doi: 10.3389/fmicb.2015.00893
Edited by:
Giovanni Di Bonaventura, Università degli Studi “G. d’Annunzio” Chieti e Pescara, Italy
Reviewed by:
Deborah R. Yoder-Himes, University of Louisville, USA Ronald Paul Rabinowitz, University of Maryland School of Medicine, USA Malgorzata Anna Mikaszewska-Sokolewicz, The Medical University of Warsaw, Poland
*Correspondence:
Yen-Hsu Chen, Division of Infectious Diseases, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, No. 100, Tzyou 1st Road, Kaohsiung 807, Taiwan infchen@gmail.com; Po-Ren Hsueh, Departments of Laboratory Medicine and Internal Medicine, National Taiwan University Hospital, National Taiwan University College of Medicine, No. 7, Chung-Shan South Road, Taipei 100, Taiwan hsporen@ntu.edu.tw
Specialty section:
This article was submitted to Infectious Diseases, a section of the journal Frontiers in Microbiology
Received: 03 March 2015 Accepted: 17 August 2015 Published: 02 September 2015 Citation:
Chang Y-T, Lin C-Y, Chen Y-H and Hsueh P-R (2015) Update on infections caused by Stenotrophomonas maltophilia with particular attention to resistance mechanisms and therapeutic options. Front. Microbiol. 6:893. doi: 10.3389/fmicb.2015.00893
Update on infections caused by
Stenotrophomonas maltophilia
with
particular attention to resistance
mechanisms and therapeutic options
Ya-Ting Chang
1, 2, Chun-Yu Lin
2, 3, Yen-Hsu Chen
2, 3, 4* and Po-Ren Hsueh
5*
1Division of Infectious Diseases, Department of Internal Medicine, Kaohsiung Municipal HsiaoKang Hospital, Kaohsiung,
Taiwan,2Division of Infectious Diseases, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung
Medical University, Kaohsiung, Taiwan,3School of Medicine, Graduate Institute of Medicine, Sepsis Research Center,
College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan,4Department of Biological Science and Technology,
College of Biological Science and Technology, National Chiao Tung University, HsinChu, Taiwan,5Departments of Laboratory
Medicine and Internal Medicine, National Taiwan University Hospital, National Taiwan University College of Medicine, Taipei, Taiwan
Stenotrophomonas maltophilia
is a Gram-negative, biofilm-forming bacterium. Although
generally regarded as an organism of low virulence, S. maltophilia is an emerging
multi-drug resistant opportunistic pathogen in hospital and community settings,
especially among immunocompromised hosts. Risk factors associated with S.
maltophilia
infection include underlying malignancy, cystic fibrosis, corticosteroid or
immunosuppressant therapy, the presence of an indwelling central venous catheter
and exposure to broad spectrum antibiotics. In this review, we provide a synthesis of
information on current global trends in S. maltophilia pathogenicity as well as updated
information on the molecular mechanisms contributing to its resistance to an array of
antimicrobial agents. The prevalence of S. maltophilia infection in the general population
increased from 0.8–1.4% during 1997–2003 to 1.3–1.68% during 2007–2012. The
most important molecular mechanisms contributing to its resistance to antibiotics
include β-lactamase production, the expression of Qnr genes, and the presence of
class 1 integrons and efflux pumps. Trimethoprim/sulfamethoxazole (TMP/SMX) is the
antimicrobial drug of choice. Although a few studies have reported increased resistance
to TMP/SMX, the majority of studies worldwide show that S. maltophilia continues
to be highly susceptible. Drugs with historically good susceptibility results include
ceftazidime, ticarcillin-clavulanate, and fluoroquinolones; however, a number of studies
show an alarming trend in resistance to those agents. Tetracyclines such as tigecycline,
minocycline, and doxycycline are also effective agents and consistently display good
activity against S. maltophilia in various geographic regions and across different time
periods. Combination therapies, novel agents, and aerosolized forms of antimicrobial
drugs are currently being tested for their ability to treat infections caused by this
multi-drug resistant organism.
Stenotrophomonas maltophilia is a Gram-negative, aerobic,
glucose non-fermenting, motile bacillus. S. maltophilia was first
isolated from pleural effusion in 1943 and initially named
Bacterium bookeri. The organism was reclassified as a member
of the genus Pseudomonas in 1961, Xanthomonas in 1983,
and then Stenotrophomonas in 1993 (
Al-Anazi and Al-Jasser,
2014
). It survives on almost any humid surface and has
been isolated from a wide variety of aquatic sources, such as
suction tubing, nebulizers, endoscopes, hemodialysis dialysate
samples, plant rhizosphere, faucets, sink drains, and shower
heads (
Brooke, 2012
). S. maltophilia is characterized by its
ability to form biofilms on various abiotic and biotic surfaces,
including lung cells (
de Oliveira-Garcia et al., 2003; Pompilio
et al., 2010
), and by its resistance to a broad array of
antimicrobial agents. The World Health Organization recently
classified S. maltophilia as one of the leading multidrug
resistant organisms (MDROs) in hospital settings (
Brooke,
2014
).
S. maltophilia is generally regarded as an organism of low
virulence and therefore an opportunistic pathogen, especially
in immunocompromised hosts. The risk factors associated
with acquiring S. maltophilia infections are well-known
and include underlying malignancy (especially hematologic
malignancy), organ transplantation, human immunodeficiency
virus (HIV) infection, cystic fibrosis, prolonged hospitalization,
intensive care unit (ICU) admission, mechanical ventilation,
indwelling catheters (vascular, urinary, biliary), corticosteroid or
immunosuppressant therapy, and recent antibiotics treatment
(
Al-Anazi and Al-Jasser, 2014
). These risk factors reflect
specific features of S. maltophilia, such as its ability to survive
on almost any humid surface, its propensity to form biofilm
and colonize humid surfaces, and its employment of several
mechanisms that confer resistance to a number of antimicrobial
agents.
S. maltophilia causes a wide range of infections including
respiratory tract infections (RTI), blood stream infections
(BSI) and, less commonly, skin and soft tissue infections
(SSTI), bone and joint infections, biliary tract infections,
urinary tract infections, endophthalmitis, endocarditis, and
meningitis (
Falagas et al., 2009a; Looney et al., 2009
). The
correlations between S. maltophilia infection and structural
abnormalities with or without obstruction or procedural
manipulation are well documented. Biliary tract infections
caused by obstruction due to hepatobiliary neoplasms (
Papadakis
et al., 1995; Chang et al., 2014
) or post-operative anastomotic
strictures of the gastrointestinal tract (
Perez et al., 2014
) have
been reported in patients with biliary S. maltophilia sepsis.
Pleural infections caused by post-surgical/tube thoracostomy
or fistula (broncho-/esophageal-/bilio-) (
Lee et al., 2014
),
post-neurosurgical meningitis (
Sood et al., 2013; Lai et al., 2014b
),
complicated urinary tract infections (
Vartivarian et al., 1996
),
and obstructive lung cancer (
Fujita et al., 1996; Vartivarian
et al., 2000
) have all been reported to create a milieu for S.
maltophilia infection. In addition, although commonly perceived
as nosocomial pathogens, community-acquired infections appear
to be on the rise (
Falagas et al., 2009a; Chang et al.,
2014
).
Prevalence
There were few data before 1970 regarding the prevalence or
clinical characteristics of S. maltophilia (previously Pseudomonas
maltophilia or Xanthomonas maltophilia) because of its rarity
and relative clinical insignificance. It was in the 1980s when S.
maltophilia became more frequently reported as an emerging
nosocomial pathogen (
Jang et al., 1992; Victor et al., 1994
),
especially in patients with post-chemotherapy neutropenia (
Kerr
et al., 1990; Labarca et al., 2000
) and in those with indwelling
central venous catheters (CVC) (
Victor et al., 1994; Lai et al.,
2006; Chen et al., 2014
). Beginning in the late 1990s worldwide
surveillance programs and multi-center studies began to provide
more comprehensive information about the pathogenicity of S.
maltophilia. Of the global surveillance programs, the SENTRY
Antimicrobial Surveillance Program initiated in 1997 and the
Study for Monitoring Antimicrobial Resistance Trends (SMART)
initiated in 2002 are the most well-known (
Jean et al., 2015
). A
number of nationwide and antimicrobial agent-targeted projects
were also launched during the late 1990s, including the Canadian
Ward Surveillance Study (CANWARD), the Surveillance and
Control of Pathogens of Epidemiologic Importance (SCOPE)
study, the British Society for Antimicrobial Chemotherapy
(BSAC) Resistance Surveillance Project, the Taiwan Surveillance
of Antimicrobial Resistance (TSAR) study, and the Tigecycline
Evaluation Surveillance Trial (TEST).
Despite the massive scale of these surveillance studies,
there are still limited integrated data on the prevalence and
susceptibility patterns of S. maltophilia. The heterogeneity
among the studies stems from the diverse patient demographics,
geographic differences, and the ratio of the isolates collected from
different sources, making inter-literature comparison difficult.
To add to the complexity, there are no worldwide guidelines
on susceptibility testing methodology and breakpoints for S.
maltophilia (
Nicodemo et al., 2004; Hombach et al., 2012
), which
results in different or absence of susceptibility breakpoints for
some antibiotics. The lack of universal references for evaluating
resistance of S. maltophilia to antimicrobial agents leads to
confusion and complications when interpreting clinical data.
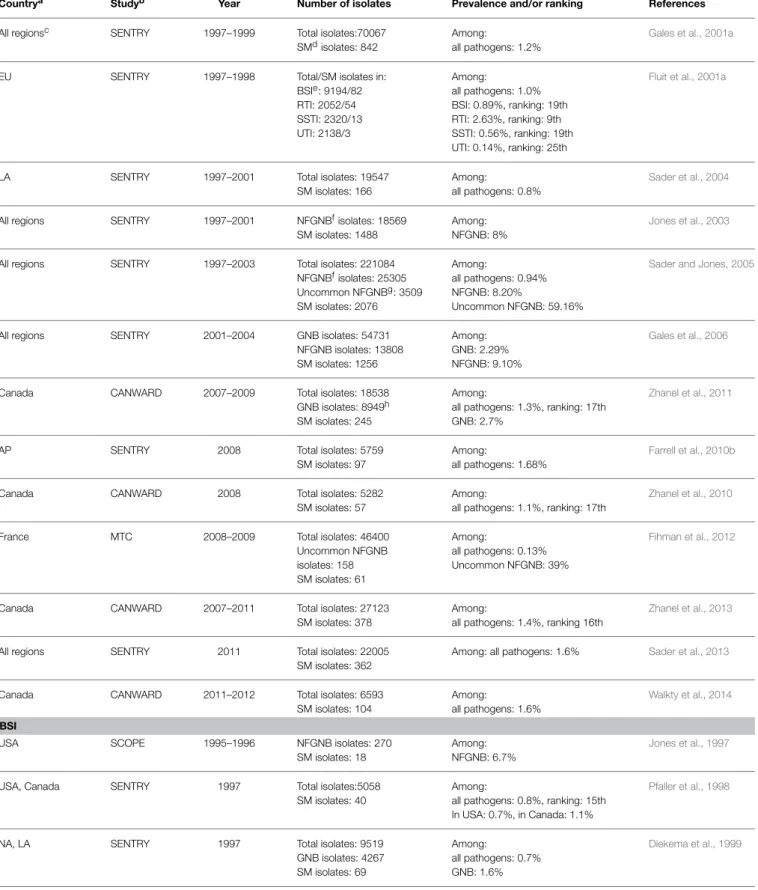
Table 1
shows the prevalence rates of infection due to S.
maltophilia, categorized by sources of infection, reported by
worldwide and nationwide surveillance projects as well as
multi-center studies. Specific patient groups such as the critically ill
in intensive care units (ICUs) and the pediatric population are
presented separately in Table 1. By comparing data gathered by
large surveillance studies over time we can estimate longitudinal
change in prevalence of S. maltophilia infection in the general
population. The frequency of occurrence among isolates from
all sources ranged from 0.8 to 1.4% in five SENTRY studies
during 1997∼2003 (
Fluit et al., 2001a; Gales et al., 2001a; Sader
et al., 2004; Sader and Jones, 2005; Fedler et al., 2006b
). During
2007–2012, the CANWARD surveillance study (
Zhanel et al.,
2011, 2013; Walkty et al., 2014
) and the SENTRY antimicrobial
surveillance program (
Farrell et al., 2010b; Sader et al., 2013
)
reported prevalence rates ranging from 1.3 to 1.68%. These data
indicate that there is an increasing trend in infections due to S.
maltophilia in the general population.
TABLE 1 | Prevalence of S. maltophilia in worldwide surveillance and multicenter studies.
Countrya Studyb Year Number of isolates Prevalence and/or ranking References
All regionsc SENTRY 1997–1999 Total isolates:70067 SMdisolates: 842
Among:
all pathogens: 1.2%
Gales et al., 2001a
EU SENTRY 1997–1998 Total/SM isolates in:
BSIe: 9194/82 RTI: 2052/54 SSTI: 2320/13 UTI: 2138/3 Among: all pathogens: 1.0% BSI: 0.89%, ranking: 19th RTI: 2.63%, ranking: 9th SSTI: 0.56%, ranking: 19th UTI: 0.14%, ranking: 25th
Fluit et al., 2001a
LA SENTRY 1997–2001 Total isolates: 19547
SM isolates: 166
Among:
all pathogens: 0.8%
Sader et al., 2004
All regions SENTRY 1997–2001 NFGNBfisolates: 18569
SM isolates: 1488
Among: NFGNB: 8%
Jones et al., 2003
All regions SENTRY 1997–2003 Total isolates: 221084
NFGNBfisolates: 25305 Uncommon NFGNBg: 3509 SM isolates: 2076 Among: all pathogens: 0.94% NFGNB: 8.20% Uncommon NFGNB: 59.16%
Sader and Jones, 2005
All regions SENTRY 2001–2004 GNB isolates: 54731
NFGNB isolates: 13808 SM isolates: 1256 Among: GNB: 2.29% NFGNB: 9.10% Gales et al., 2006
Canada CANWARD 2007–2009 Total isolates: 18538
GNB isolates: 8949h SM isolates: 245
Among:
all pathogens: 1.3%, ranking: 17th GNB: 2.7%
Zhanel et al., 2011
AP SENTRY 2008 Total isolates: 5759
SM isolates: 97
Among:
all pathogens: 1.68%
Farrell et al., 2010b
Canada CANWARD 2008 Total isolates: 5282
SM isolates: 57
Among:
all pathogens: 1.1%, ranking: 17th
Zhanel et al., 2010
France MTC 2008–2009 Total isolates: 46400
Uncommon NFGNB isolates: 158 SM isolates: 61 Among: all pathogens: 0.13% Uncommon NFGNB: 39% Fihman et al., 2012
Canada CANWARD 2007–2011 Total isolates: 27123
SM isolates: 378
Among:
all pathogens: 1.4%, ranking 16th
Zhanel et al., 2013
All regions SENTRY 2011 Total isolates: 22005
SM isolates: 362
Among: all pathogens: 1.6% Sader et al., 2013
Canada CANWARD 2011–2012 Total isolates: 6593
SM isolates: 104
Among:
all pathogens: 1.6%
Walkty et al., 2014 BSI
USA SCOPE 1995–1996 NFGNB isolates: 270
SM isolates: 18
Among: NFGNB: 6.7%
Jones et al., 1997
USA, Canada SENTRY 1997 Total isolates:5058
SM isolates: 40
Among:
all pathogens: 0.8%, ranking: 15th In USA: 0.7%, in Canada: 1.1%
Pfaller et al., 1998
NA, LA SENTRY 1997 Total isolates: 9519
GNB isolates: 4267 SM isolates: 69 Among: all pathogens: 0.7% GNB: 1.6% Diekema et al., 1999 (Continued)
TABLE 1 | Continued
Countrya Studyb Year Number of isolates Prevalence and/or ranking References
EU SENTRY 1997–1998 Total isolates: 9194
SM isolates: 82
Among:
all pathogens: 0.89%, ranking: 19th
Fluit et al., 2001a
All regions SENTRY 1997–1999 Among all pathogens in:
AP: 0.9%, Canada: 0.6% EU: 0.9%, LA: 0.8%, USA: 0.7%
Gales et al., 2001a
LA SENTRY 1997–2000 NA Among:
all pathogens: 0.7%
1997: 0.9%, 1998: 0.8%, 1999: 0.6%, 2000: 0.3%
LA SENTRY 1997–2001 Total isolates: 9058
SM isolates: 86
Among:
all pathogens: 0.95%
Sader et al., 2004
Worldwide MTC 2000–2004 All isolates: 26474
SM isolates: 203
Among:
all pathogens: 0.8%
Sader et al., 2005b RTI
NA SENTRY 1997 Total isolates: 2757
SM isolates: 99
Among:
all pathogens: 3.6%, ranking: 8th In USA: 3.5%, in Canada: 3.7%
Jones et al., 2000
LA SENTRY 1997 Total isolates: 556
SM isolates: 13
Among:
all pathogens: 2.3%, ranking: 8th
Sader et al., 1998
NA SENTRY 1998 Total isolates: 2773
SM isolates: 114
Among:
all pathogens: 4.1%, ranking: 8th In USA: 3.7%, in Canada: 5.9%
Mathai et al., 2001
EU SENTRY 1997–1998 Total isolates: 2052
SM isolates: 54
Among:
all pathogens: 2.63%, ranking: 9th
Fluit et al., 2001a
All regions SENTRY 1997–1999 Among all pathogens in:
AP: 2.8%, Canada: 5.2% EU: 3.2%, LA: 1.8%, USA: 3.3%
Gales et al., 2001a
LA SENTRY 1997–2000 Total isolates: 2505
SM isolates: 41
Among:
all pathogens: 1.6%
Gales et al., 2002
LA SENTRY 1997–2001 Total isolates: 3346
SM isolates: 60
Among:
all pathogens: 1.8%
Sader et al., 2004
NA SENTRY 2000 SM isolates: 94 Among:
all pathogens: 3.5%
Hoban et al., 2003
NA, LA, EU SENTRY 2004–2008 Isolates from HABP and
VABPi
Total cases: 31436
Regional incidence: all regions: 3.1%
USA: 3.3%, LA: 2.3%, EU: 3.2%
Jones, 2010
Canada CANWARD 2008 Total isolates: 1612
SM isolates: 42
Among:
all pathogens: 2.6%, ranking: 9th
Zhanel et al., 2010
USA and EU SENTRY 2009–2012 Total isolates: 12851
GNB isolates: 8201
Among all pathogens in: USA: 4.4%, ranking: 6th EU: 3.2%, ranking:9th GNB: 6.02%
Sader et al., 2014a
USA and EU MTC 2012 Total isolates: 2968
SM isolates: 186
Among:
all pathogens:6.3%
Farrell et al., 2014 UTI
NA SENTRY 1997 Total isolates: 1698
GNB isolates: 80% SM isolates: 6 Among: all pathogens: 0.35% GNB: 0.44% Jones et al., 1999b (Continued)
TABLE 1 | Continued
Countrya Studyb Year Number of isolates Prevalence and/or ranking References
EU SENTRY 1997–1998 Total isolates: 138
SM isolates: 3
Among:
all pathogens: 0.14%, ranking: 25th
Fluit et al., 2001a
All regions SENTRY 1997–1999 Among all pathogens in:
AP: 0.2%, Canada: 0.0% EU: 0.2%, LA: 0.0%, USA: 0.3%
Gales et al., 2001a
LA SENTRY 1997–2001 Total isolates: 1961
SM isolates: 0
Among: all pathogens: 0%
Sader et al., 2004
AP region SMARTj 2009–2010 Total GNB isolates: 1762 Among all GNB in:
China: 1.3%, Thailand: 3.3%
Lu et al., 2012
USA SMART 2009–2011 Total GNB isolates: 2135
SM isolates: 6
Among: all GNB: 0.28%
Bouchillon et al., 2013 IAI
China SMART 2002–2009 Total GNB isolates: 3420
SM isolates: 50
Among: all GNB: 1.5% NFGNB: ranking: 3rd
Yang et al., 2010
AP region SMART 2003–2010 Total GNB isolates: 20710
NFGNB isolates: 2252 SM isolates: 204 Among: all GNB: 1.0% NFGNB: 9.1% Liu et al., 2012
Taiwan SMART 2006–2010 Total GNB isolates: 2417
SM isolates: 28
Among: all GNB: 1.2%
Lee et al., 2012
Africa and middle east
TEST 2007–2012 Total isolates of cSSSI14 and IAI from TEST: 1990 and 255
GNB isolates from IAI: 225 SM isolates rom IAI: 16
Among:
all pathogens in IAI: 6.3% GNB in IAI: 7.3%
Renteria et al., 2014
SSTI
NA SENTRY 1997 Total isolates: 1562
SM isolates: 15
Among:
all pathogens: 0.96%
Doern et al., 1999
EU SENTRY 1997–1998 Total isolates: 2320
SM isolates: 13
Among:
all pathogens: 0.56%, ranking: 19th
Fluit et al., 2001a
All regions SENTRY 1997–1999 Among all pathogens in:
USA: 1.0%, Canada: 1.1% AP: 0.1%, EU: 0.6%, LA: 0.4%,
Gales et al., 2001a
LA SENTRY 1997–2001 Total isolates: 1780
SM isolates: 7
Among:
all pathogens: 0.39%
Sader et al., 2004 ICU
EU SENTRY 1997–1998 Total isolates from ICU:
3981
Among:
all pathogens: 1.6%, ranking: 14th BSI: 1.1%, ranking: 15th RTI: 3.0%, ranking: 8th UTI: 0.0%
Fluit et al., 2001b
LA SENTRY 1997–2001 Total isolates: 19547
SM isolates: 166
Among all pathogens in: ICU: 2.77%
Sader et al., 2004
NA SENTRY 2001 Total isolates from ICU:
1321 SM isolates: 40
Respiratory source: 89.0%
Among:
all pathogens: 3.0%, ranking: 10th
Streit et al., 2004
NA, LA, EU, Asia-Australia area
MTC 2000–2004 Isolates from ICU patients Total isolates: 9093 SM isolates: 131
Among:
all pathogens: 1.4%
Sader et al., 2005a
TABLE 1 | Continued
Countrya Studyb Year Number of isolates Prevalence and/or ranking References
German SARI 2003–2004 Isolates collected from 39
German ICUs Total isolates:28266 GNB isolates: 12234
Among all pathogens: Median percentage: 1.7%
Meyer et al., 2006
Canada CAN-ICU 2005–2006 Isolates from ICU patients
Total isolates:4180 SM isolates: 108
Among:
all pathogens: 2.6%
Zhanel et al., 2008
Korea MTC 2008–2009 Respiratory tract isolates
from patient with HABP in ICUs Total isolates: 372 CRGNB isolates: 82 SM isolates: 10 Among: all pathogens: 2.7% CRGNBk: 11.6% Kim et al., 2014 EU MTC(27) 9 countries
Published in 2011 Respiratory tract isolates from patient with HABP in ICUs Total isolates: 495 SM isolates: 13 Among: all pathogens: 2.6% Magret et al., 2011 PEDIATRIC POPULATION
NA SENTRY 1998–2003 Total isolates: 59826
Total isolates from pediatric patients <7 years: 4641 SM isolates: 166
Among: all pathogens in:
all ages: 1.4%, pediatric: 1.2%, both ranking: 10th
Fedler et al., 2006b
NA, LA, EU SENTRY 2004 Total isolates from pediatric
patients ≤18 years: 3537 SM isolates: 53
Among:
all pathogens: 1.5%, ranking: 15th all regions combined
Fedler et al., 2006a
COMMUNITY-ACQUIRED
USA, Canada, LA SENTRY 1997 BSI
SM isolates: 69
CAl/N/unknown: 23/28/18 CA: 33.3%
Diekema et al., 1999
UK and Ireland BSAC 2001–2006 BSI
SM isolates: 165
C/N: 31/66 CA: 33%
Livermore et al., 2008
AP region SMART 2003–2010 IAI
SM isolates: 204
CA/N: 26/125 CA: 17.2%
Liu et al., 2012
France MTC 2008–2009 All sources
SM isolates: 61
CA/N: 9/29 CA: 23.7%
Fihman et al., 2012
Taiwan SMART 2006–2010 IAI
SM isolates: 28
CA/Ni: 3/18 CA: 14.3%
Lee et al., 2012
aNA, North America; LA, Latin America; EU, Europe; USA, the United States of America; UK, United Kingdom; AP, Asia-Pacific.
bSENTRY, The SENTRY Antimicrobial Surveillance Program; SMART, Study for Monitoring Antimicrobial Resistance Trends; CAN-ICU, The Canadian Intensive Care Unit Surveillance
Study; CANWARD, The Canadian Ward Surveillance Study; SARI, Surveillance of Antibiotic Use and Bacterial Resistance in ICUs(German); BSAC, The British Society for Antimicrobial Chemotherapy Resistance Surveillance Project; TEST, Tigecycline Evaluation Surveillance Trial; TIST, Tigecycline In Vitro Surveillance in Taiwan; TSAR, Taiwan Surveillance of Antimicrobial Resistance; SCOPE, Surveillance and Control of Pathogens of Epidemiologic Importance (USA); MTC, multicenter studies.
cThe SENTRY Antimicrobial Surveillance Program has monitored the predominant pathogens and antimicrobial resistance in 5 geographic regions: Asia-Pacific, Europe, Latin America,
Canada, and the United States (Gales et al., 2001a).
dSM, Stenotrophomonans maltophilia.
eBSI, bloodstream infection; RTI, respiratory tract infection; IAI, intra-abdominal infection; UTI, urinary tract infection; SSTI, skin and soft tissue infection. fNFGNB, non-fermentative Gram-negative bacilli
gUncommon NFGNB, Acinetobacter spp. and Pseudomonas aeruginosa excluded.
hOf the 18538 organisms collected, the 20 most common represented 16780 (90.5%) of the isolates and underwent susceptibility testing, which included 8949 (53.3%) Gram-negative
bacilli.
iHABP, hospital-acquired bacterial pneumonia; VABP, ventilator-associated bacterial pneumonia.
jSMART is a global surveillance program that has monitored the in vitro susceptibility patterns of clinical Gram-negative bacilli to antimicrobial agents collected worldwide from
intra-abdominal infections since 2002 and urinary tract infections since 2009 (Morrissey et al., 2013).
kCRGNB, carbapenem-resistant Gram-negative bacteria.
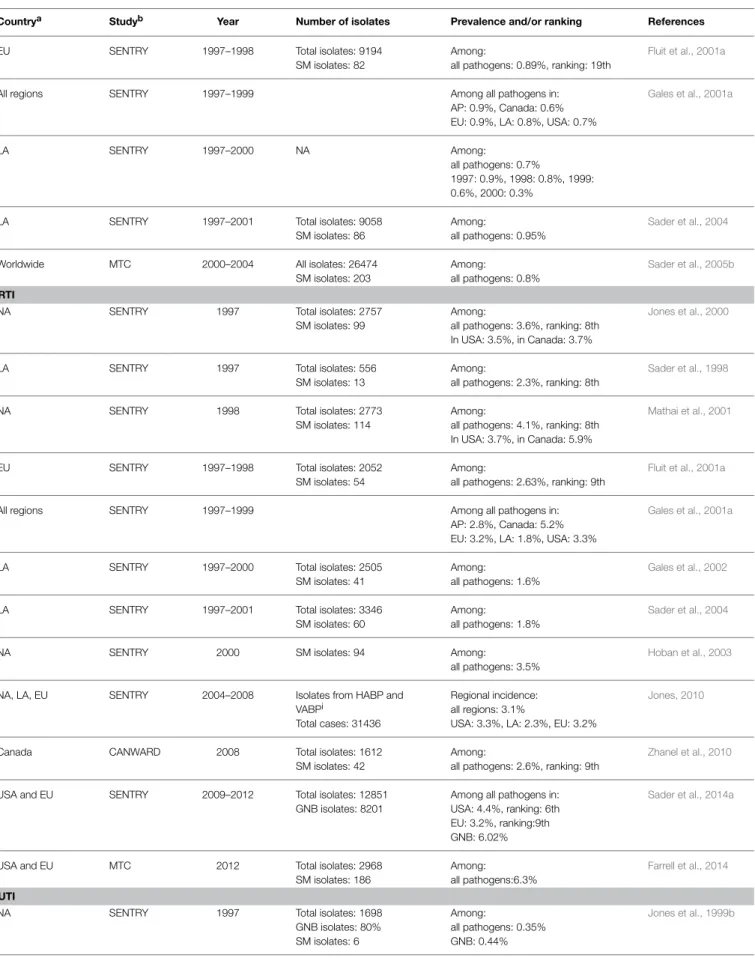
It has been observed in the general population (
Gales et al.,
2001a
) and in ICUs (
Fluit et al., 2001b
) alike that S. maltophilia
is most frequently associated with respiratory tract infections
(RTIs), followed by bloodstream infections (BSIs), and, rarely,
skin and soft tissue infections (SSTIs) and urinary tract infections
(UTI) (
Gales et al., 2001a
). The prevalence of RTIs due to S.
maltophilia is generally higher than that of other infections
caused by that pathogen, but varies widely among countries and
continents, ranging from 1.6 to 6.3% during the period 1997–
2012 (
Sader et al., 1998, 2004, 2014a; Jones et al., 2000; Fluit et al.,
2001a; Gales et al., 2001a, 2002; Mathai et al., 2001; Hoban et al.,
2003; Jones, 2010; Zhanel et al., 2010; Farrell et al., 2014
). The
United States has the most consecutive records regarding RTI
isolates collected by the SENTRY program. Based on data from
four SENTRY studies (
Gales et al., 2001a; Hoban et al., 2003;
Jones, 2010; Sader et al., 2014a
), the prevalence rates increased
from 3.3–3.5% during 1997–2004 to 4.4% during 2009–2012.
During that 15-year period, S. maltophilia went from being the
eighth to the sixth most common cause of RTI. In a large study
on 2968 RTI isolates collected from 59 medical centers in the
USA and 15 centers in European countries in 2012, 6.3% of
the pathogens were S. maltophilia (
Farrell et al., 2014
). These
observations suggest an increasing frequency of occurrence of
respiratory tract infections due to S. maltophilia.
S. maltophilia is less frequently isolated from patients with
BSIs, UTIs, or SSTIs than from patients with RTIs, with reported
isolation rates ranging from 0.7 to 1.1% for BSIs (
Jones et al.,
1997; Pfaller et al., 1998; Diekema et al., 1999; Fluit et al.,
2001a; Gales et al., 2001a; Sader et al., 2004, 2005b
), 0–0.3%
for UTIs (
Pfaller et al., 1998; Jones et al., 1999b; Fluit et al.,
2001a; Gales et al., 2001a; Sader et al., 2004, 2005b
), and 0.39–
0.96% for SSTIs (
Diekema et al., 1999; Fluit et al., 2001a; Gales
et al., 2001a; Sader et al., 2004
). SMART studies have also shown
that isolation of S. maltophilia from intra-abdominal infections
(IAIs) is also fairly uncommon, with rates ranging from 1 to
1.7% (2002–2010) (
Guembe et al., 2008; Yang et al., 2010; Lee
et al., 2012; Liu et al., 2012
). However, data from African and
Middle Eastern countries collected as part of the Tigecycline
Evaluation Surveillance Trial during 2007–2012 (
Renteria et al.,
2014
) revealed an uncommonly high rate of isolation (6.3%) of
S. maltophilia from patients with IAIs. In addition, the results
from a SMART study surveying UTIs in the Asian-Pacific region
during 2009–2010 disclosed higher rates of S. maltophilia isolated
from patients with UTIs in China (1.3%) and Thailand (3.3%)
than in other countries (
Lu et al., 2012
), although the rates were
not as high as those in certain countries in Africa and the Middle
East.
Gram-negative Bacilli (GNB) and
Non-fermenting Gram-negative Bacilli
(NFGNB)
The worldwide rate of isolation of S. maltophilia among GNB
pathogens ranges from 2.29 to 2.7% according to a SENTRY
study (2001–2004) (
Gales et al., 2006
) and a CANWARD
surveillance study (2007–2009) (
Zhanel et al., 2011
). In the US
state of Texas, however, a study at the M. D. Anderson Cancer
Center revealed an increasing trend in the ratio of S. maltophilia
among GNB isolates obtained from cancer patients during 1986–
2002 (from 2% in 1986 to 7% in 2002) (
Safdar and Rolston,
2007
).
Among NFGNB, S. maltophilia has been reported to be
the third most commonly isolated pathogen after Pseudomonas
aeruginosa and Acinetobacter baumannii. In a large survey
conducted as a part of the SENTRY program, 221,084 GNB
isolates were collected worldwide, including 25,305 (11.5%)
NFGNB isolates, of which Acinetobacter spp. and P. aeruginosa
accounted for the vast majority (87.7%). The remaining 3509
isolates were deemed unusual NFGNB species. Of them, S.
maltophilia was the most frequently isolated (n = 2076, 59.16%)
(
Sader and Jones, 2005
). A similar finding was reported in a
prospective multi-center study involving nine teaching hospitals
in France, in which S. maltophilia was the most commonly
isolated NFGNB among all unusual NFGNB species (39%)
(
Fihman et al., 2012
). Other surveillance studies, namely SCOPE
(
Jones et al., 1997
), SENTRY (
Jones et al., 2003; Gales et al.,
2006
), and SMART (
Liu et al., 2012
) showed a steady increase in
isolation of S. maltophilia among all NFGNB pathogens during
the period 1995–2010 (6.7% in 1995–1996, 8.0% in 1997–2001,
and 9.1% in 2001–2010). These findings show that S. maltophilia
is not an insignificant pathogen among disease-causing GNB and
NFGNB species.
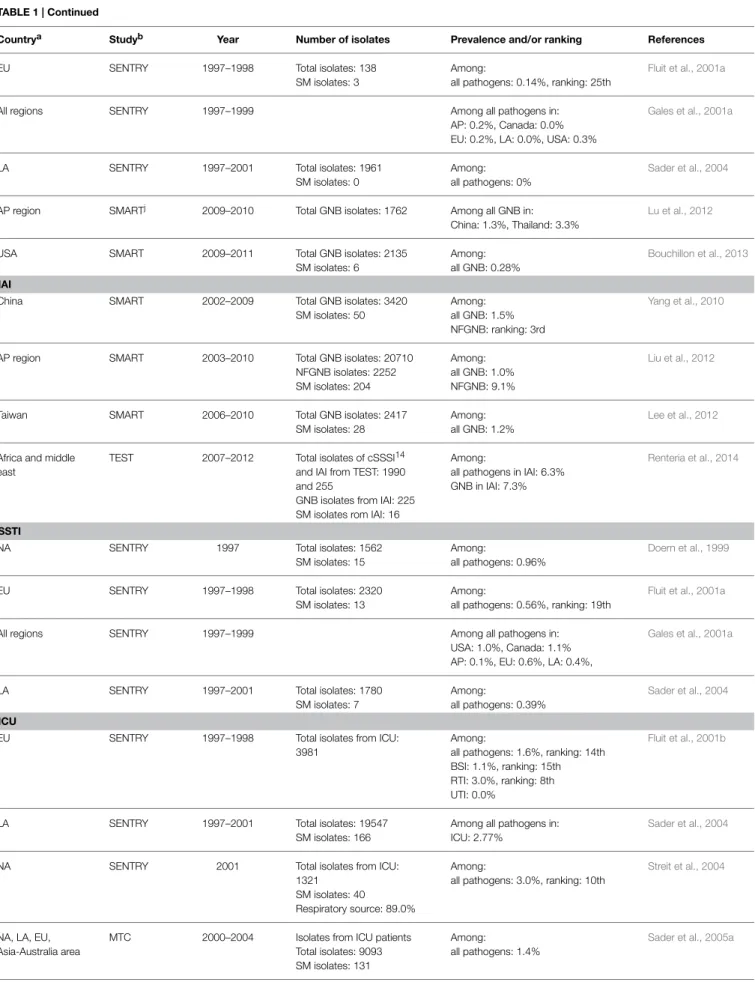
Intensive Care Units, Pediatric Population,
and Community-acquired Infections
As expected, the prevalence of infections due to S. maltophilia
is higher in intensive care units (1.4–3.0%) than in the general
population (
Fluit et al., 2001b; Sader et al., 2004; Streit et al., 2004;
Sader et al., 2005a; Meyer et al., 2006; Zhanel et al., 2008; Magret
et al., 2011; Kim et al., 2014
).
There is limited information on the worldwide prevalence
of S. maltophilia infections in the general pediatric population.
SENTRY studies conducted during 1998–2003 (
Fedler et al.,
2006b
) and in 2004 (
Fedler et al., 2006a
) showed that the
prevalence of infections due to S. maltophilia was 1.2% among
children ≤ 7 years and 1.4% among children ≤ 18 years
old. The rates are similar to those in the adult population.
A comparison of two single-center studies in China and the
USA revealed markedly different incidence rates of
ventilator-associated pneumonia due to S. maltophilia among pediatric
patients in ICUs. Ning et al. reported a rate of 20.3% among
patients aged 2 months to 16 years in a pediatric ICU in China
(
Ning et al., 2013
) whereas Arthur et al. found that the rate of
infection due to S. maltophilia among infants aged 0–6 months
in a cardiac ICU in the USA was only 0.8% (
Arthur et al., 2015
).
Several recent studies have shown that S. maltophilia is also
an emerging opportunistic pathogen in community settings
(
Falagas et al., 2009a; Chang et al., 2014
). Results of a worldwide
SENTRY study in 1997 (
Diekema et al., 1999
) and the British
Society for Antimicrobial Chemotherapy Resistance surveillance
project conducted during 2001–2006 (
Livermore et al., 2008
)
showed that 33.3 and 32%, respectively, of S. maltophilia
isolates were collected within 48 h after admission (defined
as community-acquired in these studies) from patients with
bloodstream infections. The results from two recent SMART
studies revealed that 14.3–17.2% of isolates from patients with
community-acquired IAI (also defined by a 48-h time frame
within admission) during 2003–2010 were S. maltophilia (
Lee
et al., 2012; Liu et al., 2012
). Another recent study on the
prevalence of community-acquired S. maltophilia BSI in Taiwan,
which specifically divided the patients into three categories
based on whether they had community-acquired (excluding
patients hospitalized within 90 days before admission, cared
in a nursing facility, etc.), healthcare-associated or
hospital-acquired infections, reported that 17.6% of all
community-acquired bloodstream infections were due to S. maltophilia
(
Chang et al., 2014
). A similar study in France revealed that
23.7% of all community-acquired BSIs were due to S. maltophilia
(
Fihman et al., 2012
). These studies show that
community-acquired S. maltophilia infections are far less rare than previously
thought.
Risk Factors of Mortalty
A number of risk factors for death due to S. maltophilia
infections have been reported. Paez et al. (
Paez and Costa,
2008
) reviewed the literature from 1985 to 2008 and found
that BSI and pneumonia, shock, thrombocytopenia, and
Acute Physiological Assessment and Chronic Health Evaluation
(APACHE) score >15 are independent risk factors associated
with outcome. In addition, underlying hematological malignancy
and admission to ICU are independent risk factors for cancer
patients. The impact of appropriate antimicrobial treatment and
removal of CVC on mortality were concluded to require further
clinical studies (
Paez and Costa, 2008
). The conclusion of the
review corresponds to the aforementioned studies. Falagas et al.
analyzed 15 articles for attributable mortality of S. maltophilia
infections. Only four studies provided relevant data regarding
inappropriate antibiotic treatment, and three out of the four
studies found significantly higher mortality when compared with
initial appropriate therapy (
Falagas et al., 2009b
).
Antimicrobial Susceptibility
There
are
limited
antimicrobial
options
for
infections
due to S. maltophilia because of its extensive resistance
to
most
antibiotics,
including
β
-lactam
antibiotics,
cephalosporins, macrolides, aminoglycosides, and carbapenems.
Interpretive
breakpoints
for
susceptibility
are
available
only
for
ticarcillin/clavulanate,
ceftazidime,
minocycline,
levofloxacin,
trimethoprim/sulfamethoxazole
(TMP/SMX),
and chloramphenicol (
CLSI, 2015
). Table 2 shows the rates of
susceptibility of S. maltophilia to antimicrobial agents reported
in the studies presented in Table 1. TMP/SMX is recognized
as the drug of choice (
Wang et al., 2014a
). Resistance rates
vary geographically but are generally less than 10% (
Chung
et al., 2013
). However, high and various rates of resistance
to TMP/SMX have been reported in patients with cancer
(
Vartivarian et al., 1994; Micozzi et al., 2000
), cystic fibrosis
(
Saiman et al., 2002; Cantón et al., 2003; San Gabriel et al., 2004;
Valenza et al., 2008
), and in several countries, including Taiwan,
Japan, Korea, Thailand, Spain, Mexico, Saudi Arabia, Turkey,
and Canada (16–78.8%) (
Valdezate et al., 2001; del Toro et al.,
2002; Lai et al., 2004; Gülmez and Hasçelik, 2005; Memish et al.,
2012; Wu et al., 2012; Rattanaumpawan et al., 2013; Rhee et al.,
2013; Zhanel et al., 2013; Flores-Treviño et al., 2014; Hotta
et al., 2014; Walkty et al., 2014; Wang et al., 2014a
). In the
present review, global surveillance data for the period 1997–2012
show that S. maltophilia continues to be highly susceptible to
TMP/SMX (Table 2). Over that 15-year period, the susceptibility
rates reported in worldwide SENRTY studies (
Gales et al., 2001a;
Jones et al., 2003; Gales et al., 2006; Sader et al., 2013, 2014a
),
a BSAC surveillance study (
Livermore et al., 2008
), and three
large-scale multi-national studies (
Sader et al., 2005b; Farrell
et al., 2010a, 2014
) ranged from 90 to 100%.
Ceftazidime and ticarcillin/clavulanate used to be the most
effective among β-lactam drugs against S. maltophilia. However,
recent studies have demonstrated resistance rates of more than
30% and a trend in decreasing susceptibility with ceftazidime
(47–75% during 1997–1999 to 30.5–36.8% during 2009–2012)
(Table 2) (
Gales et al., 2001a; Farrell et al., 2010a; Sader et al.,
2014b
). The same is true for ticarcillin/clavulanate. During
1997–1998, the rates of susceptibility of S. maltophilia to that
combination ranged from 71–90% but dropped to 27–46.1%
during 2003–2008.
New fluoroquinolones exhibit better potency against S.
maltophilia than ceftazidime or ticarcillin/clavulanate and have
become reasonable alternatives. Nonetheless, a comparison of
data from worldwide SENTRY studies reveals a decrease in
sensitivity of S. maltophilia to levofloxacin, from 83.4% during
the period 2003–2008 (
Farrell et al., 2010a
) to 77.3% in 2011
(
Sader et al., 2013
). Low susceptibility rates ranging from 64–
69.6% have also been reported in Canada (
Zhanel et al., 2013
),
China (
Yang et al., 2010; Tan et al., 2014
), and Korea (
Chung
et al., 2013
). Few multi-center studies have investigated the
efficacy of fluoroquinolones against S. maltophilia in patients
with UTIs. In a SMART study conducted in the Asia-Pacific
region, isolates of S. maltophilia from patients with UTIs showed
exceptionally high rates of resistance to levofloxacin (33.3%) (
Lu
et al., 2012
). Two recent reports showed low MIC50
(minimum
inhibitory concentration)values (0.5 mg/L and 0.5 mg/L) and
low MIC90
values (8 and 4 mg/L) for moxifloxacin against S.
maltophilia (
Zhanel et al., 2008; Chung et al., 2013
), indicating
that moxifloxacin could be considered an effective alternative.
Data from a number of studies demonstrate that ciprofloxacin
has poor activity against S. maltophilia, with susceptibility rates
averaging lower than 50% (Table 2).
Minocycline, doxycycline, and tigecycline have consistently
displayed good potency against S. maltophilia in studies with
various time periods, sources of specimens, and geographic
regions (
Sader et al., 2005b, 2013, 2014b; Gales et al., 2008;
Chen et al., 2012; Wu et al., 2012; Chung et al., 2013
). A TSAR
surveillance study conducted in Taiwan tested 377 isolates of
S. maltophilia obtained over a 10-year period (1998–2008) and
revealed low MIC50
(0.25 mg/L) and MIC90
values (1 mg/L) for
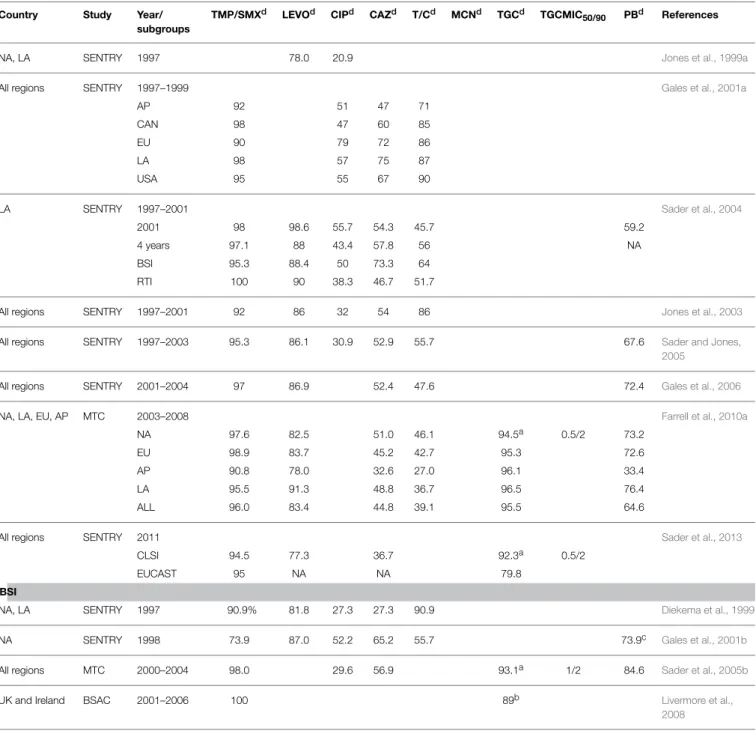
TABLE 2 | Susceptibility of S. maltophilia to various antimicrobial agents in worldwide surveillance and multicenter studies.
Country Study Year/
subgroups
TMP/SMXd LEVOd CIPd CAZd T/Cd MCNd TGCd TGCMIC
50/90 PBd References
NA, LA SENTRY 1997 78.0 20.9 Jones et al., 1999a
All regions SENTRY 1997–1999 Gales et al., 2001a
AP 92 51 47 71
CAN 98 47 60 85
EU 90 79 72 86
LA 98 57 75 87
USA 95 55 67 90
LA SENTRY 1997–2001 Sader et al., 2004
2001 98 98.6 55.7 54.3 45.7 59.2
4 years 97.1 88 43.4 57.8 56 NA
BSI 95.3 88.4 50 73.3 64
RTI 100 90 38.3 46.7 51.7
All regions SENTRY 1997–2001 92 86 32 54 86 Jones et al., 2003
All regions SENTRY 1997–2003 95.3 86.1 30.9 52.9 55.7 67.6 Sader and Jones,
2005
All regions SENTRY 2001–2004 97 86.9 52.4 47.6 72.4 Gales et al., 2006
NA, LA, EU, AP MTC 2003–2008 Farrell et al., 2010a
NA 97.6 82.5 51.0 46.1 94.5a 0.5/2 73.2
EU 98.9 83.7 45.2 42.7 95.3 72.6
AP 90.8 78.0 32.6 27.0 96.1 33.4
LA 95.5 91.3 48.8 36.7 96.5 76.4
ALL 96.0 83.4 44.8 39.1 95.5 64.6
All regions SENTRY 2011 Sader et al., 2013
CLSI 94.5 77.3 36.7 92.3a 0.5/2
EUCAST 95 NA NA 79.8
BSI
NA, LA SENTRY 1997 90.9% 81.8 27.3 27.3 90.9 Diekema et al., 1999
NA SENTRY 1998 73.9 87.0 52.2 65.2 55.7 73.9c Gales et al., 2001b
All regions MTC 2000–2004 98.0 29.6 56.9 93.1a 1/2 84.6 Sader et al., 2005b
UK and Ireland BSAC 2001–2006 100 89b Livermore et al.,
2008
aTigecycline breakpoints of ≤2 µg/mL for susceptibility and ≥8 mg/L for resistance were used for comparison purposes only, as defined by the USFDA. bSusceptibility to tigecycline at the breakpoint of 1 mg/L used for Enterobacteriaceae and Acinetobacter spp.
cResistant strains with colistin and polymyxin B MICs of ≥4 mg/L.
dAntibiotics abbreviations: TMP/SMX, trimethoprim/sulfamethoxazole; LEVO, levofloxacin; CIP, ciprofloxacin; CAZ, ceftazidime; T/C, Ticarcillin/Clavulanate; PB, polymyxin B; TGC,
tigecycline; MCN, minocycline.
tigecycline (
Wu et al., 2012
). Similar results were demonstrated
in several large-scale worldwide surveillance studies as well. A
recent SENTRY study conducted during 2009–2012 (494 isolates)
(
Sader et al., 2014a
) revealed a susceptibility of 96% and a
recent TEST study conducted during 2007–2012 (2245 isolates)
(
Renteria et al., 2014
) demonstrated low MIC50
(0.25 mg/L) and
MIC90
(1 mg/L) values.
Molecular Mechanisms in Antimicrobial
Resistance
S. maltophilia has several molecular mechanisms contributing
to its extensive antimicrobial resistance. The mechanisms are
summarized in Table 3. Detailed descriptions of the major
mechanisms are elaborated as follows.
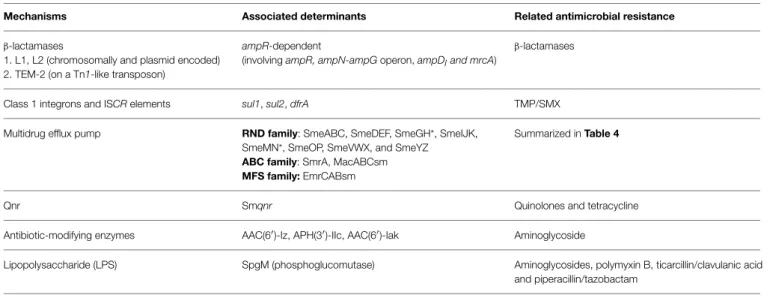
TABLE 3 | Molecular mechanisms of antimicrobial resistance in S. maltophilia.
Mechanisms Associated determinants Related antimicrobial resistance
β-lactamases
1. L1, L2 (chromosomally and plasmid encoded) 2. TEM-2 (on a Tn1-like transposon)
ampR-dependent
(involving ampR, ampN-ampG operon, ampDIand mrcA)
β-lactamases
Class 1 integrons and ISCR elements sul1, sul2, dfrA TMP/SMX
Multidrug efflux pump RND family: SmeABC, SmeDEF, SmeGH*, SmeIJK, SmeMN*, SmeOP, SmeVWX, and SmeYZ ABC family: SmrA, MacABCsm MFS family: EmrCABsm
Summarized in Table 4
Qnr Smqnr Quinolones and tetracycline
Antibiotic-modifying enzymes AAC(6′)-Iz, APH(3′)-IIc, AAC(6′)-Iak Aminoglycoside
Lipopolysaccharide (LPS) SpgM (phosphoglucomutase) Aminoglycosides, polymyxin B, ticarcillin/clavulanic acid and piperacillin/tazobactam
Mutations of bacterial topoisomerase and gyrase genes Reduction in outer membrane permeability
*not yet characterized.
β-Lactamases
S. maltophilia has two chromosomal-mediated inducible
β-lactamases, namely L1 and L2. L1 is a molecular class B
Zn
2+-dependent metallo-β-lactamase and L2 is a molecular
class A clavulanic acid-sensitive cephalosporinase. The L1 and
L2 β-lactamases are simultaneously regulated by AmpR, a
transcriptional regulator in the L2 upstream region (
Okazaki
and Avison, 2008
). The ampR-L2 module is homologous to
the ampR-ampC systems, which are widely distributed in some
members of the family Enterobacteriaceae and in P. aeruginosa
(
Lodge et al., 1990
). The regulation of chromosomal ampR-ampC
systems has been well studied in Citrobacter freundii, where
the AmpC β-lactamase induction is linked to peptidoglycan
recycling and involves several regulatory genes, such as as
ampR, ampG, and ampD (
Lindberg et al., 1985
). A similar
induction mechanism was proposed for the ampR-ampC and
the ampR-L2 modules (
Okazaki and Avison, 2008
). But unlike
P. aeruginosa, the permease system in S. maltophilia requires
an intact ampN-ampG operon for the induction of β-lactamase
(
Huang et al., 2010
). Two ampD homologs, ampDI
and
ampDII
, were found in S. maltophilia, but only ampDIappears
to be relevant to the regulation of β-lactamase (
Yang et al.,
2009
).
Penicillin-binding
proteins
(PBPs)
participate
in
peptidoglycan biosynthesis and the inactivation of PBP4 in
P. aeruginosa has been shown to confer AmpC overexpression
and β-lactam resistance (
Moya et al., 2009
). The inactivation
of a putative PBP1a gene, mrcA, recently was found to
cause basal-level L1/L2 β-lactamase hyperproduction in S.
maltophilia KJ. The inactivation of mrcA only affects basal
L1/L2 production β-lactamase, which is ampR- and
ampN-ampG-dependent, and does not augment their induction (
Lin
et al., 2011
). The universality of disruption of ampDI
or mrcA
in β-lactamase-hyperproducing S. maltophilia mutants and
clinical isolates has been proved by the existence of wild-type
ampDI
and mrcA genes. The result implicates mutation of
at least one additional gene in this phenotype (
Talfan et al.,
2013
).
Efflux Pumps
Efflux pumps in microorganisms mediate the extrusion of drugs
and are classified into five families, namely the
resistance-nodulation-cell-division (RND) family, the major facilitator
superfamily (MFS), the small multidrug resistance (SMR) family,
the ATP binding cassette (ABC) family, and the multidrug
and toxic compound extrusion (MATE) family (
Putman et al.,
2000
). Two ABC-type (SmrA, MacABCsm), one MFS-type
(EmrCABsm), a fusaric acid extrusion efflux pump (FuaABC),
and six out of the eight postulated RND-type efflux systems
have been characterized in S. maltophilia (
Alonso and Martinez,
2000; Li et al., 2002; Crossman et al., 2008; Al-Hamad et al.,
2009; Chen et al., 2011; Hu et al., 2012; Gould et al., 2013;
Huang et al., 2013a; Lin et al., 2014a,b
). The six characterized
RND-type efflux pumps in the S. maltophilia genome are
SmeABC, SmeEF, SmeIJK, SmeOP, SmeVWX, and SmeYZ
(including SmeGH and SmeMN). Table 4 provides a summary
of antimicrobial resistance associated with the abovementioned
efflux pumps.
SmeABC
The overexpression of smeABC genes confers resistance to
aminoglycosides, β-lactams, and fluoroquinolones. SmeC was
identified to function independently of SmeAB, while deletions
in smeC but not smeB compromised the antimicrobial resistance
(
Li et al., 2002
).
TABLE 4 | Genetic determinants of efflux pumps.
Efflux pumps Associated antibiotic resistance
RND FAMILY
SmeABC Quinolones, ß-lactams and aminoglycosides
SmeDEF Quinolones, tetracyclines, macrolides, chloramphenicol, novobiocin and trimethoprim/sulfamethoxazole
SmeIJK Ciproxin, levofloxacin, tetracycline and minocycline
SmeOP-TolCsm Trimethoprim/sulfamethoxazole, aminoglycosides, macrolides, doxycycline, chloramphenicol, and nalidixic acid
SmeVWX Quinolones, chloramphenicol and tetracyclines
SmeYZ Trimethoprim/sulfamethoxazole and aminoglycosides ABC FAMILY
SmrA Fluoroquinolones and tetracycline
MacABCsm Aminoglycosides, macrolides and polymyxins MFS FAMILY
EmrCABsm Nalidixic acid and erythromycin FUSARIC ACID TRIPARTITE EFFLUX PUMP
FuaABC fusaric acid