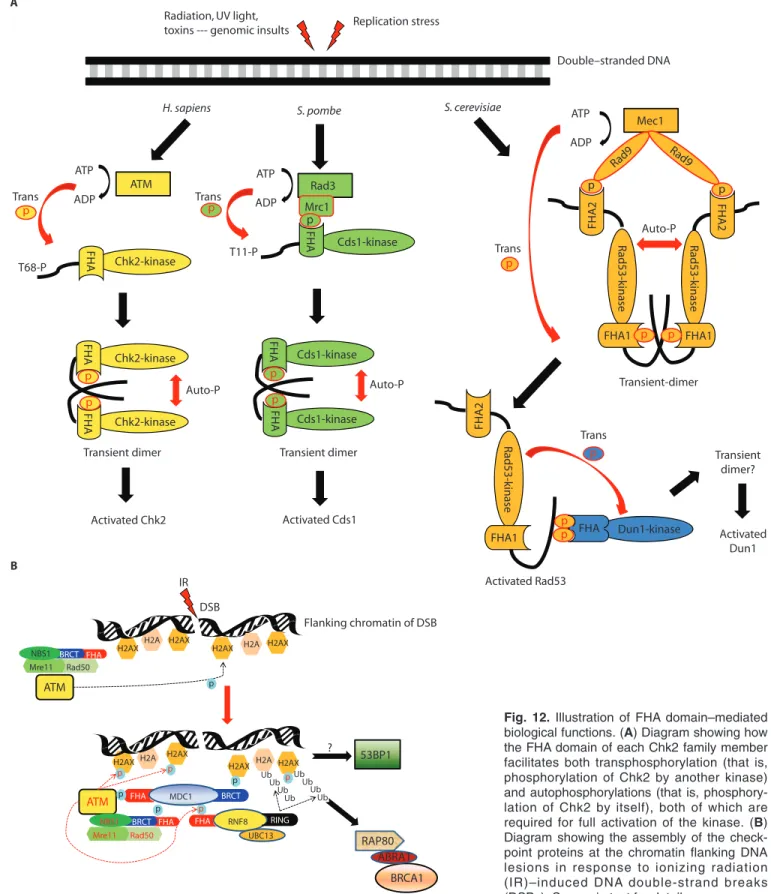
Checkpoint Signaling in Humans: Chk2, RNF8, NBS1, and MDC1 Chk2. Human Chk2 consists of an SCD and an FHA domain N-terminal to the kinase domain (Fig. 1B). Chk2, together with Chk1, plays a critical role in cell cycle regulation, DNA repair, and DNA damage–induced apoptosis at G1/S, S, and
G2/M checkpoints (65) (Fig. 2). Chk2
relays the DNA damage signal from the serine-threonine kinases ATM (ataxia telangiectasia, mutated) or ATR (ATM and Rad3-related), which phosphorylate Chk2 at Thr68in its SCD (66). Consequently, the
Chk2-FHA domain binds to the pThr68of a
second Chk2 molecule, which leads to dimerization, oligomerization, or both of Chk2, followed by autophosphorylation at the kinase activation loop for complete activation of Chk2 (16, 18) (Fig. 12A). Activated Chk2 phosphorylates and inter-acts with its downstream effectors, which leads to either halted cell cycle progression to provide time to enable repair of DNA, or the triggering of apoptosis if damage
cannot be repaired (65) (Fig. 2). Another upstream mediator, E3 identified by differ-ential display (EDD), which is a human homolog of Drosophila melanogaster “hyperplastic discs” and functions as an E3 ubiquitin ligase, associates with Chk2
through its FHA domain. Knockdown of EDD by small interfering RNA (siRNA) inhibited DNA damage–dependent phos-phorylation of Thr68, thus making Chk2
incapable of responding to DNA damage (67). Breast cancer susceptibility gene–1
β3-β4 β4-β5 β6-β7 HN OH NH2 NH2 NH2 NH2 NH -3OPO O -O -O O O pT E+1 A+2 D+3 OH OH O HN NH R83 S82 S85 N86 B A R70 T106 N107 S85 N86 R70 S6 9 605 605S6 9 G135 G133 R83 pT +1 +2 D+3 S85 N86 R70 D135 I133 R83 pT +1 +2 L+3 S85 N86 R70 A683 A681 R617 D683 I681 R617 pT +1 +2 D+3 S619 R620 R605 S619 R620 R605 pT +1 +2 L+3
FHA1 (Wild type)
(pT)XXD FHA1 (G133I/G135D)(pT)XXL
R
FHA2 (I681A/D683A) (pT)XXD
FHA2 (Wild type) (pT)XXL
+ +
Fig. 6. Schematic representation of the
structural basis of the pTXXD specificity of FHA1 for pTXXD compared with the speci-ficity of FHA2 for pTXXL. (A) Illustration of
the specificity of Thr versus Ser (highlighted by a number of interactions between pThr-γCH3and the protein). Other interactions,
demonstrated by intermolecular NOEs observed between conserved residues, non-conserved residues, or both, of the Rad53-FHA1 domain and the pThr peptide from Rad9, are also presented (32). The ionic interactions involving phosphate group, hydrophobic interactions, and the electro-static interactions involving Arg83are
indi-cated in red, black, and green, respectively. (B) Illustration of specificity at the pThr and
pThr+3 positions. Conserved and noncon-served residues among the two domains are highlighted in red and blue, respectively (41).
on June 11, 2009
stke.sciencemag.org
(BRCA1), a substrate of Chk2, interacts with Chk2 through phosphorylation-depen-dent binding with its FHA domain (13).
Chk2 is a tumor suppressor; mutations in the Chk2 gene were
identi-fied in a subset family of malig-nancy-prone Li-Fraumeni syn-dromes and in sporadic human cancers (68). The Chk2-FHA functions as both a phosphory-lation-dependent and -indepen-dent mediator to link upstream activators with downstream tar-gets, according to studies of the oncogenic mutations (Arg117 →
Gly117, Arg145 → Trp145, and
Ile157→ Thr157) within the FHA
domain (13, 16, 18, 69). The Arg117 → Gly117 mutation
occurs in the conserved region for pThr-binding and attenuates the autokinase activity in response to DNA damage (69). The locations of the other two oncogenic mutations, Arg145 →
Trp145and Ile157→ Thr157, are
remote from the binding pocket of pThr. It is interesting that the Arg145 → Trp145 mutant cannot
bind to the original pThr ligand. Arg145 is located in the core
structure (β5) of FHA β strands, and the Arg145→ Trp145
muta-tion destabilizes Chk2 and pre-vents the transphosphorylation activity of ATM (18, 70, 71). On the other hand, the Ile157 →
Thr157mutant behaves like the
wild-type (WT) protein in terms of Chk2 activation; however, this mutant fails to bind to the downstream target of ectopi-cally expressed BRCA1, p53, and cell divi-sion cycle 25A (CDC25A) (13, 72, 73).
RNF8. A recent addition to the family of checkpoint regulators is RNF8 (Fig. 1A), a novel DNA damage–response pro-tein that is recruited to the chromatin that flanks DNA lesions, where it colocalizes with phosphorylated H2AX, a variant of histone H2A, phosphor ylated ATM, MDC1, BRCA1, 53BP1, RAP80, and NBS1 (30, 74–76) (Fig. 12B). These inter-actions lead to the formation of ionizing radiation (IR)–induced foci (IRIF) in response to DNA double-strand breaks (30, 74–76). Knockdown of RNF8 by siRNA prevents adequate cell cycle arrest at the G2/M checkpoint when IR is applied (30).
RNF8 acts downstream of γH2AX (phos-phorylated H2AX) and MDC1; the
tar-geting of RNF8 to DNA lesions is facilitated by MDC1 through its phosphorylation-dependent interaction with the RNF8-FHA domain (30, 75, 76). Assembly of other
downstream checkpoint proteins, such as BRCA1, 53BP1, and RAP80, at the IRIF requires RNF8 to ubiquitinate the histones flanking the site of the DNA lesion (30, 74–76) (Fig. 12B). The RNF8-FHA domain shows a high specificity for Tyr and Phe at the pThr+3 position, as deter-mined by the aforementioned librar y screening, and the structure of the complex it forms with phosphopeptide has been solved by x-ray crystallography (30). T h e RNF8-FHA–oriented motifs of pTXX(F/Y) can be narrowed down to the four-TQ cluster–containing sequence of MDC1 from residues 698 to 768; the con-sensus sequence for the four binding sites is TQXF, which is phosphorylated by ATM (30, 76). Kdvalues for the binding of the
four synthetic phosphopeptides encom-passing the binding regions of MDC1 to the RNF8-FHA domain are in the range of 3 to 11 μM (30). Deletion of this putative
RNF8-FHA domain–binding region (residues 698 to 768) of MDC1 abolishes its interaction with RNF8 and prevents the assembly of downstream checkpoint pro-teins at sites of DNA lesions (30). Thus, the FHA domain is required for RNF8 functions in orchestrating the DNA damage response for the assembly of checkpoint proteins and signal transduction at the sites of DNA damage (30, 75, 76).
NBS1 and MDC1. NBS1 and MDC1 (also known as NFBD1) are two other important checkpoint signaling proteins, both of which contain FHA and BRCT domains (Fig. 1A). NBS1 was first identi-fied from a protein mutated in Nijmegen breakage syndrome, which is characterized by chromosome instability, radiosensitivity, and a high frequency of malignancies (77, 78). NBS1 also forms a complex with mei-otic recombination 11 (MRE11) and RAD50, which presumably functions in sensing DNA double-strand breaks (DSBs) in the DNA damage–response pathway, as well as tethering and editing two DNA ends in the homologous recombination repair pathway (79) (Fig. 12B).
NBS1 is not only a downstream target of ATM, but also acts upstream to promote optimal ATM activation and its recruitment to the flanking region of DSBs (80, 81). The unwinding of DNA ends by the MRE11:RAD50:NBS1 (MRN) complex is essential for stimulating the monomeriza-tion and activamonomeriza-tion of ATM (82). Activated ATM then phosphorylates H2AX at Ser139
neighboring the DNA lesion that serves as a docking site (83), which in turn attracts the BRCT repeat of MDC1 through its pSer-binding activity (Fig. 12B) (84). Meanwhile, the consecutive SDTD (Ser-Asp-Thr-Asp) motifs of MDC1, when phosphorylated by casein kinase 2 (CK2), are bound by the FHA domain (85, 86) and the adjacent BRCT repeat (87, 88) of NBS1; such binding directs the accumula-tion of the MRN complex to damaged chromatin. Furthermore, the recruited MDC1 acts as a molecular scaffold by accommodating additional ATM molecules through interactions of its FHA domain with phosphorylated ATM (89). This there-fore provides an important feedback loop that amplifies ATM-dependent γH2AX sig-nals at sites of damage by which MDC1 may promote downstream effector proteins that accumulate after the induction of DSBs (89). Alternatively, the FHA domain of MDC1 also interacts directly with pThr68of Chk2 (47) and with MRE11 (90),
A B C D “pT+3” ---X X X pT X X(D )---Arg “N- and C-termini to pT” ---X X XpTX X X Arg “pT – pT + 3” R102 (K100) ---pT X X pT X X S
---“pT + extended binding surface” ---X X pT X X XX--Arg R60 R62 K129 or (I/L/V)
Fig. 7. Illustration of four different mechanisms of ligand
binding by FHA domains. (A) Schematic illustration of
the “pThr+3” mechanism, in which some FHA domains recognize their targets mainly by the presence of the pThr and the residue at the +3 position (11–13, 36). (B)
The “N- and C-termini to pThr” mechanism of FHA domain interaction involves amino acid residues both N-terminal and C-N-terminal to the pThr residue (14). (C) The
“pThr-pThr+3” mechanism was recently discovered for the yeast Dun1-FHA domain. Recognition specificity was determined by two pThr residues and the +3 position of the second pThr (33). (D) The “pThr + an extended
binding surface” mechanism is required for the interac-tion between the Ki67-FHA domain and its binding partner NIFK (34).
on June 11, 2009
stke.sciencemag.org
whereas knockdown of MDC1 by siRNA attenuates the phosphorylation of p53 at Ser20 by Chk2 and decreases IR-induced
p53 stabilization, which results in a weak-ened apoptotic response (47). The knock-down also abates IRIF formation of the MRN complex, 53BP1, BRCA1, and γH2AX (91), which is suggestive of funda-mental roles for MDC1 in mediating DNA-damage checkpoints.
PNK and ATPX in the Regulation of DNA Repair Pathways
In addition to DNA-damage signaling mediated by NBS1 and/or MDC1, which presumably facilitates or is essential for the repair of DNA DSBs by homologous recombination (92–94), the FHA domain is also involved in other DNA-repair mecha-nisms. Mammalian PNK has DNA-kinase and DNA-phosphatase activities and is responsible for maintaining 5′-phosphate and 3′-hydroxyl termini at DNA-strand breaks, a prerequisite for polymerases and ligases operating in DNA repair (95). PNK is recruited to sites of base excision repair (BER) and nonhomologous end-joining (NHEJ) repair through its N-terminal FHA domain interacting with CK2-phosphory-lated pThr residues of XRCC1 and XRCC4 (x-ray repair complementing defective repair in Chinese hamster cells 1 and 4, respectively), two key components of BER and NHEJ repair pathways that complex with DNA ligases III and IV, respectively (96, 97). In support of this, a crystallo-graphical study of PNK shows that its FHA domain binds to an XRCC4-derived phos-phopeptide (14); more specif ically, it exhibits the highest selectivity for residues N-terminal of pThr, some selectivity for C-terminal residues, and no selectivity for the pThr+3 position (Fig. 9A) (96).
The FHA domain of aprataxin (APTX), a protein also involved in DNA repair and genomic stress (98), contains the same conserved residues involved in phospho-peptide recognition as does the PNK-FHA (14), which may suggest that the same ligand-sequence selectivity is shared between these two FHA domains. Indeed, APTX also forms complexes in vivo with XRCC1/ligase III and XRCC4/ligase IV and has been proposed to facilitate PNK activity during BER and NHEJ repair mechanisms (99, 100). Nevertheless, in contrast to the PNK-FHA, the APTX-FHA shows high specificity for a triply phospho-rylated peptide derived from XRCC1 with a charged residue at the pThr+3 position (99).
Taken together, these two FHA domains are likely to use different phosphopeptide-binding specificities to interact with the same binding partners in order to regulate each other’s activities. Recently, PALF [PNK and APTX-like FHA protein, also known as APLF (aprataxin and PNK-like factor)] was identified by two laboratories and shown to contain an FHA domain and a zinc finger–like CYR (Cys-Tyr-Arg) motif. This protein directly interacts with Ku86, ligase IV, and phosphorylated XRCC4 pro-teins; has endonuclease and exonuclease
activities; and thus plays roles similar to this category of proteins in DNA damage (101, 102). The binding specificities of its FHA domain have not yet been determined. Cds1, Rad53, and Dun1 in
Checkpoint Signaling in Yeast Here, we discuss Chk2 homologs in yeast: Cds1 in S. pombe and Rad53 and Dun1 in S. cerevisiae. The domain structures of these three yeast kinases are similar, but not identical, to Chk2 (Fig. 1B). Dun1 lacks an SCD preceding its FHA domain, Fig. 8. Comparison of structures of FHA1 in complex with pTXXI and pTXXD
phosphopep-tides. (A) Surface-charge diagram of Rad53-FHA1 with a pThr peptide
(SLEVpT192EADATFVQ) from Rad9 (PDB ID: 1J4Q) (32). (B) Surface-charge diagram of
Rad53-FHA1 with a pThr peptide (NDPDpT305LEIYS) from Mdt1 (PDB ID: 2A0T) (51). (C)
Stereoview showing the overlay of FHA1 structures in complex with pTXXI (PDB code 2A0T) and pTXXD (PDB code 1K3Q) phosphopeptides. The FHA1 domain is shown in a ribbon diagram colored in blue (FHA1-pTXXI complex) and green (FHA1-pTXXD complex). Several residues are highlighted: Val136 in FHA1-pTXXI, Arg83in FHA1-pTXXD, and the
peptide pThr and pThr+3 residues. The atoms are colored as for Fig. 5A. It appears that the pThr moiety is recognized in essentially the same way for both complexes. However, although there is strong ionic interaction between Asp(pT+3) and Arg83in FHA1-pTXXD, the
Ile(pThr+3) in FHA1-pTXXI contributes substantially to the binding through a hydrophobic interaction with the Val136of FHA1 and so moves the residues C-terminal to pThr from the
β6-β7 loop to the Val136-residing β10-β11 loop.
on June 11, 2009
stke.sciencemag.org
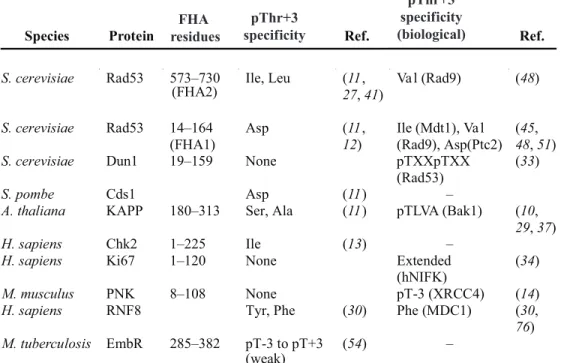
Species Protein Residues Method Phosphopeptide Source of peptide
Key FHA residues for canonical pT binding PDB code Ref. S. cerevisiae Rad53 573–730 (FHA2) NMR 1QU5 (8) S. cerevisiae Rad53 573–730
(FHA2) NMR EDI(pY)YLD† 826–832Rad9 1FHR (27)
S. cerevisiae Rad53 573–730
(FHA2) NMR EVEL(pT)QELP† 599–607Rad9 R605 (R620 (β3-β4), S619, β4-β5), T654 (β6-β7)
1K2N (36)
S. cerevisiae Rad53 2–164
(FHA1) NMR 1G3G (12)
S. cerevisiae Rad53 14–164
(FHA1) X-ray SLEV(pT)EADATFAKK† 188–198Rad9 R70 (N86 (β3-β4), S85, β4-β5) T106 (β6-β7)
1G6G (11)
S. cerevisiae Rad53 14–164
(FHA1) NMR SLEV(pT)EADATFVQ† 188–200Rad9 Same as 1G6G 1K3Q (32)
S. cerevisiae Rad53 14–164
(FHA1) NMR KKMTFQ(pT)PTDPLE† 148–160Rad9 Same as 1G6G 1K3N (32)
S. cerevisiae Rad53 14–164
(FHA1) NMR NDPD(pT)LEIYS* 301–310Mdt1 Same as 1G6G 2A0T (51)
S. cerevisiae Rad53 14–164
(FHA1) NMR NI(pT)QPTQQST* 3–12Rad53 Same as 1G6G 2JQI (33)
S. cerevisiae Dun1 19–159 NMR 2JQJ (33)
S. cerevisiae Dun1 19–159 NMR NI(pT)QP(pT)
QQST* 3–12Rad53 R60, R62 (β3-β4) 2JQL (33)
M. tuberculosis EmbR 285–382 X-ray 2FEZ (54)
M. tuberculosis EmbR 285–382 X-ray SLEV(pT)EADT† 188–197
Rad9 R312 (R327 (β3-β4) S326, β4-β5), S347 (β6-β7)
2FF4 (54)
A. thaliana KAPP 180–313 NMR 1MZK (10)
A. thaliana Y4449 1–105 NMR 1UHT
M. musculus PNK 1–106 NMR 1UJX
M. musculus PNK 1–110 X-ray YDES(pT)
DEESEKK* 229–240XRCC4 R35 (R48 (β3-β4), S47, β4-β5) 1YJM (14)
M. musculus NIPP1 1–132 NMR 2JPE (35)
M. musculus AFAD 381–487 NMR 1WLN
H. sapiens Chk2 92–207 X-ray RHFD(pT)YLIRR† Synthetic R117 (β3-β4), S140,
K141 (β4-β5) 1GXC (13)
H. sapiens CHFR 14–128 X-ray 1LGQ (15)
H. sapiens CHFR 14–128 X-ray Tungstate 1LGP (15)
H. sapiens Ki67 1–120 NMR 1R21 (40) H. sapiens Ki67 1–120 NMR KTVD(pS) QGP(pT)PVC(pT) PTFLERRKSQ VAELNDDDKDD EIVFKQPISC* 226–269 hNIFK R31 (K46 (β3-β4), S45, β4-β5) T66 (β6-β7) 2AFF (34) H. sapiens PNK 8–108 X-ray 2BRF H. sapiens RNF8 8–139 NMR 2CSW
H. sapiens RNF8 13–146 X-ray ELKpTERY Synthetic R42 (β3-β4), S60,
R61 (β4-β5) 2PIE (30)
H. sapiens KIF1C 498–599 X-ray 2G1L
H. sapiens KIF1B 531–647 NMR 2EH0
*From biological studies. †From peptide library screening.
Table 1. Structures of free FHA and FHA domain–phosphopeptide complexes. Conserved residues are underlined.
on June 11, 2009
stke.sciencemag.org
whereas Rad53 con-tains two sets of SCD-FHA domains. The discussion below will focus on the roles played by the FHA domain in the activation of these kinases in response to DNA-damage or replication stress. As described above, Chk2 is activated by a two-stage process: FHA-independent phosphorylation by an upstream kinase,
followed by FHA-dependent dimerization and autophosphorylation. The three yeast kinases largely follow the same two-stage processes; however, the molecular mecha-nisms mediated by their FHA domains vary substantially in each case (Fig. 12A).
Cds1. In S. pombe Cds1, the FHA domain is involved in both phosphorylation of Cds1 by upstream kinases and autophos-phorylation of Cds1 (46). Phosautophos-phorylation of Cds1 is mediated by the TQ repeats (Thr645to Gln646and Thr653to Gln654, Fig.
4B) of mediator of replication checkpoint protein 1 (Mrc1) (46, 103). Upon replica-tion stress, Cds1-FHA binds to pThr645and
pThr653of Mrc1, which leads to the
recruit-ment of Cds1 to the upstream kinase Rad3, Fig. 10. Stereoview of the Ki67-FHA:NIFK (226-269)3P complex
(34). (A) The ribbon diagram shows overall interactions. Four FHA
residues important for pThr234binding together with the three
phos-phorylated residues are highlighted, and the two β strands engaged in intermolecular β sheets are numbered (PDB ID: 2AFF). (B) Detailed interactions in this complex involving pThr234and the +1,
+2, and +3 residues of hNIFK and the interface between the binding loops of Ki67-FHA and the α helix of hNIFK are shown. Carbon, nitrogen, oxygen, sulfur, and phosphorus atoms are col-ored in gray, cyan, red, yellow, and blue, respectively. Some of the predicted hydrogen bonds are depicted with dashed lines.
Fig. 9. Stereoview of structures of FHA domain complexes with
phosphopeptide fragments derived from biological studies. (A)
The binding of PNK-FHA to a pThr peptide YDES(pT)DEESEKK from XRCC4 (PDB ID: 1YJM) (14). (B) The binding of EmBR-FHA
with a synthetic pThr peptide SLEV(pT)EADT (PDB ID: 2FF4) (54). (C) Structure of Rad53-FHA1 in complex with a singly
phos-phorylated Rad53-SCD1 peptide 3NI(pT)QPTQQST12(PDB ID:
2JQI). (D) Structure of Dun1-FHA in complex with a doubly
phos-phorylated Rad53 SCD1 peptide 3NI(pT)QP(pT)QQST12(PDB ID:
2JQL). Several important FHA residues are shown in ball-and-stick representation (heavy atoms and side chains only), whereas the peptide residues (heavy atoms only) are in stick representa-tion. The atoms are colored similarly to those in Fig. 5A.
on June 11, 2009
stke.sciencemag.org
which subsequently phosphorylates Thr11
of the Cds1-SCD (52, 53). The Cds1-FHA domain then binds to pThr11of the
Cds1-SCD, which leads to dimerization and autophosphorylation of Cds1 (46) (Fig. 12A). Note that both TQ repeats of Mrc1 share the consensus sequence surrounding pThr (Fig. 4B) and redundantly interact with Cds1-FHA to activate Cds1. Further-more, the Asp residue in the pThr+3 posi-tion matches that identified in the phos-phopeptide motif pTXXD in vitro, and mutation of both Asp residues to Ala (Asp648 → Ala648 and Asp658 → Ala658)
results in hydroxyurea sensitivity (46). The Kdvalues of synthetic peptides
encom-passing pThr11 within Cds1-SCD and
pThr653within Mrc1-SCD for the
Cds1-FHA domain are approximately 3.8 and 0.27 μM, respectively (46). In addition,
Cds1 inhibits the activity of Mus81, a structure-specific endonuclease that plays a role in recombination, through its FHA domain. Thus, Cds1-FHA helps to main-tain genomic integrity during cermain-tain types of replication stress (104).
Rad53. Checkpoint responses of S. cerevisiae Rad53 are required for DNA damage–induced signaling and for cell cycle arrest. They are invoked in the event of DNA damage that occurs at various stages of the cell cycle (G1/S, S-phase
pro-gression, and G2/M transitions) or
inhibi-tion of DNA replicainhibi-tion (105–107). Rad53 is also essential for cell viability (108), normal cell growth, and transcriptional regulation (109). The mechanism of trans-activation of Rad53 may be analogous to that of S. pombe Cds1 but could be sub-stantially more complicated (Fig. 12A).
Rad53 has two FHA domains, FHA1 (12) and FHA2 (8), which have become proto-typical of studies of the structure-function relationship of the FHA domain since the landmark work by Stern’s group (24). These FHA domains may play diverse and overlapping roles in regulating Rad53 acti-vation and in identifying and binding to prephosphorylated substrates (110, 111). In addition, Rad53 is recruited by two adaptor proteins, Rad9 and Mrc1 (a func-tional homolog of S. pombe Mrc1), to be phosphorylated by mitosis entry check-point protein 1 (Mec1) or telomere length–regulation protein 1 (Tel1) in response to DNA-damage and replication stress (24, 112–114). Involvement of FHA1 and FHA2 in the recruiting process remains to be firmly established, although hyperphosphorylated Rad9 preferentially binds to FHA2 (24), whereas both FHA1 and FHA2 are required for the Rad9-dependent activation of Rad53 (110, 111, 114, 115). Various SQ and TQ sequences from the Rad9-SCD (from residue 390 to 458, Fig. 4B) have redundant functions in their interactions with Rad53. The Kd values of synthetic phosphopeptides encompassing the f irst TQ sequence (pThr390) within the Rad9-SCD for FHA1
and FHA2 are 2.5 and 1.4 μM, respec-tively (48). Note that Rad9 and Mrc1 both interact with Rad53-FHA1 after the occur-rence of DNA damage, as determined in a mass spectrometry–based proteome-wide study (116). Rad9 also functions redun-dantly with Mrc1, the major adaptor in replication checkpoint signaling (112, 113), to facilitate Rad53 activation in the Δmrc1 mutant upon hydroxyurea-induced replication stress (112).
Fig. 11. Stereoviews of structures of other
protein-protein interaction domains. (A)
X-ray structure of the 14-3-3 domain in com-plex with a phosphopeptide RLYH(pS)LPA (PDB code: 1QJA) (134). (B) Solution
structure of the C-terminal SH2 domain of PLC-γ1 in complex with a phosphopeptide DND(pY1021)IPLPDPK from the PDGF
receptor (PDB code: 2PLD) (135). (C)
X-ray structure of the pin1 WW domain with doubly phosphorylated serines followed by prolines T(pS)PT(pS)PS (although only the second pSer is involved in binding) from the C-terminal domain of RNA polymerase II (PDB code: 1F8A) (136). Phosphorylated residues (Tyr, Ser, or Thr) are shown in green, prolines in red, and the remainder of the peptide in purple.
on June 11, 2009
stke.sciencemag.org
Rad9 Rad9 Chk2-kinase Chk2-kinase Chk2-kinase Cds1-kinase Cds1-kinase Cds1-kinase Replication stress Double–stranded DNA ATP ADP ATM FHA T68-P FHA FHA Auto-P Auto-P Activated Chk2 ATP ADP Rad3 Trans FHA T11-P Trans Mrc1 FHA FHA Activated Cds1 ATP ADP Mec1 FHA1 Trans FHA2 Rad53-kinase Rad53-kinase Rad53-kinase FHA1 FHA2 FHA1 FHA2 Auto-P Activated Rad53 Dun1-kinase FHA Trans Activated Dun1
H. sapiens S. pombe S. cerevisiae
p p p p p p p p p p p p p p p
Transient dimer Transient dimer Transient
dimer? Transient-dimer
Radiation, UV light, toxins --- genomic insults
A
B
ATM p
H2AX H2A p
H2AX H2A H2AX H2AX
p p Mre11 Rad50 Mre11 Rad50 NBS1 FHA RNF8 RING FHA FHA MDC1 BRCT ATM p p p Ub p Ub Ub Ub Ub Ub Ub Ub UBC13 53BP1 RAP80 BRCA1 ABRA1 H2AX H2AX H2A ? DSB Flanking chromatin of DSB IR
H2AX H2A H2AX BRCT
BRCT FHA NBS1
Fig. 12. Illustration of FHA domain–mediated
biological functions. (A) Diagram showing how
the FHA domain of each Chk2 family member facilitates both transphosphorylation (that is, phosphorylation of Chk2 by another kinase) and autophosphorylations (that is, phosphory-lation of Chk2 by itself), both of which are required for full activation of the kinase. (B)
Diagram showing the assembly of the check-point proteins at the chromatin flanking DNA lesions in response to ionizing radiation (IR)–induced DNA double-strand breaks (DSBs). See main text for details.
on June 11, 2009
stke.sciencemag.org
When DNA damage occurs in vivo, S. cerevisiae Rad53 for ms dimers or oligomers, which undergo autophosphory-lation (117). Mutations in its FHA and SCD domains compromise its intermolec-ular interactions and autophosphorylation activity (55). However, detailed molecular mechanisms of the roles of FHA1 and FHA2 in the autophosphor ylation of Rad53 remain to be established. Singly phosphorylated Rad53-SCD1 phosphopep-tides interact with the FHA1, but not FHA2, domain of Rad53 in vitro, with phosphorylation at Thr5-Gln6or Thr8-Gln9
motifs having the lowest Kdvalues (~10 to
20 μM) (33). Another study (118) showed that the topological order of FHA1 and FHA2 is critical to the autophosphoryla-tion of Rad53 and the downstream tran-scriptional activation of RNR3 (ribonu-cleotide reductase 3), whereas this order does not affect phosphorylation of Rad53 by upstream kinases. A mutant Rad53 with swapped FHA domains has no kinase activity, although it can still be recruited to upstream kinases in response to DNA damage. This evidence implies that the intermolecular interaction between the FHA domain and the phosphorylated SCD in a specific spatial arrangement might be
critical to the dimerization- or oligomeriza-tion-driven autophosphorylation of Rad53.
Evidence is accumulating that Rad53-FHA domains mediate not only the activa-tion, but also the inactivaactiva-tion, of Rad53 during cell cycle checkpoints (45, 110, 111, 114, 115). A return to normal growth conditions after stress-induced responses is crucial for the cell, and this occurs as a result of recovery (after DNA repair) or adaptation (the resumption of proliferation despite limited irreparable damage) (45). The S. cerevisiae phosphatases Ptc2 and Ptc3 interact with Rad53 and are involved in checkpoint inactivation in response to DSBs induced by site-specific homothallic switching endonuclease (49). The interac-tion of Ptc2 and Ptc3 with Rad53 occurs through the FHA1 domain of Rad53 in a phosphorylation-dependent manner. The Kd value for binding of the synthetic phospho-peptides encompassing pThr376 of Ptc2
(pT376DAD) to Rad53-FHA1 is 2.3 μM
(45). In vivo, this binding occurs when Ptc2 is phosphorylated by CK2 on Thr376,
which leads to dephosphorylation of Rad53 and the resumption of the cell cycle after DNA damage (45).
Dun1. Last, but not least, is the dra-matic finding of an FHA domain with two
specific pThr-binding sites (33) (Fig. 7C). S. cerevisiae Dun1 is a kinase that is phos-phorylated and activated by Rad53 (119, 120). In vivo studies have indicated that the phosphorylated SCD1 of Rad53 interacts with Dun1-FHA (56) and leads Rad53 to phosphorylate Thr380at the Dun1 activation
loop, thus activating Dun1 (120) (Fig. 12A). Mutation of all four Thr residues to Ala in the TQ motifs of SCD1 abolishes the kinase activity of Rad53 and renders cells hypersensitive to DNA damage– causing reagents. The autokinase activity of Rad53 and Rad53-dependent survival in response to genotoxic stress can be restored by reverting any one of these mutated residues to Thr, whereas transduc-tion of signals downstream of Dun1 remains retarded (33, 56). In vitro binding studies also indicate that the Rad53-FHA1 recognizes and binds to singly phosphory-lated Rad53-SCD1 peptides. In contrast, the Dun1-FHA domain shows strongest binding to synthetic phosphopeptides of Rad53-SCD1 that contain simultaneously phosphorylated Thr5and Thr8 residues
(33). As discussed above, the results of structural and biological studies reveal a hierarchical regulation of the Rad53-Dun1 signaling cascade by a “phospho-counting
Species Protein Ref. Ref.
S. cerevisiae Rad53 573–730
(FHA2) Ile, Leu (11,27, 41) Va l (Rad9) (48)
S. cerevisiae Rad53 14–164
(FHA1)
Asp (11,
12) Ile (Mdt1), Va l(Rad9), Asp(Ptc2) (45, 48, 51)
S. cerevisiae Dun1 19–159 None pTXXpTXX
(Rad53)
(33)
S. pombe Cds1 Asp (11) –
A. thaliana KAPP 180–313 Ser, Ala (11) pTLVA (Bak1) (10,
29, 37)
H. sapiens Chk2 1–225 Ile (13) –
H. sapiens Ki67 1–120 None Extended
(hNIFK)
(34)
M. musculus PNK 8–108 None pT-3 (XRCC4) (14)
H. sapiens RNF8 Tyr, Phe (30) Phe (MDC1) (30,
76) M. tuberculosis EmbR 285–382 pT-3 to pT+3 (weak) (54) – FHA residues pThr+3 specificity pThr+3 specificity (biological)
Table 2. Summary of ligand specificities derived from chemical (combinatorial library) and biological approaches.
on June 11, 2009
stke.sciencemag.org