以高重力填充床吸收揮發性有機物質之研究
Absor ption of VOCs in Rotating Packed Bed 計 劃 編 號 : NSC 89-2214-E-002-034 執 行 期 限 : 88/08/01~89/07/31 主 持 人 : 劉 懷 勝 台 灣 大 學 化 工 系 教 授 中文摘要 (關鍵詞:吸收、有機揮發物、轉填 充床、高重力場、質傳係數) 本研究主要是利用旋轉填充床吸收空氣中 的揮發性有機物。新發展的旋轉填充床,是藉 由氣、液在高重力場下,使流動處於較高的加 速狀態。因此,旋轉填充床可降低系統溢流可 能性、縮短氣液兩相平衡時間、增加處理量、 改善質傳效果、降低空間需求。實驗結果顯示, 質傳係數會隨著轉速的增加而增大,約為轉速 的 0.28 到 0.51 次方;若與傳統填充床的經驗式 和實驗值比較,則高重力場下的 KGa 則大約高 了一個數量級之多。
英文摘要 (keyword: Absorption, VOC, Rotating
Packed Bed, Higee, Mass Transfer Coefficient)
A Higee absorption process was developed
for removing VOCs (Volatile Organic
Compounds) from air into aqueous phase under a centrifugal field. A rotating packed bed, which replaces gravity with centrifugal force, was adopted in this study. Under a centrifugal field, the operating range can be covered in higher gas and liquid flow rates due to the reduced tendency to flood, and mass transfer was also enhanced. The experimental results showed that the mass transfer coefficient was increased with increasing rotor speed, to the power ranging from 0.28 to
0.51. Comparing with the model-predicted values and the experimental data of conventional packed beds, KGa obtained from our experiment was
approximately an order of magnitude higher.
INTRODUCTION
In recent years, there have been many studies on mass transfer intensification of various systems in the high gravity system (i.e. Higee),
such as absorption, stripping, adsorption,
distillation, and extraction. The higee system, developed by Ranshaw and Mallinson (1981), basically is a rotating packed bed that replaced gravity with centrifugal force. Under a centrifugal field, the liquid film and the mass transfer
boundary layer may become thinner.
Consequently, the effective wetted surface area of the packing may be increased. Moreover, the system may be operated in higher ratio of gas and liquid rates due to the reduced tendency of flooding. These features would contribute to process intensification; mainly by enhancing mass transfer dramatically and reducing the equipment size significantly.
Absorption, a separation technique to remove VOCs, is to transfer gaseous pollutants to liquid absorbent in a packed column or a bubble
column. Several literatures have been published about the adoption of centrifugal field in the packed bed for absorption. Ramshaw and Mallinson (1981) performed an experiment of absorbing NH3 into water, and found that the gas
phase mass transfer coefficients were 4-9 times higher than those conventional packed beds. Keyvani and Gardner (1989) investigated an air-CO2-H2O system. The liquid phase volumetric
mass transfer coefficient increased with rotational speed to the power of 0.6-0.7. And an average height of transfer unit could be reduced to 1.5-4.0 cm, which means a reduction of the packed tower height up to two orders of magnitude. Kumer and Rao (1990) also adopted a CO2-NaOH solution
system and found that the overall liquid phase volumetric mass transfer coefficients were an order of magnitude higher than those in conventional packed beds. Guo et al. (1997) constructed a cross-flow rotating packed bed. They performed experiments of absorption of NH3 in water and absorption of SO2 in
ammonium sulfite solution. Their results showed that the overall gas phase volumetric mass transfer coefficients were not influenced by rotational speed when centrifugal acceleration was above 15g. However, there is no literature concerning absorbing VOCs in a rotating packed bed. Thus, the purpose of this study is to evaluate the centrifugal force on the efficiency of VOCs removal by absorption.
EXPERIMENT
Fig. 1 shows the diagram of the
experimental setup. Fresh water at 25℃ was pumped into the rotating packed bed. An air stream was introduced to a bubbler containing liquid VOCs, and was diluted by another air stream to the desired VOC concentration. The buffer flask in fact is an empty flask to maintain
stable gas concentration. Then the water and the gas stream contacted countercurrently in a rotating packed bed. Two VOCs, i.e. isopropyl alcohol and acetone, were used in this study. The concentration of inlet and outlet gas streams were measured by a gas chromatography. In the rotating packed bed, the packings used in this study were 2-mm diameter plastic beads, whose total specific surface area, at, was 1200 m
2
/m3
, and porosity,ε, was 0.4. The axial height of the bed was 2 cm. The inner and outer radiuses of the bed were 2 and 4 cm, respectively. The bed can be operated from 150 to 2100 rpm.
RESULTS AND DISSCUSION
Fig. 2 shows the dependence of VOC removal efficiency on rotor speed. It is obvious that the removal efficiency increases as rotor speed increases within less than 1 second contact time (Guo et al., 2000). This characteristic implies better mass transfer under a high centrifugal field.
The design equation, similar to the one derived by Liu et al. (1996), can be also obtained using mass balance and the transfer unit concept:
(
)
1 A 1 A 1 X H Y X H Y A 1 1 ln r r ð zp G a K 2 y 2 2 y 1 2 1 2 2 t m G − + − − − − = (1)where A is the absorption factor defined as
m y m G H L A= (2)
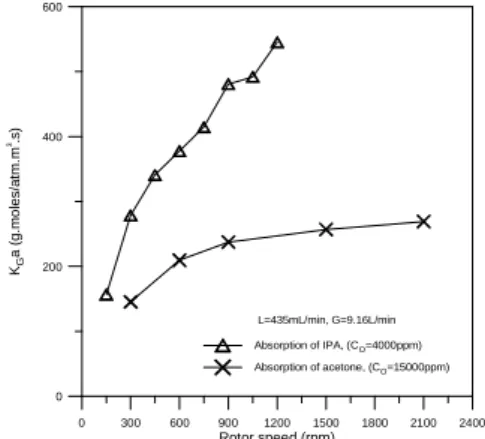
Fig. 3 shows the dependence of KGa on rotor
speed. The mass transfer coefficient could be increased by increasing rotor speed. In other words, the centrifugal force reduces mass transfer resistance. If KGa is expressed as a power law of
x
Ga ù
K ∝ (3) The values of x generally increase with the increase of liquid and gas flow rates and ranges from 0.28 to 0.51.
For conventional packed beds, there have been many correlations to predict a mass transfer coefficient. Eq. (4) is the correlation for the gas phase mass transfer coefficient provided by Onda et al. (1968).
(
)
2 P t 1/3 G 0.7 G G t G d a Sc 2Re D a k = − (4)The gas-liquid interfacial area, a, was also provided by Onda et al. (1968).
− − = − 0.2 L 0.05 L 0.1 L 0.75 C t We Fr Re ó ó 1.45 exp 1 a a (5)
An overall gas phase volume mass transfer coefficient, KGa, may be expressed in terms of the
two film coefficients as follows:
a k H a k 1 a K 1 L G G + = (6) where kL is given by Onda et al. (1968).
( )
0.4 p t 0.5 L L L 2/3 L 1/3 L L L ad D ñ ì aì L' 0.0051 g ì ñ k − = (7)With Eq. (4) to Eq. (7), the model-predicted value for conventional packed beds, (KGa)c, can be
calculated. The comparison of KGa vs. (KGa)c is
shown in Fig. 4. It is obvious that these calculated values for conventional packed beds are lower than our higee results. The ratio of the higee experimental values to conventional correlations ranges from 1.15 to 7. This indicates that centrifugal force did enhance the mass transfer of the VOC absorption.
Houston and Walker (1950) performed an experiment of absorbing acetone by water in a conventional packed bed. The height and
diameter of the bed were 2 ft and 12 inch. The packings were 1-inch Raschig rings. Their results are shown in Fig. 5. The KGa obtained from
Houston and Walker is approximately an order magnitude lower than this higee experimental results.
CONCLUSION
According to the results of our experiment, it is clear that centrifugal force can intensify the mass transfer during VOC absorption in a rotating packed bed. The concentration of the outlet VOC would be reduced to very low level with aid of a centrifugal force during very short contact time. Compared with conventional packed beds, KGa of our experimental rotating
packed bed can be up to several folds higher than the model-predicted values, and is an order magnitude higher than the experimental data for a conventional bed. This result is confirmed to Ramshaw’s (1981) higee experiment, which showed the gas phase mass transfer coefficients were 4-9 times higher than the conventional ones. Therefore, a rotating packed bed indeed is a potential process intensification apparatus for absorption of VOCs.
REFERENCE
Guo, F., C. Zheng, K. Guo, Y. Feng, and N. C. Gardner, “Hydrodynamics and Mass Transfer in
Cross-flow Rotating Packed Bed”, Chem. Eng.
Sci. 21/22, 3853 (1997).
Guo, K., F. Guo, Y. Feng, J. Chen, C. Zheng, N. C. Gardner, “Synchronous Visual and RTD Study on Liquid Flow in Rotating Packed-Bed
Air In Compressor Rotameter Water Bath Gas Out Rotameter Water Liquid Out Pump Rotor Bubbler
Gas-Collecting Tube VOC
Buffer Flask
0.01 0.10 1.00
gas mass flux (Kg/m2
.s) 0 200 400 600 KG a (g .m o le s /a tm .m 3.s ) L'=0.68 Kg/m2 .s L'=1.36 Kg/m2 .s L'=2.71 Kg/m2 .s L'=4.07 Kg/m2 .s L'=1.19 Kg/m2 .s, w=900rpm L'=1.19 Kg/m2 .s, w=2100rpm L'=2.81 Kg/m2 .s, w=900rpm L'=2.81 Kg/m2 .s, w=2100rpm
(rotating packed bed)
(conventional packed bed)
Houston, R. W. and C. A. Walker, “Absorption in Packed Tower-Effect of Molecular Diffusivity on Gas Film Coefficient”, Ind. Eng. Chem., 42, 1105
(1950).
Keyvani, M. and N. C. Gardner, “Operating Characteristics of Rotating Beds”,Chem. Eng. Prog., 85, 48 (1989).
Kumar, M. P. and D. P. Rao, “Studies on a High-Gravity Gas-Liquid Contactor”, Ind. Eng. Chem. Res. 29, 917 (1990).
Liu, H. S., C. C. Lin, S. C. Wu, and H. W. Hsu, “Characteristics of a Rotating Packed Bed”, Ind. Eng. Chem. Res. 35, 3590 (1996).
Onda, K., H. Takeuchi, and Y. Okumoto, “Mass Transfer Coefficient between Gas and Liquid Phases in Packed Columns”, J. Chem. Eng. of Japan, 1, 56 (1968)
Ramshaw, C. and R. H. Mallison, “Mass Transfer Process”, United States Patent 4383255 (1981).
Fig. 1. The diagram of the experimental setup.
Fig. 2. The dependence of VOC removal efficiency on rotor speed.
Fig. 3. The dependence of KGa on rotor speed.
Fig. 4. Comparison of KGa of the experimental higee values with the
calculated values for conventional packed beds.
Fig. 5. The mass transfer coefficient of absorption of acetone in a rotating packed bed and in a conventional packed bed.
0 300 600 900 1200 1500 1800 2100 2400 Rotor speed (rpm) 80 85 90 95 100 R e m o v a l e ff ic ie n c y (% ) L=435mL/min, G=9.16L/min Absorption of IPA, (CO=4000ppm) Absorption of acetone, (CO=15000ppm) 0 300 600 900 1200 1500 1800 2100 2400 Rotor speed (rpm) 0 200 400 600 KG a (g .m o le s /a tm .m 3.s ) L=435mL/min, G=9.16L/min Absorption of IPA, (CO=4000ppm) Absorption of acetone, (CO=15000ppm) 0 200 400 600 KGa (g.moles/atm.m 3 .s) 0 200 400 600 (K G a )C (g .m o le s /a tm .m 3.s ) Absorption of IPA Absorption of acetone