Familial Aggregation of Olfactory Impairment and Odor
Identification in Older Adults
Laura A. Raynor, M.S. M.P.H,
Department of Epidemiology and Community Health, University of Minnesota, Minneapolis, MN, United States
James S. Pankow, Ph.D.,
Department of Epidemiology and Community Health, University of Minnesota, Minneapolis, MN, United States
Karen J. Cruickshanks, Ph.D.,
Department of Ophthalmology and Visual Sciences, University of Wisconsin-Madison, Madison, WI, United States
Carla R. Schubert, M.S.,
Department of Ophthalmology and Visual Sciences, University of Wisconsin-Madison, Madison, WI, United States
Michael B. Miller, Ph.D.,
Department of Psychology, University of Minnesota, Minneapolis, MN, United States Ronald Klein, MD, MPH, and
Department of Ophthalmology and Visual Sciences, University of Wisconsin-Madison, Madison, WI, United States
Guan-Hua Huang, Ph.D.
National Chiao Tung University, Hsinchu, Taiwan
Abstract
Objectives/Hypothesis—The objective of this analysis was to estimate the genetic contributions to olfactory impairment.
Study Design—Population-based
Methods—Olfactory impairment was measured using the San Diego Odor Identification Test (SDOIT) at the 5-year follow-up examination for the population-based Epidemiology of Hearing Loss (EHLS) study. Subjects were classified as impaired if they correctly identified fewer than 6 out of 8 odorants. In order to reduce confounding by age, analysis was restricted to subjects who were 60-79 years of age. Familial aggregation was evaluated by heritability estimates, tetrachoric correlations, and odds ratios in 207 sibling pairs from 135 sibships.
Results—The prevalence of olfactory impairment was 20.2% overall and was higher in men. After adjustment for sex, age, and smoking, heritability of olfactory impairment was moderate (h2=0.55), although not statistically significantly different from zero (p=0.09). By contrast, the
Corresponding Author: Laura A. Raynor, M.S. M.P.H, University of Minnesota, Division of Epidemiology & Community Health, 1300 South Second Street, Suite 300, Minneapolis, MN 55454-1015, Phone Number: 612-626-0027 Fax Number,
NIH Public Access
Author Manuscript
Laryngoscope. Author manuscript; available in PMC 2011 August 1. Published in final edited form as:
Laryngoscope. 2010 August ; 120(8): 1614–1618. doi:10.1002/lary.20989.
NIH-PA Author Manuscript
NIH-PA Author Manuscript
adjusted heritability estimate for bubble gum, one of the individual odorants, was significant (h2=0.51, p=0.01).
Conclusion—Genetic factors may contribute to general olfactory impairment in older adults, but the strength of familial aggregation differs for individual odorants, a finding consistent with prior research.
Keywords
Heritability; genetics; olfaction
Introduction
The human sense of smell is the least well characterized of all the senses. Olfaction involves a cascade of biochemical and electrophysiological processes that convert the molecular information of an odorant into an odor sensation.1 Biologically, the human olfaction system consists of millions of olfactory sensory neurons arranged in a sensory epithelium inside the nasal cavity.2 The ciliated dendritic ends of the olfactory sensory neurons receive and amplify chemosensory signals by generating electrical impulses that are transmitted to the synaptic complexes of the olfactory bulb of the brain.1 The olfactory bulb glomerulus receives the input of the sub-group of neurons that are all expressing the same odorant receptor protein.1
Odorant receptors are essential components of the molecular decoding device of the nose and have been well documented via genomic sequencing and annotation.1,2 Odorant receptors comprise the largest gene-family in the human genome and the genes associated with odorant receptors have been clustered on almost every chromosome.1,3,4,5,6,7 The organization of human odorant receptors is highly conserved in most species.1 However, as primates have become less reliant on olfaction for their survival, the number of odorant receptor genes has been reduced, leading to an extensive accumulation of pseudogenes and copy number variants.1,2 Thus there appears to be a lower degree of purifying selection in human olfaction, resulting in enhanced inter-individual genomic diversity.
The presence of widespread diversity in olfaction has led to many studies that seek a genetic basis for the variation in both the threshold and perception of odorants.1 However, although variation in odorant receptor genes may contribute to differences in sensitivity between individuals, evidence supporting heritability of olfactory impairment has been mixed.5,8 For example, the results of twin studies are contradictory and the presence of significant heritability varies with the odorants being tested.9,10,11,12 A recent family study also found that the presence of significant heritability was smell-specific.8 In the present study we evaluated familial aggregation of olfactory impairment in a well-characterized cohort of older adults from Beaver Dam, Wisconsin.
Materials and Methods
Participants
During 1987-1988, a private census was conducted to identify residents of the city or township of Beaver Dam, Wisconsin who were ages 43-84 years. These residents were subsequently invited to participate in the Beaver Dam Eye Study, a study of age-related ocular disorders. Of the 5,924 eligible individuals, 4,926 participated in the eye examination phase (1988-1990). Some of the participants were later determined to be part of nuclear or extended families, and pedigree structures were collected. Details regarding the original cohort and baseline examination have been described in earlier publications.13,14
NIH-PA Author Manuscript
NIH-PA Author Manuscript
Participants who were alive as of March 1, 1993 (n=4,541) were eligible to participate in the first EHLS examination that occurred at the time of the 5-year follow-up visit for the eye study, conducted between 1993-5.15 Of those eligible, 3,753 participated in first EHLS examination. A 5-year follow-up EHLS examination was conducted from 1998-2000 and had 2,800 participants.15 EHLS was approved by the Human Subjects Committee of the University of Wisconsin-Madison and informed consent was obtained from each participant at the beginning of each examination.
The San Diego Odor Identification Test (SDOIT) and related olfaction questions were a component of the 5-year follow-up examination.16,17,18 Participants with cognitive impairment (defined as a Mini-Mental State Examination score <24 or reported dementia) were not excluded from participating; however, they were less likely to have complete olfaction data (n=97 with olfaction data).19 Complete olfaction data, gathered via in-person examinations, was obtained from 2,527 participants (85%) from the EHLS 5-year follow-up study.
Procedures
A standardized questionnaire that included relevant questions about potential covariates was administered and participants had their olfaction tested by trained personnel via the SDOIT. The SDOIT is a standardized odor identification test that uses eight common, natural odors typically found in the home (coffee (Folgers Coffee, J.M. Smucker Co., Orrville, Ohio), chocolate (Hershey’s Kisses (milk chocolate), Hershey Company, Hershey, PA), bubble gum (Bazooka, Topps Company, Inc., New York, NY), baby powder (Johnson’s Baby Powder, Johnson and Johnson, New Brunswick, NJ), cinnamon (McCormick and Co., Inc., Sparks, MD), mustard (French’s Classic Yellow, Reckitt Benckiser, Slough, Berkshire, U.K.) play dough (Play-Doh, Hasbro, Inc, Pawtucket, RI), and peanut butter (Skippy Creamy, Unilever, London, UK)).16,17,18 Brand-specific odorants were prepared (wrapped in gauze and stored in opaque containers) and replaced following a standardized protocol. A picture board with illustrations of the eight target items and 12 distracters was presented prior to the presentation of the odorants. Participants were asked to name or identify each picture. If participants were unable to successfully name or identify at least 18 out of 20 pictures in this array, the odorants were not administered.
Odors were presented in a random order and participants were tested with their eyes closed to prevent visual clues. Participants used the picture board to aid identification of odorants. If incorrect, they are told the correct name, and the odor is re-presented later in the test sequence. An inter-stimulus interval of 45 seconds was used to minimize adaptation Participants correctly identifying fewer than 6 of 8 odorants after two trials were considered to have impaired olfaction. The cut-point corresponds to approximately two standard deviations less than the mean olfaction score in a group of 75 healthy adults aged 20 to 40 years of age.17 Test-retest reliability coefficient of the SDOIT was 0.86 when tested with a mean delay of five days in a sample of 92 subjects, and test-retest agreement of olfactory impairment over an average of three weeks was 95% in a sample of 90 subjects.17,20 In a recent study, there was excellent agreement between olfaction impairment on the SDOIT and the Brief Smell Identification Test, another test commonly used in research studies (96% agreement, kappa=0.81).20
Statistical Analyses
Olfactory impairment and identification of individual odorants were modeled as
dichotomous traits. We chose to restrict our heritability analyses to individuals aged 60-79 to reduce confounding by age and ensure sufficient variability in the olfactory measures, as
NIH-PA Author Manuscript
NIH-PA Author Manuscript
previous research indicates that the majority of individuals ≥80 years of age exhibit reduced sensitivity to odors on common olfactory tests whereas the majority of individuals <60 years of age are not impaired.21
Heritability was calculated as the proportion of the total variance in olfactory measures explained by additive genetic effects after controlling for covariates and it ranges from 0 to 100%, with 0 signifying no additive genetic influence and 100% implying that all individual differences are due to genetic influence. Heritability estimates for olfactory impairment were calculated as follows: (a) unadjusted (b) adjusted for age and sex and (c) adjusted for age, sex, and smoking status. Heritability of olfactory impairment was estimated by a liability threshold model, whereby individuals whose liability is above a certain threshold are affected. If the condition runs in families, the siblings of these individuals will have a higher average liability than the population mean and a greater proportion of them will have liability exceeding the threshold.22 The liability threshold model was implemented in the Sequential Oligogenic Linkage Analysis Routines (SOLAR) software package
(http://www.sfbr.org/solar/index.html; Version 2.1.5).23
In addition, familial aggregation was estimated in sibships by tetrachoric correlations and pairwise odds ratios. The tetrachoric correlation estimates likeness between relatives and ranges from 0 (no correlation) to 1 (perfect correlation).24 The calculation of the tetrachoric correlation is also defined under the liability threshold model, where it is assumed that genes and environmental factors result in an underlying liability to the disease and there exists a threshold that divides the population into affected and unaffected subjects. This threshold is inferred from the disease prevalence in the sample and the tetrachoric correlation is the correlation between siblings for the liability.25 The pairwise odds ratio for any two siblings is the ratio of the odds that, given that any sibling selected at random has the disorder, a second sibling selected at random will also have the disorder over the odds that, given that any sibling selected at random does not have the disorder, a second sibling picked at random will have the disorder.26 Odds ratios that are greater than one indicates sibling aggregation.
Results
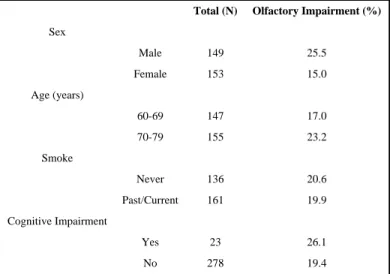
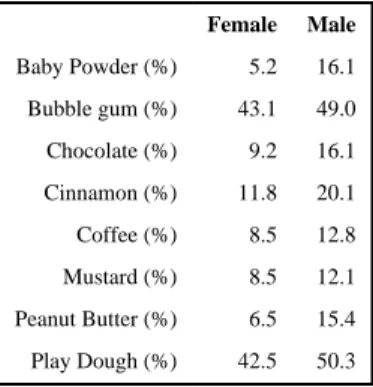
Olfaction measurements were available for 207 full sibling pairs from 135 sibships. Within these families there were more female pairs (n=63) than male pairs (n=58). The prevalence of olfactory impairment in the 60-79 year old age group was 20%. Subjects with olfactory impairment were more likely to be males and cognitively impaired (Table 1). Of the individual odorants, subjects were most likely to incorrectly identify bubble gum and play dough (Table 2). Men were more likely than women to incorrectly identify each of the individual odorants.
Estimates of familial aggregation for olfactory impairment are presented in Table 3. Heritability estimates for olfactory impairment were moderate in size but not statistically significantly different from zero in an unadjusted model (h2=0.37; p=0.18), when adjusted for age and sex (h2=0.49; p=0.12), or additionally adjusted for smoking history (h2=0.55; p=0.09). Further adjustment for self-reported deviated septum, nasal polyps, or heavy alcohol consumption had little material effect on these heritability estimates. Sibling odds ratios and tetrachoric correlations for general olfactory impairment also were not statistically significant.
Familial aggregation was also evaluated for two individual odorants with a prevalence of incorrect identification >20% (i.e., bubble gum and play dough). Heritability for the identification of bubble gum was statistically significant both before (h2=0.46; p=0.02) and
NIH-PA Author Manuscript
NIH-PA Author Manuscript
after (h2=0.51; p=0.01) covariate adjustment (Table 3). Heritability estimates for play dough were lower (h2=0.29; p=0.09) in the adjusted model.
Discussion
We conducted a study of the hereditary basis of olfaction loss in a population-based sample making use of available SDOIT measurements and questionnaire data from the EHLS study. To our knowledge, this is the first study to estimate familial aggregation for olfactory impairment or identification of individual odorants in a population-based sample. The use of a population-based sample, irrespective of disease status, allows inferences about all the genetic variation that exist in the general population. In comparison, other studies that sample families by selecting cases of a specific diseases limit statistical inference to
statements relevant to the sub-population sampled.27 Furthermore, sampling families that are not population-based requires that the sampling method be accounted for in the analyses, yet many studies fail to do so leading to biased estimates of heritability.27
We found significant heritability for identification of one of the individual odorants (bubble gum) measured in the SDOIT. By contrast, heritability estimates for general olfactory impairment were not statistically different from zero. It is possible the bubble gum odorant was less familiar to participants in this age range, as it was not commercially available until after World War II. Although the test protocol provides an opportunity to learn the correct identification, it may be a more sensitive indicator of olfactory dysfunction.
Past research into genetic influences on olfaction has been conducted using twins and families.8,9,10,11,12 Studies have focused on individual odorants, which have varied widely between studies, but failed to quantify the heritability of the traits. The studies that have included heritability calculations found mixed results. Finkel et al. found moderate heritability for total odor identification and perceived intensity and non-significant
heritability for odor detection and perceived pleasantness of six odorants; however, Knaapila et al. found a low heritability for total odor identification but pleasantness and intensity of individual odorants were significant.8,12,28 Our own study found evidence of familial aggregation of general olfactory impairment using a population-based sample; however, heritability estimates were not significantly different from zero.
Although the aforementioned studies used different smell tests, which make it difficult to replicate findings across studies, multiple research studies have yielded evidence for genetic control of olfactory-related traits. For example, Knaapila et al. found evidence of genetic linkage for the perceived intensity of paint thinner odor on chromosome 2p14 and the pleasantness of cinnamon odor on chromosome 4q32.3.8 A recent study conducted by Pinto et al. also found evidence of genetic linkage over a region of chromosome 4
(4q22.3-4q31.22) for hyposmia.29 Future research is needed to further identify and characterize the specific genes responsible for olfactory impairment in aging populations. Instruments such as the SDOIT that require identification of common household odorants may reflect both sensory and cognitive processes as subjects must not only detect the presence of the scent but also correctly identify it from a set of possibilities. Recent results from the EHLS cohort indicate that olfactory impairment at baseline is a strong predictor of cognitive decline suggesting that the SDOIT may be sensitive to early changes in cognition. 21 However, it is unlikely that cognitive impairment contributed to the low heritability estimates as only 23 of the participants included in these analyses had cognitive impairment. Moreover the test protocol was developed to minimize misclassification due to naming difficulties, an early sign of cognitive impairment, through the use of the picture board and by basing the olfaction score on the number correctly identified after two trials. It is a
NIH-PA Author Manuscript
NIH-PA Author Manuscript
strength of the study that the entire population-based cohort was tested, as excluding cognitively impaired people might lead to under-estimating heritability.
Limitations of this study include the small number of relative pairs that were ages 60-79 and had impaired olfaction at the EHLS 5-year follow-up examination. Likewise small sample sizes inhibited the ability to estimate familial aggregation for six of the eight odorants, as the prevalence of impaired olfaction for these scents was much lower when compared to the bubble gum and play dough odorants. Future analyses with larger sample sizes should yield more precise estimates of heritability.
The SDOIT is a standardized test using common odorants, is inexpensive and efficient to administer in research settings. It has a high reliability and there is excellent agreement between impairment measured with the SDOIT and scratch-and-sniff measures of olfaction such as the Brief Smell Identification Test (96% agreement, kappa=0.81).20 It is possible that it underestimates olfactory impairment, as the number of odorants is small and may miss some specific anosmias, In addition, it may fail to identify people with only mild threshold losses who may be able to detect sufficient signal to correctly identify the odorants.
It is important to note that olfactory impairment is likely a multi-factorial disorder involving both genetic and environmental factors. While we controlled for the potential confounding effects of age, sex, and cigarette smoking in heritability analyses, there may be additional environmental exposures that impact estimates of hereditary influence. Further research into environmental causes of olfaction loss is warranted to understand how genes and
environment contribute to the development of the phenotype, both individually and in conjunction with each other. While this study supports the contributions of genetic factors to olfactory impairment in older adults from a population-based cohort, a greater knowledge of environmental determinants would allow us to refine our estimates of heritability.
Conclusion
This study used a population-based sample to estimate familial aggregation for olfactory impairment and identification of individual odorants, finding that general olfactory impairment in older adults may be genetically influenced, but the strength of familial aggregation differs for individual odorants. The results of this study are consistent with prior research.
Acknowledgments
Funding: The project described was supported by R01AG021917 (KJC) from the National Institute on Aging, National Eye Institute, and National Institute on Deafness and Other Communication Disorders and R37AG11099 (KJC) from the National Institute on Aging. The content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Institute on Aging or the National Institutes of Health.
References
1. Hasin-Brumshtein Y, Lancet D, Olender T. Human olfaction: from genomic variation to phenotypic diversity. Trends Genet. 2009; 25(4):178–84. [PubMed: 19303166]
2. Keller A, Vosshall LB. Human olfactory psychophysics. Curr Biol. 2004; 14(20):R875–8. [PubMed: 15498475]
3. Zozulya S, Echeverri F, Nguyen T. The human olfactory receptor repertoire. Genome Biol. 2001; 2(6):research0018.1–research0018.12. [PubMed: 11423007]
4. Glusman G, Yanai I, Rubin I, Lancet D. The complete human olfactory subgenome. Genomic Res. 2001; 11(5):685–702.
NIH-PA Author Manuscript
NIH-PA Author Manuscript
5. Olender T, Feldmesser E, Atarot T, et al. The olfactory receptor universe—from whole genome analysis to structure and evolution. Genet Mol Res. 2004; 30(4):545–53. [PubMed: 15688320] 6. Malnic B, Godfrey PA, Buck LB. The human olfactory receptor gene family. Proc Natl Acad Sci
USA. 2004; 101(8):2585–9.
7. Niimura Y, Nei M. Evolution of olfactory receptor genes in the human genome. Proc Natl Acad Sci USA. 2003; 100(21):12235–12240. [PubMed: 14507991]
8. Knaapila A, Keskitalo K, Kallela M, et al. Genetic component of identification, intensity, and pleasantness of odours: a Finnish family study. Euro J Hum Genet. 2007; 15(5):596–602. 9. Hubert HB, Fabsitz RR, Feinleib M, et al. Olfactory sensitivity in humans: genetic versus
environmental control. Science. 1980; 208(4444):607–9. [PubMed: 7189296]
10. Gross-Isseroff R, Ophir D, Bartana A, et al. Evidence for genetic determination in human twins of olfactory thresholds for a standard odorant. Neurosci Lett. 1992; 141(1):115–8. [PubMed: 1508392]
11. Segal NL, Topolski TD, Wilson SM, et al. Twin analysis of odor identification and perception. Physiol Behav. 1995; 57(3):605–9. [PubMed: 7538679]
12. Finkel D, Pederson NL, Larsson M. Olfactory functioning and cognitive abilities: a twin study. J Gerontol Psychol Sci Soc Sci. 2001; 56(4):P226–33.
13. Klein R, Klein BE, Lee KE. Changes in visual acuity in a population. The Beaver Dam Eye Study. Ophthalmol. 1996; 103(8):1169–78.
14. Linton KL, Klein BE, Klein R. The validity of self-reported and surrogate-reported cataract and age-related macular degeneration in the Beaver Dam Eye Study. Am J Epidemiol. 1991; 134(12): 1438–46. [PubMed: 1776618]
15. Cruickshanks KJ, Tweed TS, Wiley TL, et al. The five-year incidence and progression of hearing loss: The Epidemiology of Hearing Loss Study. Arch Oto Head Neck Surg. 2003; 129(10):1041–6. 16. Murphy, C.; Anderson, J.; Markinson, S. Psychophysical assessment of chemosensory disorders in
clinical populations. In: Kurihara, K.; Suzuki, N.; Ogawa, H., editors. Olfaction and Taste XI. Tokyo, Japan: Springer-Verlag Tokyo; 1994. p. 609-13.
17. Murphy C, Schubert CR, Nondahl DM, et al. Prevalence of olfactory impairment in older adults. JAMA. 2002; 288(18):2307–12. [PubMed: 12425708]
18. Morgan CD, Nordin S, Murphy C. Odor identification as an early marker for Alzheimer’s disease: impact of lexical functioning and detection sensitivity. J Clin Exp Neuropsychol. 1995; 17(5):793– 803. [PubMed: 8557819]
19. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state,” a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975; 12:189–198. [PubMed: 1202204]
20. Ekman G, Berglund B, Berglund U, et al. Perceived intensity of odor as a function of time of adaptation. Scand J Psychol. 1967; 8(3):177–186. [PubMed: 6079317]
21. Schubert CR, Carmichael LL, Murphy C, et al. Olfaction and the 5-year incidence of cognitive impairment in an epidemiological study of older adults. J Am Geriatr Soc. 2008; 56(8):1517–21. [PubMed: 18662205]
22. Strachan, T.; Read, A. Human Molecular Genetics. New York: Wiley-Liss; 1996.
23. Almasy L, Blangero J. Multipoint quantitative trait linkage analysis in general pedigrees. Am J Hum Genet. 1988; 62(5):1198–1211. [PubMed: 9545414]
24. Neale, MC.; Cardon, LR. Methodology for genetic studies of twins and families. Boston: Kluwer Academic Publishers; 1992.
25. Sham, P. Statistics in human genetics. London: Hodder Arnold Publication; 1998.
26. Hunt SC, Hasstedt SJ, Williams RR. Testing for familial aggregation of a dichotomous trait. Gen Epi. 1986; 3(5):299–312.
27. Hopper JL, Bishop DT, Easton DF. Population-based family studies in genetic epidemiology. Lancet. 2005; 366(9494):1397–406. [PubMed: 16226618]
28. Knaapila A, Tuorila H, Silventoinen K, et al. Environmental effects exceed genetic effects on perceived intensity and pleasantness of several odors: a three-population twin study. Behav Genet. 2008; 38(5):484–92. [PubMed: 18543092]
NIH-PA Author Manuscript
NIH-PA Author Manuscript
29. Pinto JM, Sanguansak T, Hayes MG, et al. A genome-wide screen for hyposmia susceptibility loci. Chem Sens. 2008; 33(4):319–29.
NIH-PA Author Manuscript
NIH-PA Author Manuscript
NIH-PA Author Manuscript
NIH-PA Author Manuscript
NIH-PA Author Manuscript
Table 1 Prevalence of olfactory impairment by selected characteristics
Total (N) Olfactory Impairment (%)
Sex Male 149 25.5 Female 153 15.0 Age (years) 60-69 147 17.0 70-79 155 23.2 Smoke Never 136 20.6 Past/Current 161 19.9 Cognitive Impairment Yes 23 26.1 No 278 19.4
NIH-PA Author Manuscript
NIH-PA Author Manuscript
NIH-PA Author Manuscript
Table 2 Prevalence of incorrect identification of odorants by sex
Female Male Baby Powder (%) 5.2 16.1 Bubble gum (%) 43.1 49.0 Chocolate (%) 9.2 16.1 Cinnamon (%) 11.8 20.1 Coffee (%) 8.5 12.8 Mustard (%) 8.5 12.1 Peanut Butter (%) 6.5 15.4 Play Dough (%) 42.5 50.3
NIH-PA Author Manuscript
NIH-PA Author Manuscript
NIH-PA Author Manuscript
Table 3
Estimates of familial aggregation for general olfactory impairment and identification of individual odorants in 207 sibling pairs
Model a h 2 p-value Odds Ratio p-value Tetrachoric Correlation p-value Olfactory Impairment 1 0.37 0.18 1.27 0.57 0.08 0.48 2 0.49 0.12 3 0.55 0.09 Bubble Gum 1 0.46 0.02 1.78 0.64 0.22 0.05 2 0.46 0.02 3 0.51 0.01 Play Dough 1 0.32 0.07 1.49 0.60 0.15 0.17 2 0.25 0.13 3 0.29 0.09