which can affect 5% to 20% of pregnant women.2–8
Poor attendance to prenatal clinics, substance misuse, low birth weight, and premature delivery are observed in pregnant women with depressed mood.9,10
Untreated depression during pregnancy also increases the risk of negative preg-nancy outcomes,1,11
and psychopathologic symptoms dur-ing pregnancy could have further physiologic conse-quences to the fetus.12
As is well known, pharmacotherapy for depression during pregnancy is a clinical dilemma. Although it has been suggested that the relapse rates for depression are high during pregnancy13
and taking antidepressants does not increase the risk of malformation or miscarriage,1
more recent data have raised the concern that taking se-lective serotonin reuptake inhibitor (SSRI) antidepres-sants during the third trimester may be associated with considerably increased risk of perinatal complications.14,15
In addition, antidepressant use during pregnancy in women with a history of major depression is associated
Omega-3 Fatty Acids for
Major Depressive Disorder During Pregnancy:
Results From a Randomized, Double-Blind,
Placebo-Controlled Trial
Kuan-Pin Su, M.D.; Shih-Yi Huang, Ph.D.; Tsan-Hung Chiu, M.D., Ph.D.;
Kuo-Cherh Huang, Dr.P.H., M.B.A.; Chieh-Liang Huang, M.D.;
Hui-Chih Chang, M.S.; and Carmine M. Pariante, M.D., Ph.D.
Background: Perinatal depression is common, and treatment remains challenging. Depression has been reported to be associated with the abnormality of omega-3 polyunsaturated fatty acids (PUFAs). A pro-found decrease of omega-3 PUFAs in the mother during pregnancy is associated with the higher demand of fetal development and might precipitate the occurrence of depression. In this study, we examined the efficacy of omega-3 PUFA monotherapy for the treatment of depression during pregnancy.
Method: From June 2004 to June 2006, we con-ducted an 8-week, double-blind, placebo-controlled trial comparing omega-3 PUFAs (3.4 g/d) with placebo in pregnant women with major depressive disorder (DSM-IV criteria). No psychotropic agent was given 1 month prior to or during the study period. The Hamilton Rating Scale for Depression (HAM-D) was scored every other week as the primary measurement of efficacy, while the Edinburgh Postnatal Depression Scale (EPDS) and Beck Depression Inventory (BDI) were secondary measures.
Results: Thirty-six subjects were randomly assigned to either omega-3 PUFAs or placebo, and 33 among them were evaluated in more than 2 visits. A total of 24 subjects completed the study. As compared to the placebo group, subjects in the omega-3 group had sig-nificantly lower HAM-D scores at weeks 6 (p = .001) and 8 (p = .019), a significantly higher response rate (62% vs. 27%, p = .03), and a higher remission rate, although the latter did not reach statistical significance (38% vs. 18%, p = .28). At the study end point, subjects in the omega-3 group also had significantly lower de-pressive symptom ratings on the EPDS and BDI. The omega-3 PUFAs were well tolerated and there were no adverse effects on the subjects and newborns.
Conclusions: Omega-3 PUFAs may have therapeutic benefits in depression during pregnancy. In regard to the safety issue and psychotherapeutic effect, as well as health promotion to mothers and their newborns, it is worthy to conduct replication studies in a larger sample with a broad regimen of omega-3 PUFAs in pregnant women with depression.
Trial Registration: clinicaltrials.gov Identifier: NCT00618865
(J Clin Psychiatry 2008;69:644–651)
Received Aug. 7, 2007; accepted Nov. 7, 2007. From the Department of Psychiatry and Mind-Body Interface Research Centre (Drs. Su and C-L Huang) and the Department of Obstetrics and Gynecology (Dr. Chiu), China Medical University Hospital, Taichung, Taiwan; the School of Nutrition and Health Sciences (Drs. Su and S-Y Huang) and the School of Health Care Administration (Drs. K-C Huang and Chang), Taipei Medical University, Taipei, Taiwan; and the Institute of Psychiatry, King’s College London, United Kingdom (Drs. Su and Pariante and Ms. Chang).
The work was supported by the grants of NSC 93-2320-B-039-001 and 95-2320-B-039-037-MY3 from the National Science Council; DOH94F044 and DOH95F022 from the Department of Health; and DMR-94-10, DMR-94-46, and CMU-95-143 from the China Medical University and Hospital in Taiwan.
The authors report no additional financial or other relationships relevant to the subject of this article.
Corresponding authors and reprints: Kuan-Pin Su, M.D., Mind-Body Interface Research Centre, Department of General Psychiatry, China Medical University Hospital, No. 2, Yuh-Der Rd., Taichung 404, Taiwan (e-mail: cobolsu@gmail.com); and Shih-Yi Huang, Ph.D., School of Nutrition and Health Sciences, Taipei Medical University, Taipei, Taiwan (e-mail: sihuang@tmu.edu.tw).
W
omen during pregnancy and after childbirthwith a higher risk of premature delivery and lower gesta-tional age at birth compared with women who elected to discontinue medication during pregnancy, and the adverse effect of antidepressants remained after the analyses con-trolled for severity and duration of depressive symptoms during pregnancy.16
To date, the U.S. Food and Drug Ad-ministration has not approved any antidepressant agents during pregnancy. Furthermore, most mothers are exceed-ingly anxious about accepting antidepressant medication, and despite moderate to severe depressive symptoms and impaired functioning, they tend to focus on the risks of in utero exposure to medication rather than on the risks of untreated depression.17
Most mothers-to-be choose not to take medications, a problem that was highlighted in the lay press U.S. News and World Report article, “The Baby or the Drug? It’s a Choice That Many Pregnant Women Often Face—But Shouldn’t.”18
Considering the potential impact on mothers and newborns, the development of safe and ef-fective management is critical for pregnant women with depression.
Omega-3 polyunsaturated fatty acids (PUFAs), includ-ing eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), are nutritional compounds with widely estab-lished health benefits.19–21
Since essential fatty acids can-not be synthesized by the human body, they are considered an indispensable dietary component.22
Pregnancy is asso-ciated with a decrease in the biochemical PUFA status, and it returns to normal slowly after delivery.23,24
Recently, a deficit of omega-3 PUFAs was hypothesized to be etio-logically important in depression.25
Societies in which a large amount of omega-3 PUFAs are consumed appear to have a lower prevalence of major depressive disorder.26–28
Consistent with the above-mentioned fact, it is found that patients with major depressive disorder have lower levels of omega-3 PUFAs,29–33
and the level of omega-3 PUFAs is significantly negatively correlated with the severity of depressive symptoms.31,33
The abnormalities in PUFA composition on cell membranes can alter membrane mi-crostructure, cause abnormal signal transduction and im-munologic dysregulation, and possibly increase the risk of developing depression.25,34
More importantly, 2 meta-analytic reviews35,36
and several clinical trials37–41
have re-ported an antidepressant effect of PUFAs. In addition, the use of omega-3 fatty acids in psychiatric patients during the perinatal stage was supported by case reports of preg-nant women with depression42
and schizophrenia43
and by a small, open-label, flexible-dose trial in 15 pregnant de-pressed subjects.44
Due to the advantage of lack of terato-genesis45
and its essentiality for the central nervous system development,46–48
omega-3 PUFAs might be a promising alternative treatment for pregnant women with depression. In this study, we conducted an 8-week, double-blind, placebo-controlled trial. Our hypothesis is that omega-3 PUFAs are effective and safe in treating major depressive disorder in pregnant women.
METHOD Subjects
This 8-week, randomized, parallel-group, placebo-controlled, double-blind study was approved by the insti-tutional review board and conducted at China Medical University Hospital, Taiwan. Eligible participants were pregnant women, aged 18 to 40 years, with DSM-IV ma-jor depressive disorder onset between their 16th week (second trimester) and 32nd week (third trimester) of ges-tation seen at the Department of Obstetrics during the 24-month study period (June 2004 to June 2006). They were screened with the Taiwanese version of the Edinburgh Postnatal Depression Scale (EPDS) by a psychiatric re-search nurse and then interviewed by experienced psychiatrists using the structured Mini-International Neuropsychiatric Interview (MINI).49
The information on the translation, validation, and instruction of the Tai-wanese version of the MINI can be accessed on the Web site of the Taiwanese Society of Psychiatry (http:// www.sop.org.tw/dow_a.htm). The Taiwanese version of the EPDS has been validated in screening depression dur-ing pregnancy.50
Subjects were excluded if they had a DSM-IV diagno-sis of bipolar disorder, psychotic disorder, or substance abuse/dependence or any Axis II diagnosis of borderline or antisocial personality disorder. Participants were re-quired to be free from any psychotropic agents at least 1 month, to have a score of at least 18 on the 21-item Ham-ilton Rating Scale for Depression (HAM-D) at screening phase, and to have good physical health as determined by medical history, physical examination, blood laboratory results, electrocardiogram, chest radiography, and urinal-ysis. The supply of open-label omega-3 fatty acids could be continued, and they were available upon subjects’ re-quests even if the study were to end before the delivery. All participants were informed of other treatment options, including antidepressant medications and psychotherapy, and provided written consent before entering the study. Study Design
At the baseline visit (week –1), the detailed psychiat-ric, obstetpsychiat-ric, and medical histories were obtained, and the HAM-D, Taiwanese version of the EPDS, and 21-item Beck Depression Inventory (BDI) were used for assess-ment. Before random assignment, all the consenting par-ticipants received a single-blind placebo lead-in trial for 1 week. Those who showed a decrease of 20% or more in HAM-D scores (placebo responders) would not proceed to the randomization phase. After the placebo lead-in phase, participants were randomly assigned (at week 0) to receive 5 identical gelatin capsules per day containing ei-ther omega-3 fatty acids or placebo (olive oil ethyl esters) for 8 weeks. The capsules for the treatment group con-tained a total daily dosage of omega-3 fatty acid with 2.2
g of EPA and 1.2 g of DHA, which were produced from menhaden fish body oil concentrate. The capsules (omega-3 fatty acid and placebo) were vacuum deodor-ized, amended by blending with orange flavor, and supplemented with tertiary butylhydroquinone, 0.2 mg/g, and tocopherols, 2 mg/g, as antioxidants.
We performed the HAM-D, EPDS, BDI, and a brief adverse-effect checklist at week –1 (placebo lead-in phase), week 0 (baseline), and weeks 2, 4, 6, and 8. Par-ticipants taking any antidepressants, antipsychotics, or mood stabilizers 1 month prior to the study were ex-cluded, and no psychotropic agents were given during the study period. Blood samples were taken for omega-3 PUFA analysis at week –1 and week 8, with which each individual red blood cell fatty acid was analyzed with gas chromatography of methyl esters. The detailed step-by-step laboratory procedures were described elsewhere.51
Outcome Measures and Statistical Analysis
All participants after randomization were included in the analysis of illness characteristics and the incidence of treatment-emergent adverse events. The primary mea-surement of efficacy was the HAM-D, while the EPDS and BDI were the secondary measures, and statistical
analyses were done between 2 groups. The intention-to-treat (ITT) population included all patients who had a baseline and at least 1 postbaseline observation, while the per-protocol population included all patients who completed 8 weeks of treatment. Biweekly changes in HAM-D total scores from baseline to endpoint were ana-lyzed as the primary outcome. Treatment responders were characterized descriptively as those who improve at least 50% from baseline in HAM-D. Patients with remission were defined as those who had a HAM-D score of 7 or less. Categorical variables, such as the remission rate and response rate, were analyzed by using χ2
tests.
Differences of rating scores between placebo and omega-3 groups at each visit point were assessed by an independent-samples t test. The statistical model used to compare differences of outcome measurements at end-point between groups was the analysis of covariance with treatment group as the main effect and the baseline score as the covariate. The effect of the omega-3 PUFAs was examined with repeated-measures analysis of variance us-ing time as the repeated factor, treatment groups (placebo or omega-3 PUFA) as the independent factor, and clinical data (age, years of education, duration of current episode, number of previous depressive episodes, number of previ-Figure 1. Flowchart of Subject Screening and Enrollment
Abbreviations: DSM-IV = Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; EPDS = Edinburgh Postnatal Depression Scale.
Screened by Psychiatric Research Nurse
(N = 1048)
EPDS Score ≥12 (N = 126)
Met DSM-IV Criteria for Major Depressive Disorder
(N = 71) Randomization (N = 36) Omega-3 (N = 18) Placebo (N = 18) Completers (N = 13) Completers (N = 11) Excluded (N = 922)
1. Did not complete the EPDS (12/1048) 2. EPDS Score < 12 (910/1036)
Excluded (N = 55)
1. Refused to meet psychiatrists for structured interview (N = 40) 2. Structurally interviewed but did not meet DSM-IV criteria for
major depressive disorder (N = 15)
Excluded (N = 35)
1. Refused to participate (N = 31): Disagreement with diagnosis (15/31) Disagreement with necessity of treatment (9/31) Concerns of adverse effects of treatment (7/31) 2. Placebo responders at placebo lead-in phase (4/40)
Discontinued (N = 5)
1. Did not return for any follow-up visit (excluded from intent-to-treat analysis, N = 1) 2. Did not return before completion (N = 1) 3. Unsatisfactory response (N = 3)
Discontinued (N = 7)
1. Did not return for any follow-up visit (excluded from intent-to-treat analysis, N = 2) 2. Did not return before completion (N = 1) 3. Unsatisfactory response (N = 3) 4. Severe nausea (N = 1)
ous instances of gestation and/or abortion, and baseline red blood cell omega-3 PUFA compositions) as covar-iates. A mixed-model analysis of variance approach was applied for repeated-measurement analysis. The missing values in the ITT population were handled by using the PROC MI procedure of the SAS statistical software to implement the expectation-maximization algorithms. Data were analyzed using SAS statistical software, ver-sion 8.02 (SAS Institute, Inc., Cary, N.C.). A value of p less than .05 is considered statistically significant.
RESULTS Disposition and Demographics
One thousand forty-eight pregnant women were screened by a psychiatric research nurse; 86 of 126 women with EPDS score more than 12 agreed to be structurally interviewed by psychiatrists, and 71 met the DSM-IV diagnosis of major depressive disorder. Forty women consented to participate in the study initially. The reasons for refusal to participate were patients’ ment with having a depressive disorder (15/31), disagree-ment with necessity of treatdisagree-ment (9/31), and concerns of adverse effects of treatment (7/31).
Figure 1 provides a detailed flow chart summarizing study recruitment. Four placebo responders (10%) were withdrawn by the investigators because of a greater than
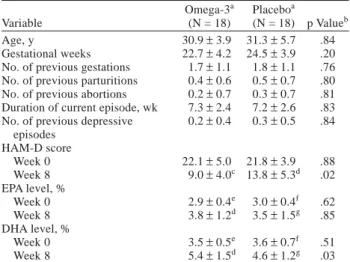
20% decrease in the HAM-D score at placebo lead-in phase. The remaining 36 subjects were then randomly as-signed to either the omega-3 group (N = 18) or the pla-cebo group (N = 18). Among these, a total of 33 patients (N = 17 in the omega-3 group, N = 16 in the placebo group) who had been evaluated in more than 2 visits were included in the ITT analysis. With a dropout of 12 sub-jects, 24 patients (N = 13 in the omega-3 group, N = 11 in the placebo group) completed this 8-week study, and these data were used for per-protocol analysis. Five of the dropouts were from the omega-3 group (2 failed to return in follow-up and 3 withdrew consent for unsatisfactory response), while 7 were from the placebo group (3 failed to return, 3 withdrew consent for unsatisfactory response, and 1 withdrew consent for severe nausea). Nine of the omega-3 group and 6 of the placebo group were willing to take open-label omega-3 fatty acids after the study period (including early dropout) until the delivery. Table 1 shows no statistical differences in demographics, clinical charac-teristics, or omega-3 PUFA composition on red blood cells.
Therapeutic Outcomes
As shown in Figure 2, participants in the omega-3 group differed significantly from those in the placebo group in the mean HAM-D score at week 6 and week 8. We used the mixed-model analysis of variance approach for repeated-measurement analyses in the ITT population, and the results indicated that biweekly changes in the scores of HAM-D (F = 7.467, p = .010), EPDS (F = 4.976, p = .033), and BDI (F = 4.695, p = .038) in the Table 1. Demographics, Clinical Characteristics, and
Outcomes (HAM-D, erythrocyte EPA, and DHA) of Pregnant Women Before and After Treatment for Major Depressive Disorder
Omega-3a Placeboa
Variable (N = 18) (N = 18) p Valueb
Age, y 30.9±3.9 31.3±5.7 .84
Gestational weeks 22.7±4.2 24.5±3.9 .20
No. of previous gestations 1.7±1.1 1.8±1.1 .76 No. of previous parturitions 0.4±0.6 0.5±0.7 .80 No. of previous abortions 0.2±0.7 0.3±0.7 .81 Duration of current episode, wk 7.3±2.4 7.2±2.6 .83 No. of previous depressive 0.2±0.4 0.3±0.5 .84
episodes HAM-D score Week 0 22.1±5.0 21.8±3.9 .88 Week 8 9.0±4.0c 13.8±5.3d .02 EPA level, % Week 0 2.9±0.4e 3.0±0.4f .62 Week 8 3.8±1.2d 3.5±1.5g .85 DHA level, % Week 0 3.5±0.5e 3.6±0.7f .51 Week 8 5.4±1.5d 4.6±1.2g .03
aMean±SD; case numbers of both groups were 18 unless otherwise
specified.
bIndependent samples t test results. cN = 13.
dN = 11. eN = 15. fN = 14. gN = 10.
Abbreviations: DHA = docosahexaenoic acid, EPA = eicosapentaenoic acid, HAM-D = 21-item Hamilton Rating Scale for Depression.
Figure 2. Evolution of the 21-Item Hamilton Rating Scale for Depression (HAM-D) Scores in Pregnant Women With Depression Treated With Omega-3 PUFAs or Placebo During the Study Perioda
aThe significant differences were noted at week 6 (p = .001)* and
week 8 (p = .019)** by independent-samples t test. All values represent the intent-to-treat population. Biweekly changes in HAM-D scores illustrate a significantly greater decline in the omega-3 group by the mixed-model analysis of variance approach (F = 7.467, p = .010). *p = .001. **p = .019. 25 20 15 10 5
Mean HAM-D Score
Placebo (N = 16) Omega-3 (N = 17) –1 0 1 2 3 4 5 6 7 8 Week of Treatment ** *
omega-3 group showed significantly greater declines. On the other hand, the interactions between time and treat-ment assigntreat-ment for those aforetreat-mentioned outcome mea-surements were not significant.
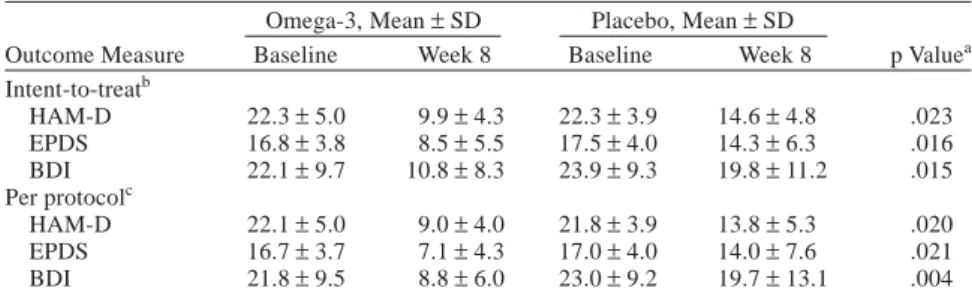
In Table 2, primary and secondary outcome measures at the final study end point are shown with ITT and per-protocol analysis. Also, Table 3 presents a significantly higher response rate in the omega-3 group at weeks 6 and 8 (compared with placebo) and a higher remission rate for the omega-3 group at weeks 4, 6, and 8, but the differences in remission rate did not reach statistical significance. Adverse Events
No participant was withdrawn because of adverse events by investigators’ decision, and 12 of 18 in the pla-cebo group and 10 of 18 in the omega-3 fatty acid group reported no adverse events. The events attributed to treat-ment were insomnia (2 in the placebo group and 3 in the omega-3 group), nausea (4 in the placebo group and 6 in the omega-3 group), and diarrhea (2 in the placebo group and 1 in the omega-3 group). Reported events were mostly mild and self-limited, except 1 occurrence of severe nau-sea in the placebo group that terminated the treatment. There was no effect found in any blood laboratory
param-eter, such as abnormal bleeding time or liver function. No obstetric complication was noted in any participant, and all the newborns were normal in general physical and neurobehavioral examination at birth.
DISCUSSION
This is the first double-blind placebo-controlled trial of omega-3 PUFAs in pregnant women with depression. This is also the first double-blind placebo-controlled trial to demonstrate omega-3 PUFAs’ monotherapy effect on depression, as several other double-blind placebo-controlled trials were done as adjunct therapy.37–41
We found that omega-3 PUFA monotherapy significantly im-proved depressive symptoms in pregnant women with major depressive disorder. The antidepressant effect of omega-3 PUFAs was significantly better than placebo af-ter week 6, a result that appeared to show a delayed effec-tiveness of omega-3 PUFAs, compared to the results of 2 to 4 weeks in nonpregnant patients with major depression reported in previous studies.37–39
The remission rate did not reach a significant difference between the omega-3 and placebo groups. Furthermore, the early dropout rate was high (8/36 for the first 4 weeks) in this study. One Table 2. Effects of Treatment on Total Scores of HAM-D, EPDS, and BDI
Omega-3, Mean±SD Placebo, Mean±SD
Outcome Measure Baseline Week 8 Baseline Week 8 p Valuea
Intent-to-treatb HAM-D 22.3±5.0 9.9±4.3 22.3±3.9 14.6±4.8 .023 EPDS 16.8±3.8 8.5±5.5 17.5±4.0 14.3±6.3 .016 BDI 22.1±9.7 10.8±8.3 23.9±9.3 19.8±11.2 .015 Per protocolc HAM-D 22.1±5.0 9.0±4.0 21.8±3.9 13.8±5.3 .020 EPDS 16.7±3.7 7.1±4.3 17.0±4.0 14.0±7.6 .021 BDI 21.8±9.5 8.8±6.0 23.0±9.2 19.7±13.1 .004
ap Values are based on the pairwise comparisons from the analysis of covariance model with
treatment as the main effect and baseline as the covariate.
bN = 17for the omega-3 group; N = 16 for the placebo group. cN = 13 for the omega-3 group; N = 11 for the placebo group.
Abbreviations: BDI = 21-item Beck Depression Inventory, EPDS = Edinburgh Postnatal Depression Scale, HAM-D = 21-item Hamilton Rating Scale for Depression.
Table 3. Response Rate and Remission Rate Defined by the Change of Hamilton Rating Scale for Depression Score
Variable Week 0 Week 2 Week 4 Week 6 Week 8
Patients with 50% reduction, N/N (%)
Omega-3 0/18 (0) 3/17 (18) 6/14 (43) 9/14 (64) 8/13 (62)
Placebo 0/18 (0) 3/16 (19) 2/14 (14) 2/12 (17) 3/11 (27)
p Valuea … .94 .09 .01 .03
Patients with score≤7, N/N (%)
Omega-3 0/18 (0) 1/17 (6) 2/14 (14) 5/14 (36) 5/13 (38)
Placebo 0/18 (0) 1/16 (6) 1/14 (7) 2/12 (17) 2/11 (18)
p Valuea … .97 .54 .28 .28
aχ2 test results.
possible explanation is that the antidepressant effect of omega-3 PUFAs was not as effective in pregnant women as in nonpregnant depressed patients. The other explana-tion is that the participants in this study were aware and expecting the continuous open-label omega-3 PUFA treat-ment when the study ended. We also observed that most participants expressed a high interest in the “nutrition therapy” option when entering this study, which might lead to a choice not to continue the trial eventually if they did not experience the improvement they expected and guessed they were in the placebo arm.
There are 2 potential mechanisms of omega-3 PUFAs’ effect on depression during pregnancy. First, a profound decrease of omega-3 PUFAs in pregnant women due to the high demand of fetal development23,24
might precipi-tate depression43
because omega-3 PUFAs’ deficit can in-duce serotonergic dysfunctions52–54
and has been reported extensively in patients with major depressive disorder.29–33
Another possible mechanism is that omega-3 PUFAs play an important role in mood stabilization by targeting parts of the “arachidonic acid cascade.”55
The arachidonic acid cascade hypothesis in mood disorders has been supported by other studies, including the higher levels of arachi-donic acid and increased activity of phospholipase A2, a
major metabolic enzyme of arachidonic acid, in patients with mood disorders32,33,51,56
and the inhibitory effect on phospholipase A2 activity of mood stabilizers.
57–60
The treatment was well tolerated with few adverse events. The only patient who withdrew from the study did so due to severe nausea and was in the placebo group. This finding is consistent with the finding in the Freeman et al.44
open-label trial that no dropout occurred due to omega-3 PUFAs’ side effects in 15 pregnant depressed patients. Also, there was no obstetric or newborn compli-cation noted, which is very important because the adverse effects of medication to the fetus are the major concern for women when considering pharmacotherapy during pregnancy.61,62
In the light of an ideal treatment for perina-tal depression, which should be harmless for both mothers and newborns, in uterus and even during breastfeeding,63
omega-3 fatty acids have established their safety and ben-efits during pregnancy45,64
and thus deserve further at-tention of clinical study and application as a treatment modality for perinatal depression.
There are matters needing further attention when ap-plying the findings of this study to clinical practice. First, the optimal composition and the dosage of EPA and DHA for treatment of depression during pregnancy require fur-ther exploration. It has been suggested that fur-there is a pos-sible dose-dependent relation of the antidepressant effect of EPA; however, heterogeneity in the severity of depres-sion, difference in the body EPA and DHA composition, and dietary intake of fish should be taken into account.36
Taiwan is a high fish-consuming country,28
and pregnant women generally consume even more fish for a better
source of nutrition as suggested.65
When compared with previous clinical trials for nonpregnant depressed popula-tions, the daily dose used in this study (3.4 g per day) was higher than the effective dose of clinical studies from the United Kingdom (1 to 2 g/day)39
or Israel (2 g/day),38
but lower than that of our previous study (6.6 g/day) in Tai-wan.37
Being aware of the relationship between depres-sion and low fish intake in some epidemiologic studies, it will be of interest to know if pregnant depressed patients, who had a low content of bodily omega-3 PUFAs, will re-spond to a lower dose than nonpregnant depressed pa-tients with normal contents. Second, the supplement of omega-3 PUFAs in this study induced a significant in-crease in the DHA level, but not the EPA level. This could result from the small sample size; however, it might imply that DHA level had a stronger relationship with depres-sion than EPA did. The correlations of omega-3 fatty acid concentrations and behavioral parameters in the rat model of depression have been examined, and it was found that the level of brain DHA, not EPA, was negatively cor-related to depression-like behaviors.66
Furthermore, the changes of EPA and DHA levels in this study might not reach a steady state because that is expected to take more than 12 weeks.67
Finally, caution should be taken because the high discontinuation rate (33%) and the lack of infor-mation about compliance might bias our results.
To date, no psychotropic drugs have been approved to be safe during pregnancy and breast-feeding, which chal-lenges psychiatrists with the difficult task of recommend-ing pharmacotherapy to pregnant patients.68
Knowledge of risks of psychotropic medications to the fetus of prena-tal exposure is still limited, and it is neither feasible nor ethical to conduct prospective, case-control studies for the benefits and risks of antidepressant agents during pregnancy. Thus, the appropriate data from alternative, nondrug treatments are important. In regard to the safety issue and psychotherapeutic effect, as well as health pro-motion to mothers and newborns, omega-3 PUFAs are a promising agent for patients with major depressive dis-order during pregnancy. Replicated studies in a larger sample with a broad regimen of dose and composition of omega-3 PUFAs in pregnant depression are needed before we could suggest omega-3 PUFAs as the first-line treat-ment for depressive disorders during pregnancy.
REFERENCES
1. Wisner KL, Zarin DA, Holmboe ES, et al. Risk-benefit decision making for treatment of depression during pregnancy. Am J Psychiatry 2000;157: 1933–1940
2. Lee DT, Chan SS, Sahota DS, et al. A prevalence study of antenatal depression among Chinese women. J Affect Disord 2004;82:93–99 3. Evans J, Heron J, Francomb H, et al. Cohort study of depressed mood
during pregnancy and after childbirth. BMJ 2001;323:257–260 4. Kitamura T, Sugawara M, Shima S, et al. Temporal variation of validity
of self-rating questionnaires: improved validity of repeated use of Zung’s Self-Rating Depression Scale among women during the perinatal period. J Psychosom Obstet Gynaecol 1999;20:112–117
5. Kumar R, Robson KM. A prospective study of emotional disorders in childbearing women. Br J Psychiatry 1984;144:35–47
6. Gaynes BN, Gavin N, Meltzer-Brody S, et al. Perinatal depression: prev-alence, screening accuracy, and screening outcomes. Evid Rep Technol Assess (Summ) 2005;119(Feb):1–8
7. Gotlib IH, Whiffen VE, Mount JH, et al. Prevalence rates and demo-graphic characteristics associated with depression in pregnancy and the postpartum. J Consult Clin Psychol 1989;57:269–274
8. O’Hara MW, Neunaber DJ, Zekoski EM. Prospective study of postpar-tum depression: prevalence, course, and predictive factors. J Abnorm Psychol 1984;93:158–171
9. Hedegaard M, Henriksen TB, Sabroe S, et al. Psychological distress in pregnancy and preterm delivery. BMJ 1993;307:234–239
10. Pagel MD, Smilkstein G, Regen H, et al. Psychosocial influences on new born outcomes: a controlled prospective study. Soc Sci Med 1990;30: 597–604
11. Wadhwa PD, Sandman CA, Porto M, et al. The association between prenatal stress and infant birth weight and gestational age at birth: a prospective investigation. Am J Obstet Gynecol 1993;169:858–865 12. Teixeira JM, Fisk NM, Glover V. Association between maternal anxiety
in pregnancy and increased uterine artery resistance index: cohort based study. BMJ 1999;318:153–157
13. Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA 2006;295:499–507
14. Moses-Kolko EL, Bogen D, Perel J, et al. Neonatal signs after late in utero exposure to serotonin reuptake inhibitors: literature review and implications for clinical applications. JAMA 2005;293:2372–2383 15. Chambers CD, Johnson KA, Dick LM, et al. Birth outcomes in pregnant
women taking fluoxetine. N Engl J Med 1996;335:1010–1015 16. Suri R, Altshuler L, Hellemann G, et al. Effects of antenatal depression
and antidepressant treatment on gestational age at birth and risk of preterm birth. Am J Psychiatry 2007;164:1206–1213
17. Freeman MP. Antenatal depression: navigating the treatment dilemmas. Am J Psychiatry 2007;164:1162–1165
18. Rubin R. The baby or the drug? it’s a choice that many pregnant women often face—but shouldn’t. US News and World Report Mar 27, 1995:59 19. Torpy JM, Lynm C, Glass RM. JAMA patient page. Eating fish: health
benefits and risks. JAMA 2006;296:1926
20. Akabas SR, Deckelbaum RJ. Summary of a workshop on n-3 fatty acids: current status of recommendations and future directions. Am J Clin Nutr 2006;83:1536S–1538S
21. Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA 2006;296:1885–1899 22. Burr GO, Burr MM. A new deficiency disease produced by the rigid
exclusion of fat from the diet. J Biol Chemistry 1929;82:345–367 23. Hornstra G. Essential fatty acids in mothers and their neonates. Am J
Clin Nutr 2000;71:1262S–1269S
24. Al MD, van Houwelingen AC, Hornstra G. Long-chain polyunsaturated fatty acids, pregnancy, and pregnancy outcome. Am J Clin Nutr 2000;71:285S–291S
25. Horrobin DF, Bennett CN. Depression and bipolar disorder: relationships to impaired fatty acid and phospholipid metabolism and to diabetes, cardiovascular disease, immunological abnormalities, cancer, ageing and osteoporosis: possible candidate genes. Prostaglandins Leukot Essent Fatty Acids 1999;60:217–234
26. Tanskanen A, Hibbeln JR, Tuomilehto J, et al. Fish consumption and depressive symptoms in the general population in Finland. Psychiatr Serv 2001;52:529–531
27. Tanskanen A, Hibbeln JR, Hintikka J, et al. Fish consumption, depres-sion, and suicidality in a general population. Arch Gen Psychiatry 2001;58:512–513
28. Hibbeln JR. Fish consumption and major depression. Lancet 1998;351:1213
29. Peet M, Murphy B, Shay J, et al. Depletion of omega-3 fatty acid levels in red blood cell membranes of depressive patients. Biol Psychiatry 1998;43:315–319
30. Adams PB, Lawson S, Sanigorski A, et al. Arachidonic acid to eicosapentaenoic acid ratio in blood correlates positively with clinical symptoms of depression. Lipids 1996;31(suppl):S157–S161 31. Edwards R, Peet M, Shay J, et al. Omega-3 polyunsaturated fatty acid
levels in the diet and in red blood cell membranes of depressed patients. J Affect Disord 1998;48:149–155
32. Maes M, Smith R, Christophe A, et al. Fatty acid composition in major depression: decreased omega 3 fractions in cholesteryl esters and in-creased C20: 4 omega 6/C20:5 omega 3 ratio in cholesteryl esters and phospholipids. J Affect Disord 1996;38:35–46
33. Maes M, Christophe A, Delanghe J, et al. Lowered omega3 polyunsatu-rated fatty acids in serum phospholipids and cholesteryl esters of depressed patients. Psychiatry Res 1999;85:275–291
34. Logan AC. Neurobehavioral aspects of omega-3 fatty acids: possible mechanisms and therapeutic value in major depression. Altern Med Rev 2003;8:410–425
35. Freeman MP, Hibbeln JR, Wisner KL, et al. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry. J Clin Psychiatry 2006;67:1954–1967
36. Lin PY, Su KP. A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. J Clin Psychiatry 2007;68:1056–1061
37. Su KP, Huang SY, Chiu CC, et al. Omega-3 fatty acids in major depres-sive disorder: a preliminary double-blind, placebo-controlled trial. Eur Neuropsychopharmacol 2003;13:267–271
38. Nemets B, Stahl Z, Belmaker RH. Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. Am J Psychiatry 2002;159:477–479
39. Peet M, Horrobin DF. A dose-ranging study of the effects of ethyl-eicosapentaenoate in patients with ongoing depression despite apparently adequate treatment with standard drugs. Arch Gen Psychiatry 2002;59: 913–919
40. Frangou S, Lewis M, McCrone P. Efficacy of ethyl-eicosapentaenoic acid in bipolar depression: randomised double-blind placebo-controlled study. Br J Psychiatry 2006;188:46–50
41. Nemets H, Nemets B, Apter A, et al. Omega-3 treatment of childhood depression: a controlled, double-blind pilot study. Am J Psychiatry 2006;163:1098–1100
42. Chiu CC, Huang SY, Shen WW, et al. Omega-3 fatty acids for depression in pregnancy. Am J Psychiatry 2003;160:385
43. Su KP, Shen WW, Huang SY. The use of omega-3 fatty acids for the management of depression and psychosis during pregnancy and breast-feeding. In: Peet M, Glen I, Horrobin DF, eds. Phospholipid Spectrum Disorder in Psychiatry and Neurology. 2nd ed. Carnforth, United Kingdom: Marius Press; 2003:391–399
44. Freeman MP, Hibbeln JR, Wisner KL, et al. An open trial of omega-3 fatty acids for depression in pregnancy. Acta Neuropsychiatrica 2006;18:21–24
45. Olsen SF, Sorensen JD, Secher NJ, et al. Randomised controlled trial of effect of fish-oil supplementation on pregnancy duration. Lancet 1992;339:1003–1007
46. McNamara RK, Carlson SE. Role of omega-3 fatty acids in brain devel-opment and function: potential implications for the pathogenesis and prevention of psychopathology. Prostaglandins Leukot Essent Fatty Acids 2006;75:329–349
47. Cohen JT, Bellinger DC, Connor WE, et al. A quantitative analysis of prenatal intake of n-3 polyunsaturated fatty acids and cognitive develop-ment. Am J Prev Med 2005;29:366–374
48. Uauy R, Hoffman DR, Mena P, et al. Term infant studies of DHA and ARA supplementation on neurodevelopment: results of randomized controlled trials. J Pediatr 2003;143:S17–S25
49. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998;59(suppl 20):22–33
50. Su KP, Chiu TH, Huang CL, et al. Different cutoff points for different trimesters? the use of Edinburgh Postnatal Depression Scale and Beck Depression Inventory to screen for depression in pregnant Taiwanese women. Gen Hosp Psychiatry 2007;29:436–441
51. Chiu CC, Huang SY, Su KP, et al. Polyunsaturated fatty acid deficit in patients with bipolar mania. Eur Neuropsychopharmacol 2003;13:99–103 52. Delion S, Chalon S, Guilloteau D, et al. Age-related changes in
phospho-lipid fatty acid composition and monoaminergic neurotransmission in the hippocampus of rats fed a balanced or an n-3 polyunsaturated fatty acid-deficient diet. J Lipid Res 1997;38:680–689
53. Delion S, Chalon S, Guilloteau D, et al. Alpha-linolenic acid dietary deficiency alters age-related changes of dopaminergic and serotoninergic neurotransmission in the rat frontal cortex. J Neurochem 1996;66: 1582–1591
54. Delion S, Chalon S, Herault J, et al. Chronic dietary alpha-linolenic acid deficiency alters dopaminergic and serotonergic neurotransmission in rats. J Nutr 1994;124:2466–2475
55. Rapoport SI, Bosetti F. Do lithium and anticonvulsants target the brain arachidonic acid cascade in bipolar disorder? Arch Gen Psychiatry 2002;59:592–596
56. Noponen M, Sanfilipo M, Samanich K, et al. Elevated PLA2 activity in schizophrenics and other psychiatric patients. Biol Psychiatry 1993;34: 641–649
57. Chang MC, Contreras MA, Rosenberger TA, et al. Chronic valproate treatment decreases the in vivo turnover of arachidonic acid in brain phospholipids: a possible common effect of mood stabilizers. J Neurochem 2001;77:796–803
58. Rintala J, Seemann R, Chandrasekaran K, et al. 85 kDa cytosolic phospholipase A2 is a target for chronic lithium in rat brain. Neuroreport 1999;10:3887–3890
59. Chang MC, Jones CR. Chronic lithium treatment decreases brain phospholipase A2 activity. Neurochem Res 1998;23:887–892 60. Ghelardoni S, Tomita YA, Bell JM, et al. Chronic carbamazepine
selectively downregulates cytosolic phospholipase A2 expression and cyclooxygenase activity in rat brain. Biol Psychiatry 2004;56:248–254 61. Lamberg L. Risks and benefits key to psychotropic use during
pregnancy and postpartum period. JAMA 2005;294:1604–1608
Editor’s Note: We encourage authors to submit papers for
consideration as a part of our Focus on Women’s Mental Health section. Please contact Marlene Freeman, M.D., at mfreeman@psychiatrist.com.
62. Einarson A, Selby P, Koren G. Abrupt discontinuation of psychotropic drugs during pregnancy: fear of teratogenic risk and impact of counsel-ling. J Psychiatry Neurosci 2001;26:44–48
63. Freeman MP. Omega-3 fatty acids and perinatal depression: a review of the literature and recommendations for future research. Prostaglandins Leukot Essent Fatty Acids 2006;75:291–297
64. Blanchard DS. Omega-3 fatty acid supplementation in perinatal settings. MCN Am J Matern Child Nurs 2006;31:250–256
65. Hsu CS, Liu PL, Chien LC, et al. Mercury concentration and fish consumption in Taiwanese pregnant women. BJOG 2007;114:81–85 66. Huang SY, Yang HT, Chiu CC, et al. Omega-3 fatty acids on the
forced-swimming test. J Psychiatr Res 2008;42:58–63
67. Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am J Clin Nutr 2006;83: 1467S–1476S
68. Hampton T. Antidepressants and pregnancy: weighing risks and benefits no easy task. JAMA 2006;295:1631–1633