Diffusivity of Microporous Carbon for Benzene and Methyl.Ethyl Ketone A d s o r p t i o n
H. L. Chiang*, P. C. Chiang **, Y. C. Chiang** and E.E. Chang*** *Department of Environmental Engineering, Fooyin Institute of Technology, 151,
Chin-Hsueh Road, Ta-Liao Hsiang, Kaoshsiung Hsien, 831,Talwan, ROC.
**Graduate Institute of Environmental Engineering, National Taiwan University, 71 Chou-Shan Road, Talpei, Talwan,R.O.C.
** * Department of Analytical Chemistry, Taipei Medical College, Talwan, ROC. (Received in Germany 4 June 1998; accepted 28 August 1998)
ABSTRACT
Kinetic studies results indicate that the pore diffusion coefficient is from 10 -6 to 10 -8 cm2/sec for both benzene and MEK in the temperature range of 30-120 oC. Under similar adsorbate concentration, MEK exhibits an effective pore diffusion coefficient greater than benzene. This may be attributed to its smaller molecular size being a polar molecular structure attracted to the activated carbon surface. In general, the effective diffusivity of MEK was greater than benzene under the same temperature and similar concentration. The influence of in fluent concentration on effective diffusion coefficient at low temperature is greater than that at high temperature. ©1999 Elsevier Science Ltd. All rights reserved
Key words : diffusivity, microporous carbon, benzene, methyl-ethyl ketone(MEK), adsorption
INTRODUCTION
1.Macropore diffusion
The importance of diffusion of heterogeneous catalysis in macroporc has made it a popular research subject. There are four kinds of macropore diffusion (1)molecular diffusion, (2)Knudsen diffusion, (3)Poiseuille flow and (4)surface diffusion. The diffusion mechanisms concern the pore structure distribution of adsorbents(I).
Mathematical relationships for each kind of macroporc diffusion arc shown below: (1)Molecular Diffusion. Dp - D, (1)
r
where Dp
is pore diffusivity, D,n is molecular diffusivity and x is the tortousity factor.Fuller, et al, derived the molecular diffusivity(Dm) of two components system as follows(2-5): 2733
D, 1.0xl0-3xT~7' ( 1 i )
(zoL +(zoL
where P the pressure, T the absolute temperature, (ZV)A and (ZU)B are the molecular volumes of gases A and B, respectively, and MAand M s are the gas molecular weights. Generally, the mean free path of molecules is smaller than the pore diameter of adsorbent and the transport mechanism is molecular diffusion.
(2)Knudsen Diffusion. Knudsen showed that under these conditions the diffusivity per unit cross-sectional area of pore is given by
Dk = ~ r v = 9700r (3)
where D k ,v ,T and M are Knudsen diffusivity, mean molecular velocity, absolute temperature and molecular weight; respectively.
(3) Poiseuille Flow. The Poiseuille flow diffusivity is shown as:
p-r I
D o - 8u
(4)
where Dpo is Poiseuille diffusivity, P is absolute pressure, rp is pore radius and p. is viscosity coefficient.
(4)Surface Diffusion. The overall diffusion coefficient is shown as:
+(1-6~] .KDs
(5)
where D,Dk,~p,K and D s are overall diffusivity, Knudsen diffusivity, adsorbent porosity, equilibrium constant, and surface diffusivity.
2.Micropore diffusion
The micropore diffusion is derived as follows(6):
8q
I d / 2 ~',
" 7 7 / " D -g) (6)
The initial and boundary condition are shown as follows:
q(r,0) = qo, & c , t ) - oo, ,.0
then
qo_qo=M~ --~ e x p - ~.Dot. (8)
and
3.Adsorption kinetic
Regarding transient diffusion and sorption, a mass balance on a small volume element within the grain combined with Fick~ law give the following differential material balance(7-8):
G O I ~ cE'\
where 6p is adsorbent porosity, C is the gas-phase concentration, t is time,pp is the density of the adsorbent solid part, q is sorbed mass, r is the sphere or cylinder radial coordinate, ~p is a shape factor equal to one for the cylindrical system and two for the spherical system and Dp is the gas diffusivity of sorbate molecules through the intragranular pore space.
If the sorbed-phase is favored in activated carbon system, then the first term in equation can be discarded. The local adsorption equilibrium, following the Freundlich equation, is assumed to describe the partitioning between gas and sorbed phases:
q = kC ~ (11)
where k and n are empirical constant.
8C' "C 'I-" 1 0
"=~-- = De(~oo ] • ~-;'~ (r" =-~) (12, where
6pDp C1-" (13)
is an effective diffusivity and
C o
is the influent concentration of adsorbates. The equation can be dimensionless as follows:~
c~0o,-°. 1 - - - L x ?-
X ~ ~X2
C X - r , o - ~ a ~ a n d
a
is the grain or fiber radius. The appropriate initial andwhere Q - C---~' boundary
conditions for adsorption procedure with external film resistance are
:
Q(O < X < i, o = O) = 0(-~-ff ) X.l " B (1- Qs )
. o( x.o.o)
For a desorption procedure, the corresponding conditions are:
Q(0 ,: x ,: 1, o - 0) - 0
(-~)X.l " B(l-Qs)
o .) - °
where Qs represents Q at the surface(X=l),B ..
kla
is the Biot number of mass transfer and k I is the gas- DpE t,film mass transfer coefficient. The relative mass uptake for adsorption (----~) ,or the loss for
where M® is the total mass uptake at a gas-phase concentration of C O and M o is the cumulative mass uptake at dimensionless time 0.
4Adsorption mechanism
A possible sequence of reaction steps in removing adsorbate from the air mixture, flowing into a granular activated carbon, is as foUows(9): mass transfer, surface diffusion, intragranular diffusion, physical adsorption, gas desorption, chemical reaction and surface renewal. Where the pro ducts of chemical reaction are volatile and poorly adsorbed, leaving the macropore region and entering the fluid stream surrounding the carbon granule where they are swept away from the activated carbon by mass transport.
Tien(10) modified the results of Jonas, and denoted the diffusion was the predominant mechanisms in activated carbon. Recent research on sorption by dry soil grains revealed that a diffusion process governs the rate at which gas-phase species reach the intragranular soil surface. The structural similarity between porous soil grains and activated carbon suggests that similar mechanisms may be controlling in both cases, and several related studies support this conclusion(11-15).
EXPERIMENTAL METHODS
KOWA COSMOS carbons(8x30 mesh) were used and made of coconut shells in this study. Five two- liters bottles made of polyethylene were each filled with 500 grams of activated ,-arbon and a liter each 1-5 N NaOH solution then placed in a rotating vibrator for 24 hours. The activated carbons were then dried in an oven at 105 o c for 48 hours. The activated carbon after NaOH treatment was divided into two parts by weight. One part inflow ozone concentration was held at 40 mg/L with a flow rate set at 2.5 L/min for 30 minutes. These procedures were performed in a water tank to control the reaction temperature of ozone and carbon below the combustion temperature. Activated carbons were washed with distilled water until the concentration of sodium ion of the rinsed water attained that of the distilled water. The carbons were stored in a dryer until analysis. The activated carbon was treated with the following: ozone, 1N NaOH and ozone, 2N NaOH and ozone, 3N NaOH and ozone, 4N NaOH and ozone and 5N NaOH and ozone. These were
presented as AC, AC(O3), AC(1N NaOH+O3), AC(2N NaOH+O3), AC(3N NaOH+O3), AC(4N NaOH+O3) and AC(5N NaOH+O3) , respectively.
Procedures for adsorption kinetic experiments and adsorption conditions are as follows: Fifty mg of activated carbon was placed in the balance connected to a data acquisition system. The influent concentration of benzene was from 372 to 2486 ppm and that of methyl ethyl ketone(MEK) was from 500 to 1470 ppm. Inflow rate of benzene and MEK vapor was set at 2.0L/rain and the adsorption temperatures were 30, 70 and 120°C. The adsorption kinetic test-run was repeated four times for all experimental concentrations.
Similar governing model equations with the boundary and initial conditions have been solved by several investigations. In this research, numeric method and least square of a Fortran program were used to solve the governing equation.
RESULTS AND DISCUSSIONS
Macropore diffusion
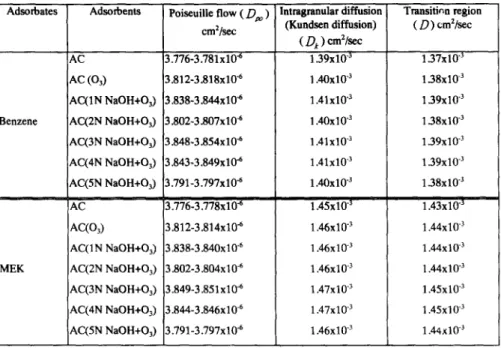
The diffusion of adsorbates in macropore- molecular diffusion, surface diffusion, Knudsen diffusion, and Poiseuille flow- are considered the diffusion mechanisms. The results of macropore diffusivity of benzene and MEK flow into seven kinds of activated carbon (AC, AC(O3), AC(1N NaOH+O3), AC(2N NaOH+O3), AC(3N NaOH+O3) , AC(4N NaOH+O3) and AC(5N NaOH+O3) ) that are shown as Table 1.
Molecular diffusion.
According to the Equation 2 ,molecular diffusivity concerns temperature, pressure, molecular weight and adsorbate characteristics. In this research, the molecular diffusivity of N2-C6H6 and N2-C4HsO system are 0.107 and 0.112 cm2/sec; respectively. The molecular weight and volume of MEK is smaller than benzene, so the molecular diffusivity of MEK is greater than benzene.Knudsen diffusion.
When the mean free path of adsorbate is greater than the pore diameter of activated carbon, the collision of molecules and the pore wall can inhibit molecule transport. Equation 3 shows that the Knudsen diffusivity is proportional to the pore radius, temperature and molecular weight. The analysis indicated that the Knudsen diffusivity of benzene is between 1.39"10 -3 and 1.41"10 -3 cm2/sec and that of MEK is between 1.45"10 .3 and 1.47"10 .3 cm2/sec. The Knudsen diffusivity is directly proportional to theTable 1. Macropore diffusivit~ o f benzene and M E K adsorb on activated carbons Adsorbates A d s o r b e n t s Poiseuille flow ( Dro ) Intragranular diffusion Transitic, n region
cm2/se c (Kundsen diffusion) (D) cm2/see
( D, ) cm2/sec
AC 3.776-3.781x10 "6 1.39x10 -3 1.37x10 "3
AC ( 0 3 ) 3.812-3.818x10 "6 1.40X10 -3 1.38x10 -3
AC(1N NaOH+O3) 3.838-3.844x10 "6 1.41x10 "3 1 39x10 "3
Benzene AC(2N NaOH+O3) 3.802-3.807xlff 6 1.40x10 "3 1.38x10 "3
AC(3N NaOH+O3) 3.848-3.854x10 "6 1.41x10 -~ 1 39x10 -3 AC(4N NaOH+O3) 3.843-3.849x10 "6 1.41x10 -3 1.39x10 -3 AC(5N NaOH+O3) 3.791-3.797x10 "6 1.40x10 "~ 1.38x10 -3 AC 13.776-3.778x10 "6 1.45x10 "3 1.43x10 "3 A C ( O 3 ) 3.812-3.814x10 "6 1.46x10 "3 1.44x10 3 AC(IN NaOH+O3) 3.838-3.840x10 "6 1.46x10 "3 1.44x10 3
MEK AC(2N NaOH+O3) 3.802-3.804x10 -6 1.46x10 3 1 . 4 4 x 1 0 "3
AC(3N NaOH+O3) 3.849-3.851x10 ~s 1.47x10 "3 1.45x10 -3
AC(4N NaOH+O3) 3.844-3.846x10 6 1.47x10 "3 1.45x10 3
AC(5N NaOH+O3) 3.791-3.797x10 "6 1.46x10 "3 1.44x10 -3
* The influent concentration of benzene is between 372 and 2486 ppm and that of M E K is between 500 and 1470 ppm.
Diffusion of transient region. The diffusivity of transient region calculates as: 1 - 1 + D ~ - f [ 1 - ( l + ~ ) y ~ ] (17)
where D is diffusivity of transient region, JA and JB are molecular transport flux of A and B, and YA is the molar fraction of A. Assuming the molecular transport flux of A is equal to that of B(JA=JB), SO the equation simplifies as:
1 1 1
- ~ = ~ + ~ (18)
The diffusivity of benzene is between 1.37"10 -3 and 1.39'10 .3 cm2/sec and that of MEK is between 1.43"10 .3 and 1.45"10 .3 cm2/sec in transient region.
Poiseuille flow. Several kinds of mixture systems of nitrogen-benzene and nitrogen-MEK are in this research, so the viscosity of mixture gas to calculate the poiseuille diffusivity should be consider. At 303 OK, the viscosity of nitrogen, benzene and M E K are 178.8, 74.2 and 77.0 p.P, respectively. The research indicated that the viscosity of nitrogen is greater than that of benzene and MEK at the same temperature, which in turn indicates a lower influent concentration of adsorbates and higher viscosity of the mixture gas. The Poiseuille diffusivity of benzene is between 3.776"10 °6 and 3.854"10 -6 cm2/sec; that of MEK is between 3.776"10 -6 and 3.851"10 -6 cm2/sec for seven kinds of activated carbon. The influent concentration of benzene decreased from 2,468 ppm to 372 ppm and the Poiseuille diffusivity decreased from 3.854" 10 -6 and 3.848" 10-6 cm2/sec.
Adsorption kinetic
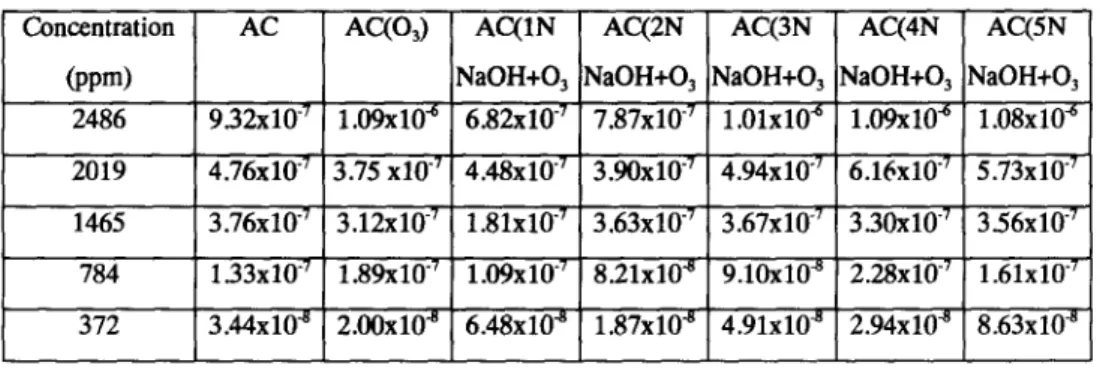
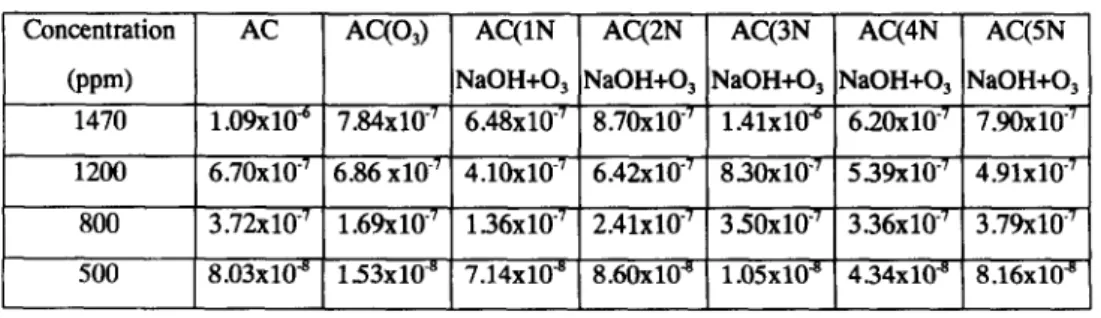
The adsorption kinetic curves of benzene and MEK on seven kinds of activated carbon are shown as Tables 2 and 3.
Table 2. Effective diffusivity of benzene on activated carbons
Concentration A C AC(O3) AC(1N AC(2N AC(3N AC(4N AC(5N
(ppm) NaOH+O3 NaOH+O3 NaOH+O3 NaOH+O 3 NaOH+O3
2486 9.32x10 "7 1.09x10 "6 6.82x10 "7 7.87x10 "7 1.01xl0 ~ 1.09x10 "6 1.08x10 "6 2019 4.76x10 "7 3.75x10 "7 4.48x10 "7 3.90x10 "7 4.94x10 "7 6.16x10 "7 5.73x10 "7 1465 3.76x10 "7 3.12x10 -7 1.81x10 "7 3.63x10 "7 3.67x10 "7 3.30x10 "7 3.56x10 "7 784 1.33x10 "7 1.89x10 "7 1.09x10 "7 8.21x10 "8 9.10x10 ~ 2.28x10 "7 1.61x10 "7 372 3.44x10 8 2.00x10 s 6.48x10 s 1.87x10 s 4.91x10 ~ 2.94x10 ~ 8.63x10 8
Effective diffusivity of benzene adsorbs on activated carbon. The effective diffusivity of benzene was adsorbed on activated carbon that was between 1.87"10 -8 and 1.09"10-6 cm2/sec. While the influent concentration of benzene was decreasing from 2486 ppm to 372 ppm, the effective diffusivity decreased on: A C from 9.32"10 -7 to 3.44"10 -8 cm2/sec.
AC(O3) from 1.09" 10-6 to 2.00* 10 -8 cm2/sec.
AC(1N NaOH+O3) from 6.82* 10 -7 to 6.48* 10 -8 cm2/sec. AC(2N NaOH+O3) from 7.87* 10 -7 to 1.87" 10 -8 cm2/sec.
AC(5N NaOH+O3) from 1.08"10 -6 to 8.63"10-8 cm2/sec.
If the influent concentration of benzene was decreasing from a maximum of 2,486 ppm to a minimum 372 ppm, then the ratio of minimum and maximum of effective diffusivity of AC is 3.7%, AC(O3) is 1.8%, AC(1N NaOH+O3) is 9.5%,AC(2N NaOH+O3) is 2.3%, AC(3N NaOH+O3) is 4.9%, AC(4N NaOH+O3) is 2.7% and AC(5N NaOH+O3) is 8.0%.
If the influent concentration of benzene was 2,486 ppm, 2,019 ppm, 1,465 ppm, 784 ppm and 372 ppm, the ratio of minimum and maximum of effective diffusivity of seven kinds of activated carbon were 1.60, 1.64, 2.07, 2.78 and 4.61, respectively. Results indicated the lower the influent concentration of benzene, the more varied the effective diffusivity.
Effective diffusivity of MEK adsorbs on activated carbon. If the influent concentration of MEK was
decreasing from 1470 ppm to 500 ppm, the effective diffusivity decreased on: AC from 1.09* 10 -6 to 8.03" 10 -8 cm2/sec.
AC(O3) from 7.84' 10 -7 to1.53" 10 -8 crn2/sec.
AC(1N NaOH+O3) from 6.48* 10-Tto 7.14" 10-8cm2/sec. AC(2N NaOH+O3) from 8.70" 10 -7 to 8.60" 10 -8 cm2/sec. AC(3N NaOH+O3) from 1.41" 10 -6 to 1.05" 10-8 cm2/sec. AC(4N NaOH+O3) from 6.20* 10 -7 to 4.34* 10 -8 cm2/sec. AC(5N NaOH+O3) from 7.90* 10 -7 to 8.16" 10 -8 cm2/sec.
Table 3. Effective diffusivity of MEK on activated carbons
Concentration AC AC(O3) AC(1N AC(2N AC(3N AC(4N AC(5N
(ppm) NaOH+O3 NaOH+O 3 NaOH+Oa NaOH+O3 NaOH+O3
1470 1.09x10 6 7.84x10 7 6.48x10 7 8.70x10 7 1.41x10 "6 6.20x10 7 7.90x10 "7 1200 6.70x10 "7 6.86x10 -7 4.10x10 -7 6.42x10 "7 8.30x10 "7 5.39x10 "7 4.91x10 "7 800 3.72x10 "7 1.69x10 "7 1.36x10 "7 2.41x10 "7 3.50x10 "7 3.36x10 "~ 3.79x10 "7 500 8.03x10 "8 1.53x10 -s 7.14x10 "~ 8.60x10 "8 1.05x10 "s 4.34xlff a 8.16x10 "s
The influent concentration of MEK was decreasing from 1,470 ppm to 500 ppm, then the ratio of the minimum to maximum effective diffusivity of AC is 7.4%, AC(O3) is 2.0% , AC(1N NaOH+O3) is ll%,AC(2N NaOH+O3) is 9.9%, AC(3N NaOH+O3) is 0.7%, AC(4N NaOH+O3) is 7.0% and AC(5N NaOH+O3) is 10%.
If the influent concentration of MEK was 1,470 ppm, 1,200 ppm, 800 ppm and 500 ppm then the ratio of minimum and maximum effective diffusivity of seven kinds of activated carbon were 2.27, 2.02, 2.79and 8.19; respectively. Results indicated the lower the influent concentration of MEK, the more varied the effective diffusivity.
Comparing the similar influent concentration of benzene and MEK, the effective diffusivity of MEK was greater than that of benzene. (The concentration of benzene is 1465 ppm to MEK is 1470 ppm, and the concentration of benzene is 784 ppm to that of MEK is 800 ppm). This can be attributed to MEK lesser molecular weight, so the molecules transport to the active sites of MEK is grt ater than that of benzene. Furthermore, the activated carbon was treated with ozone and sodium hydroxide that would increase the oxygen functional groups and polarization of the surface of activated carbon, so that the adsorption potential of polar molecular would increase at the same influent concentration. This is why, the effective diffusivity of MEK(dipole moment is 3.3D) is greater than that of benzene (dipole moment is zero).
Effective diffusivity at different temperature
This research selected three kinds of adsorption temperature that included 30oc(303oK), 70oC(343oK) and 120oc(393OK) to present as room temperature, middle temperature and high temperature; respectively and adsorbents AC, AC(O3) and AC(2N NaOH+O3) to adsorb benzene and MEK. The analysis results of effective diffusivity are shown as Figures 1 and 2.
The effective diffusivity of benzene on AC was between 3.44*10-8and 2.59"10 -6 cm2/sec, that on AC(O3) was between 2.00*10-Sand 2.25"10 -6 cm2/sec and that on AC(2N NaOH+O3)was between 1.87*10-Sand 9.97"10 -7 cm2/sec under the influent concentrations were between 372 ppm and 2486 ppm and the adsorption temperatures were between 30oc (303oK) and 120oc (393OK). These comparisons showed that the effect of influent concentration at low temperature (30°C) was greater than at high temperature (120oC).
3.0E-06- ~ O 3 0 3 K Q 3 4 3 K ~, 393 K ! ~ 2.0E-06 - ~ " 1 . 0 E - 0 6 - O.OE+O0 0 1000 1 2 0 0 0 2 5 0 0 3 0 0 0 Concentration (ppmv) AC(O3)-Ben 4 . 0 E - 0 6 O 303 K O 343 K A 393 K 3 . 0 E - 0 6 - 2 . 0 E - 0 6 1.0E-06 0 . 0 E + 0 0 ~ o ~ ~ 1500 ' 2 0 ~ ' ~ 3 ~ Concenr.radon (ppmv) AC(2N NaOH+O3)-Ben 4.0E-06 O 303 K Q 343 [~ A 393 K 3.0E-O6 - 2.0E-06 - c~ ]..0E-06 0.0E+00 O L 0 500 I 0 0 0 [500 2000 ~ 3 0 0 0 Conceut.~tion (ppmv) Figure 1. E f f e c t i v e d i f f u s i v i t y o f b e n z e n e a d s o r b e d o n A C , AC(O3), a n d A C ( 2 N N a O H + O ~ ) at d i f f e r e n t t e m p e r a t u r e s .
A C - M E K 2 . 0 E - 0 6 ,_, 1.5E-06 - 1.0E-06 - 5 . 0 E - 0 7 - O . O E + O 0 - , 0 500 1(200 1500 2O00 o 303 K r~ 343 K ~. 393 K Concentration (ppmv) A C ( O 3 ) - M E K 1 . S E - 0 ~ ~" 5.0E-0"7 - rn O.OE+00 - 500 1000 1500 2000 O 303 K ISl 343 K n 393 K Concentration (ppmv) A C ( 2 N N a O H + O 3 ) - M E K 2.0E-06 -, 1.0E-06 - 5.0E-0"7 0.OE+O0 0 5~0 10(30 1- 1 5 ~ 21300 O 303 K Q 343 K A 393 K Concentration (ppmv) F i g u r e 2. E f f e c t i v e d i f f u s i v i t y o f M E K a d s o r b e d o n A C , A C ( O a ) , a n d A C ( 2 N N a O H + O 3 ) at different t e m p e r a t u r e s .
was between 8.03"10 -8 and 1.79"10 .6 cm2/sec, that on AC(O3) was between 1.53"10-8 and 1.40"10.6 cm2/sec and that on AC(2N NaOH+O3)was between 8.6*10-8and 1.68"10 .6 cm2/sec. In general, the effective diffusivity of MEK was greater than that of benzene under the same temperature and similar concentration. The influence of influent concentration on effective diffusion coefficient at low temperature was greater than at high temperature.
Sailis selected Kureha granular activated carbon as adsorbent to study the adsorption kinetic of benzene at 23oC. As the influent concentration of benzene was increasing from 100 ppm to 10000 ppm, the adsorption capacity increased from 200mg/g to 300mg/g and the effective diffusivity increased from 2* 10-8 to 5"10 -7 cm2/sec. The effective diffusivity and influent concentration had a liner relationship(16). The adsorption procedures, conditions and results were similar to those found in this research..
CONCLUSIONS
When the adsorption temperature range was from 30oC to 120oc and the influent concentration of benzene was from 372 ppm to2486 ppm and that of MEK was from 500 ppm to 1470 ppm, the effective diffusion coefficient was from 10-8 to 10.6 crn2/sec. MEK exhibits an effective pore diffusion coefficient greater than that of benzene at a similar adsorbates concentration.. This may be due to MEK smaller molecular size, and a polar molecular structure that has a strong affinity toward the activated carbon surface. In general, the effective diffusivity of MEK was greater than that of benzene under the same temperature and similar concentration. The influence of influent concentration on effective diffusion coefficient at low temperature than that at high temperature.
REFERENCES
1 .D.M. Ruthven, Principles of Adsorption & Adsorption Process, John Wiley and Sons, New York(1984). 2.R.B.Bird etal., Transport Phenomena, John Wiley and Sons, New York(1960).
3.T.K. Sherwood, Mass Transfer, McGRAW-Hill, Inc.,(1975).
4.A.L. Hines and R.N. Maddox, Mass Transfer: Fundamentals and Application, Prentice-Hall Inc.,(1985). 5 J. Szekely, J.W.Evans and H.Y. Sohn, Gas-Solid Reactions, Academic Press,(1976).
Lewis Publishers, INC. Michigan, USA, (1992).
7.T.F. Lin, J.C. Little and W. W. Nazaroff, Transport and Sorption of Volite Organic Compounds and Water Vapor within Dry Soil Grains, Environ. Sci Technol.,28, 322-330, (1994).
8.T.F. Lin and W. W. Nazaroff, Transport of Vinyl Chloride with Activated Carbon: Predicting Isotherms and Breakthrough Curves from a Single Kinetic Sorption Experiment, Proceeding of the 7th IUAPPA Regional Conference on Air Pollution and Waste Issues, Volume I, 363-370,(1994).
9.L.A. Jonas, Reaction Steps in Gas Sorption by Impregnated Carbon, Carbon,16 115- 119, (1978). 10.C. Tien, Adsorption Calculations and Modeling, Butterworth-Heineman,(1994).
ll.D.R. Garg and D. M. Ruthven, The Effect of the Concentration Dependence of Diffusivity on Zeolite Sorption Curves, Chem. Eng. Sci., 27, 95-99,(1972).
12.D.M. Ruthven and R.I. Derrah, Sorption in Davison 5a Molecular Sieves, Can. J. Chem. Eng. 50, 743- 747, (1972).
13.P.G. Gray and D.D. Do, Adsorption and Desorption of Gaseous Sorbates on a Bidispered Particle with Freundilch Isotherm:I. Theory Analysis. Gas Sep. Purif. 3,193-200, (1989).
14.P.G. Gray and D.D. Do, Adsorption and Desorption of Gaseous Sorbates on a Bidispered Particle with Freundilch Isotherm:lI. Experimental Study of Sulphur Dioxide Sorption on Activated Carbon Panicles Gas Sep. Purif. 3,201-208, (1989).
15.P.G. Gray and D.D. Do, Adsorption and Desorption Dynamics of Sulphur Dioxide on a Single Large Activated Carbon Particles, Chem. Eng. Comm., 96, 141-154, (1990).
16.D.H. Everett and R.H. Ottewill, Surface Area Determination, Processdings of the International Symposium on Surface Area Determination, University of Bristol, K. 16-18, July, (1969).