143 Bulletin of Marine Science
© 2005 Rosenstiel School of Marine and Atmospheric Science of the University of Miami
SEXUAL REPRODUCTION OF THE ALCYONACEAN CORAL
LOBOPHYTUM PAUCIFLORUM IN SOUTHERN TAIWAN
Tung-Yung Fan, Yu-Hsiang Chou, and Chang-Feng Dai
ABSTRACT
Sexual reproduction of the alcyonacean octocoral Lobophytum pauciflorum (Eh-renberg) in Nanwan Bay, southern Taiwan was studied by histological examinations of gonad development on monthly samples collected from tagged colonies between March 2001 and November 2002. A sample of 15 aggregations of L. pauciflorum in an area of 10 × 30 m was also examined in July 2002 to determine reproductive traits. Lobophytum pauciflorum is a gonochoric broadcast spawner and the sex ratio of the population is 1:1. Lobophytum pauciflorum often forms aggregations in which all of the colonies have the same sex. This suggests that the colonies in an aggrega-tion are likely formed by asexual fission. Most (>50%) of the colonies in the female and male aggregations had gonads when their diameters were 10–15 and 5–10 cm, respectively. The average maximum fecundity was 16 eggs polyp−1. Mature eggs
were 400–870 µm in diameter. Oogenesis and spermatogenesis took about 12 mo. Spawning occurred from July–September during late summer to early autumn. This spawning season is consistent with the environmental conditions such as warmer water temperatures and new bare substrate created by typhoon disturbances that favor the survival and settlement of coral larvae.
Research on sexual reproduction of soft corals has increased enormously in the
last two decades. These studies have revealed a wide variety of reproductive
pat-terns among coral species and geographical regions. However, most studies have
been concentrated in relatively few geographical locations, mainly the Red Sea (e.g.,
Benayahu, 1997a) and the Great Barrier Reef, Australia (e.g., Alino and Coll, 1989).
Other regions with abundant soft corals, particularly the West Pacific islands, have
received little attention. Research on soft coral reproduction in these areas is needed
for a global perspective of life history patterns and a better understanding of the
adaptive significance of reproductive traits in soft corals (Benayahu, 1997a).
The characteristics of sexual reproduction in soft corals are diverse. Benayahu
(1997a) concluded that there are at least three modes of sexual reproduction in
al-cyonacean corals; i.e., broadcasting of gametes, external surface brooding, and
in-ternal brooding of planulae. The majority of tropical Alcyoniidae studied to date are
gonochoric broadcast spawners with external fertilization and larval development
(e.g., Alino and Coll, 1989; Benayahu, 1997a). Many broadcast spawning
alcyona-ceans have an annual spermatogenic cycle while their oogenesis is completed over a
prolonged period with overlapping oogenic cycles (Yamazato et al., 1981; Alino and
Coll, 1989; Benayahu, 1997a).
Broadcast spawning soft corals have been found to have short, seasonal, and
syn-chronized spawning episodes (Alino and Coll, 1989; Benayahu, 1997a), similar to
those of scleractinian corals (Harrison and Wallace, 1990). On the Great Barrier
Reef, Australia, the soft corals spawn during the multispecies mass spawning event
(Babcock et al., 1986; Alino and Coll, 1989). In contrast, the Red Sea soft corals
ex-BULLETIN OF MARINE SCIENCE, VOL. 76, NO. 1, 2005 144
hibit an extended reproductive season (Benayahu, 1997a), as do the scleractinian
cor-als in that region (Shlesinger and Loya, 1985).
Lobophytum pauciflorum (Ehrenberg, 1834) is abundant and widely distributed
in Indo-West Pacific reefs (Tursch and Tursch, 1982; Verseveldt, 1983; Benayahu,
1997b, 2002). It forms aggregations of physiologically discrete colonies (Fan, pers.
obs.). The colonies encrust reef surfaces with low stalks and erect lobes. As is typical
for this genus, polyps of L. pauciflorum are dimorphic with autozooids functioning
as feeding polyps and bearing gonads. Little information is available on its
reproduc-tive biology other than that it is a gonochoric spawner and that it participates in a
mass spawning event on the Great Barrier Reef (Alino and Coll, 1989). Lobophytum
pauciflorum is one of the most abundant soft corals on coral reefs of southern
Tai-wan. The present study describes sexual reproductive characteristics of L.
pauciflo-rum in southern Taiwan, including its sexuality, size structure, reproductive mode,
spermatogenic and oogenic cycles, month of spawning, colony size at sexual
matu-rity, and relationship between colony size and fecundity. The results are compared
with reproductive data for L. pauciflorum from the Great Barrier Reef and other
Lobophytum species.
MATERIALS AND METHODS
STUDY SITE.—This study was conducted on coral reefs in Nanwan Bay, southern Taiwan (21°55.950´ N; 120°44.691´E) from March 2001–November 2002. All corals used in this study were collected monthly or bimonthly from the reef slope at 7–9 m depth.
SEXUAL REPRODUCTION.—Large colonies (>13 cm in diameter) of L. pauciflorum were randomly selected and tagged in March, April, and July 2001. Twenty-three female and five male colonies were identified after examination of spicules and gonads in these tagged colo-nies. Pieces, each 2 cm in diameter, were cut off at least 3 cm from the edge of tagged large colonies. To prevent excessive damage to the colonies, not all of the tagged colonies were sampled on each collection date. Each sample was placed in a plastic bag with a waterproof label. The color of eggs and sperm sacs of each sample was scored when they were alive. Then, the samples were fixed with 10% formalin in seawater for at least 24 hrs. Polyps were dissected by fine-point forceps under a binocular microscope. Wet preparations of their mesenteries were made and examined under a microscope. The sex of each colony was determined and the diameters of oocytes or sperm sacs were measured from each colony using a calibrated ocular micrometer. Only oocytes with apparent color and > 400 μm in diameter were scored as mature. Histological sections were used to confirm the developmental stage of oocytes and sperm sacs. For histological preparation, samples were fixed with 10% formalin in seawater for at least 24 hrs, rinsed in freshwater, decalcified in 8% formic acid, and stored in 70% alco-hol. Tissue samples were dehydrated with increasing concentrations of alcohol, cleared with xylene, and embedded in Paraplast. Serial sections 6–8 µm thick were prepared and stained with Mayer’s hematoxylin and eosin. These slides were examined for gamete development under a compound microscope at magnifications up to 1000×. The diameters of oocytes and sperm sacs were assigned to arbitrary size classes in 50 µm intervals (i.e., 0–49, 50–99 µm, etc.) to determine the size frequencies of gonads. The monthly variation in size frequencies for oocytes and sperm sacs of different size classes as well as the monthly variation in percent-ages of colonies containing mature gonads were used to determine the seasonal pattern of gametogenesis and spawning among colonies.
A sample of 15 aggregations of L. pauciflorum in an area of 10 × 30 m was collected and examined before the predicted spawning month; i.e., in July 2002, to determine sex ratio, size structure of male and female colonies, and the relationship between colony size and repro-ductive traits such as sexual maturity and fecundity. In total, 146 colonies (78 female and 68
male) in the aggregation were measured and sampled. Two perpendicular diameters across the colony center were measured and their average was taken as the diameter of the colony. A piece ≥ 2 cm was collected from the center of each colony. All colonies in an aggregation were counted and sexed. The samples were fixed with 10% formalin in seawater for at least 24 hrs. Samples were examined at 64× magnification under a dissecting microscope. Colonies were considered sexually mature if oocytes or sperm sacs were visible under a dissecting micro-scope. Five polyps selected randomly from each female colony were dissected and the mean number of eggs per polyp was used to estimate polyp fecundity.
RESULTS
Histological sections revealed that all colonies of L. pauciflorum are gonochoric.
Female and male gonads develop along mesenteries within polyp cavities in the
autozooids of separate colonies. Autozooids bear gonads on up to six of the eight
mesenteries, with early stage oocytes developing proximally and mature oocytes
dis-tally on mesenteries relative to the oral disc of polyps. Gonad maturation occurred
synchronously within and between colonies. Maturation of oocytes was
accom-panied by a gradual color change from white to deep purple. Mature oocytes were
first recorded around May 2001 and 2002, 3–4 mo prior to spawning. Maturation of
sperm sacs was accompanied by a gradual color change from transparent to white.
Mature sperm sacs were first recorded around June 2001 and 2002, 2–3 mo prior
to spawning. The mature eggs and sperm sacs reached a diameter of 400–870 and
200–525 µm, respectively. The maturity of gonads before spawning was confirmed
by histological sections. For mature eggs, the nuclei migrated toward the periphery
of oocytes and became dented at one side. The mature sperm sacs had lumens.
Sper-matozoa were arranged with the heads located peripherally and the tails projecting
toward the lumen.
GAMETOGENESIS.—Monthly variation of size-frequency distributions in oocyte
diameters and the percentage of colonies containing mature eggs in L. pauciflorum
from March 2001–November 2002 indicated a clear annual oogenic cycle (Figs. 1,3A).
Early oocytes appeared in mesenteries in March 2001, and continued to grow until
September. Mature eggs with purple coloration appeared in May 2001 and 2002 (Fig.
3A). The color gradually deepened with time. All colonies (n = 11) had mature eggs
on 4 September 2001 (lunar day 17, full moon phase) and had spawned, as evidenced
by the disappearance of eggs in the tagged colonies when sampled on 21 September
2001 (lunar day 5, new moon phase). In 2002, all samples contained mature eggs in
June (n = 12) and July (n = 9), 73% had mature eggs in August, 46% had mature eggs
in September, and none of the samples had mature eggs in November. The sharp
decline in colonies containing mature eggs in September 2001 as well as the gradual
decline from July–September 2002 were probably the result of spawning.
Monthly variation in size-frequency distributions of sperm sac diameters and
percentages of colonies containing mature sperm sacs also indicated a clear annual
spermatogenic cycle (Figs. 2, 3B). Early sperm sacs appeared in mesenteries in April
2001, and continued to grow until August. All colonies (n = 3) had mature sperm sacs
in June 2001, 75% had mature sperm sacs in September, and none of the samples had
mature sperm sacs in November. The gradual decline in colonies containing mature
sperm sacs from August–November 2002 was probably the result of spawning.
SEX RATIO.—The 15 aggregations in an area of 10 × 30 m were composed of seven
female and eight male aggregations. The number of colonies in each of the female
BULLETIN OF MARINE SCIENCE, VOL. 76, NO. 1, 2005 146
and male aggregations ranged from 2–49 and 4–20, respectively. All colonies in each
aggregation were either of the same sex or lacked gonads. The total number of
colo-nies belonging to the seven female and eight male aggregations was 92 (78 with eggs
and 14 with no gonads) and 78 (68 with testes and 10 with no gonads) colonies,
re-Figure 1. Monthly size-frequency distributions of oocyte diameters of Lobophytum pauciflorum in Taiwan. Numbers in parentheses indicate number of oocytes measured each month.spectively. The sex ratio did not differ from 1:1 for both aggregations ( χ
2= 0.07, P =
0.77) and colonies (78: 68, χ
2= 0.68, P = 0.41).
SIZE STRUCTURE OF FEMALE AND MALE COLONIES.—The size-frequency
distribu-tions of female and male colonies in the 15 aggregadistribu-tions ranged from 9.5–57.5 and
6–42 cm in diameter with the modes at size class 15–20 and 20–25 cm, respectively
(Fig. 4). The distributions of female and male colony sizes were similar ( χ
2= 16.95,
P = 0.11).
COLONY SIZE AT SEXUAL MATURITY.—Most (>50%) of the colonies in female
ag-gregations bore gonads when they reached 10–15 cm in diameter or above, while
those in male aggregations occurred at 5–10 cm in diameter (Fig. 5). The minimum
Figure 2. Monthly size-frequency distributions of sperm sac diameters of LobophytumBULLETIN OF MARINE SCIENCE, VOL. 76, NO. 1, 2005 148
colony size at sexual maturity was 8.0 and 5.5 cm in diameter for female and male
colonies, respectively. Colonies < 5.0 cm in diameter were not found or examined in
this study.
FECUNDITY.—Fifty-two female colonies ranging from 9.5–53.5 cm in diameter
were examined. There was a positive relationship between colony size and the
num-ber of eggs per polyp (Fig. 6; r
2= 0.30, F = 21.8, P < 0.001). An exponential equation
(y = 15.7(1 − e
−0.05x)) describes a curve increasing to an asymptote at a value of 15.7.
Thus, the predicted average maximum fecundity was 16 eggs polyp
−1.
Figure 3. Monthly changes in percentage of colonies of Lobophytum pauciflorum containing ma-ture gonads in (A) female colonies and (B) male colonies. Numbers above bars indicate number of colonies sampled. Letters refer to months; S1 and S2 indicate sampling at 4 and 21 September 2001, respectively.
DISCUSSION
S
imilar to the other congeneric species, Lobophytum pauciflorum is a
gonochor-ic broadcast spawner (e.g., Alino and Coll, 1989; Benayahu, 1997a; Table 1). Gonad
structure of L. pauciflorum is also similar to that of other congeneric species from
the Indo-Pacific reefs, such as Lobophytum crassum von Marenzeller, 1886 (see
Yamazato et al., 1981) and Lobophytum compactum Tixier-Durivault, 1956 (see
Mi-chalek-Wagner and Willis, 2001). In these species, gonads are borne in all the
mesen-teries of a polyp except a pair of dorsal directives, with early stage oocytes developing
Figure 4. Size-frequency distribution of (A) female colonies and (B) male colonies from 15 ag-gregations of Lobophytum pauciflorum in an area of 10 × 30 m in southern Taiwan.
BULLETIN OF MARINE SCIENCE, VOL. 76, NO. 1, 2005 150
proximally and mature oocytes distally on mesenteries relative to the oral disc of the
polyp (Yamazato et al., 1981).
The oogenic cycle of L. pauciflorum in southern Taiwan requires 1 yr, while that
of L. pauciflorum in the Great Barrier Reef, Australia and other Lobophytum species
requires > 1 yr with large and small eggs, representing two cohorts, existing
simulta-neously within autozooids (Shinkarenko, 1981; Yamazato et al., 1981; Alino and Coll,
1989; Michalek-Wagner and Willis, 2001). It seems that the time required for oocyte
maturation varies greatly in different alcyonacean species (Benayahu, 1997a).
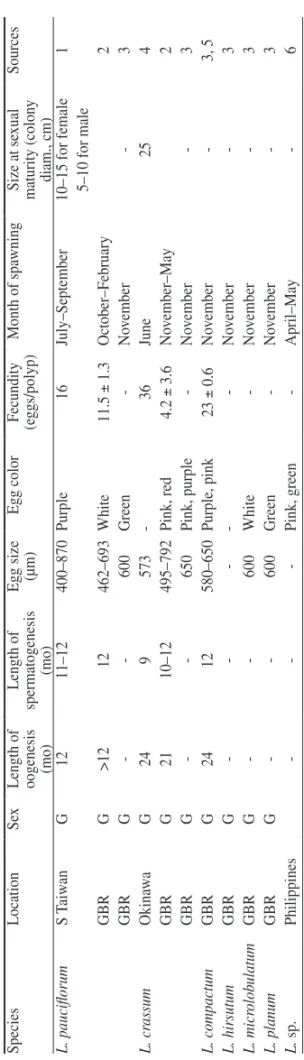
Lobophytum pauciflorum produced larger mature eggs (400–870 µm in diameter)
than did other Lobophytum species (Table 1). The greater egg size of L. pauciflorum
may represent an increased investment in energy storage and survivorship of larvae
(Sier and Olive, 1994; Fan and Dai, 1995). However, the fecundity of L.
pauciflo-rum was lower (16 eggs polyp
−1) than that of L. compactum (23 eggs polyp
−1) and L.
crassum (36 eggs polyp
−1). It may be regarded as a trade-off between egg size and
fecundity in L. pauciflorum.
The coloration of the eggs seems to vary for a given soft coral species, even within
the same colony on occasion (Alino and Coll, 1989). Mature colored oocytes of L.
pauciflorum were found 3–4 mo before spawning. Mature oocytes of L. compactum
appeared 2–3 mo prior to spawning (Michalek-Wagner and Willis, 2001). This is
lon-ger than that of most stony corals whose colored eggs appeared several weeks before
spawning (Harrison and Wallace, 1990).
The length of the spermatogenic cycle of L. pauciflorum in southern Taiwan lasted
about 11–12 mo, which resembles that of L. pauciflorum in the Great Barrier Reef,
Australia (Shinkarenko, 1981) and L. compactum (see Michalek-Wagner and Willis,
Figure 5. Percentage of colonies of Lobophytum pauciflorum with oocytes or sperm sacs per size class (mean colony diameter). Numbers above bars indicate number of colonies sampled.
2001), but is 2 mo longer than that of L. crassum (see Yamazato et al., 1981). Studies
on gametogenic cycles of alcyonaceans have demonstrated that spermatogenesis is
completed within 1 yr and is usually shorter than oogenesis (Benayahu, 1997a).
The colony size at sexual maturity of L. pauciflorum is smaller than that of L.
crassum (25 cm, Yamazato et al., 1981). In addition, females of L. pauciflorum attain
sexual maturity at a larger colony size (10–15 cm) than males (5–10 cm). A similar
phenomenon was also found in Sarcophyton glaucum (Quoy & Gaimard, 1833) (see
Benayahu and Loya, 1986) and Parerythropodium fulvum fulvum (Forskal, 1775) (see
Benayahu and Loya, 1983).
The sex of colonies in an aggregation of L. pauciflorum was the same, indicating
that small colonies may have been derived from fission of nearby large colonies.
Fur-ther, L. pauciflorum tends to exist as many small colonies in dense aggregations
in-stead of few large colonies (Fan, pers. obs.). This aggregation pattern of colonies may
originate from the growth and fission of original founder colonies. McFadden (1986)
demonstrated that colonies of an alcyonacean soft coral on the rocky shores from
northern California to British Columbia can increase their rates of particle capture
at high flow velocities by forming aggregations of small, clonally replicated colonies.
In addition, alcyonaceans may increase their space acquisition by fission because
relative growth rates of small colonies are greater than larger ones (Fabricius, 1995).
Fission of L. pauciflorum may be a strategy to occupy a favorable substratum at faster
rates than sexual recruits.
The reproductive season of soft corals in southern Taiwan is April–June for
Sarco-phyton crassocaule Moser, 1919 (see Chou, 2002), May and June for both Sinularia
scabra Tixier-Durivault, 1970 and Sinularia exilis Tixier-Durivault, 1970 (see Wu,
1994), June–August for Sarcophyton trocheliophorum von Marenzeller, 1886 (see
Chou, 2002), July and August for Sinularia nanolobata Verseveldt, 1977 (see Wu,
1994), and July–September for L. pauciflorum. The reproductive season of soft
cor-als in the Red Sea cor-also occurs in extended periods and this may lead to temporal
Figure 6. Relationship between polyp fecundity (number of mature oocytes polyp−1) and colony
BULLETIN OF MARINE SCIENCE, VOL. 76, NO. 1, 2005 152
Table 1. Comparison of reproducti
ve characteristics among
Lobophytum
corals. G: gonochoric. -: no data.
Species
Location
Se
x
Length of oogenesis (mo)
Length of spermatogenesis (mo) Egg size (µm) Egg color Fecundity (eggs/polyp) Month of spa wning Size at se xual maturity (colon y diam., cm) Sources L. pauciflorum S Taiw an G 12 11–12 400–870 Purple 16 July–September 10–15 for female 1 5–10 for male GBR G >12 12 462–693 White 11.5 ± 1.3 October–February 2 GBR G -600 Green -No vember -3 L. cr assum Okina w a G 24 9 573 -36 June 25 4 GBR G 21 10–12 495–792 Pink, red 4.2 ± 3.6 No vember–May 2 GBR G -650 Pink, purple -No vember -3 L. compactum GBR G 24 12 580–650 Purple, pink 23 ± 0.6 No vember -3, 5 L. hir sutum GBR G -No vember -3 L. micr olob ulatum GBR G -600 White -No vember -3 L. planum GBR G -600 Green -No vember -3 L. sp. Philippines -Pink, green -April–May -6
1: Present study; 2: Shinkarenk
o, 1981; 3:
Alino and Coll, 1989; 4:
Y
amazato et al. 1981; 5: Michalek-W
agner and
W
reproductive isolation among alcyonaceans (Benayahu, 1997a). However, in contrast,
alcyonaceans in the Great Barrier Reef spawn in multispecific spawning episodes
(Alino and Coll, 1989). The geographic variation in reproductive timing seen in L.
pauciflorum from southern Taiwan and the Great Barrier Reef is also found in the
scleractinian corals Echinopora lamellose (Esper, 1795), Merulina ampliata (Ellis
and Solander, 1786), Mycedium elephantotus (Pallas, 1766), and Echinophyllia aspera
(Ellis and Solander, 1786). These coral species participate in the mass spawning
dur-ing early summer in the Great Barrier Reef (Babcock et al., 1986), but delay spawndur-ing
until the late summer and autumn in southern Taiwan (Fan and Dai, 1995, 1998;
Fan 1996; Dai et al., 2000). The timing of reproduction may reflect environmental
conditions favorable for the survival of larvae (Giese and Pearse, 1974). The possible
advantage for these species of breeding near the end of seasonal disturbances
(ty-phoons and heavy rainfalls) is to increase the substrate availability for settling larvae
and to avoid high mortality caused by these disturbances, thus increasing their
re-productive success (Shlesinger and Loya, 1985; Fan and Dai, 1995, 1998; Mendes and
Woodley, 2002). The favorable environmental conditions for the survival of soft coral
larvae during these months is supported by other sympatric alcyonaceans including
S. trocheliophorum (see Chou, 2002) and S. nanolobata (see Wu, 1994), which also
spawned during this period. Delay in the reproductive timing of these species in
Nanwan Bay may be an adaptation to the local environment.
ACKNOWLEDGEMENTS
We thank P.-J. Liu for his assistance in the field. We appreciate the anonymous reviewers who provided critical comments on the mansucript. This study was supported by grants from the National Science Council, R.O.C. (NSC 90-2621-B-291-002 and 91-2621-B-291-004).
LITERATURE CITED
Alino, P. M. and J. C. Coll. 1989. Observations of the synchronized mass spawning and post settlement activities of octocorals on the Great Barrier Reef, Australia: biological aspect. Bull. Mar. Sci. 45: 697–707.
Babcock, R. C., G. D. Bull, P. L. Harrison, A. J. Heyward, J. K. Oliver, C. C. Wallace, and B. L. Willis. 1986. Synchronous spawnings of 105 scleractinian coral species on the Great Barrier Reef. Mar. Biol. 90: 379–394.
Benayahu, Y. 1997a. Developmental episodes in reef soft corals: ecological and cellular determi-nants. Proc. 8th Int. Coral Reef Symp., Panama 2: 1213–1218.
__________. 1997b. A review of three alcyonacean families (Octocorallia) from Guam. Microne-sica 30: 207–244.
__________. 2002. Soft corals (Octocorallia: Alcyonacea) of the southern Ryukyu Archipelago: the families Tubiporidae, Clavulariidae, Alcyoniidae and Briareidae. Galaxea, JCRS, 4: 11– 32.
__________ and Y. Loya. 1983. Surface brooding in the Red Sea soft coral Parerythropodium
fulvum fulvum (Forskal 1775). Biol. Bull. 165: 353–369.
__________ and ______. 1986. Sexual reproduction of a soft-coral: synchronous and brief an-nual spawning of Sarcophyton glaucum. Biol. Bull. 170: 32–42.
Bermas, N. A., P. M. Alino, M. P. Atrigenio, and A. Uychiaoco. 1992. Observations on the reproduction of scleractinian and soft corals in the Philippines. Proc. 7th Int. Coral Reef
BULLETIN OF MARINE SCIENCE, VOL. 76, NO. 1, 2005 154
Chou, Y. H. 2002. Sexual reproduction of three alcyonacean species in southern Taiwan. M.S. Th esis, National Taiwan University, Taipei. 49 p.
Dai, C. F., T. Y. Fan, and J. K. Yu. 2000. Reproductive isolation and genetic diff erentiation of a scleractinian coral Mycedium elephantotus. Mar. Ecol. Prog. Ser. 201: 179–187.
Fabricius, K. E. 1995. Slow population turnover in the soft coral genera Sinularia and
Sarco-phyton on mid- and outer-shelf reefs of the Great Barrier Reef. Mar. Ecol. Prog. Ser. 126:
145–152.
Fan, T. Y. 1996. Life histories and population dynamics of foliaceous corals in northern and southern Taiwan. P.h. D Th esis, National Taiwan University, Taipei. 173 p.
________ and C. F. Dai. 1995. Reproductive ecology of the scleractinian coral Echinopora
lam-ellosa in northern and southern Taiwan. Mar. Biol. 123: 565–572.
________ and _______. 1998. Sexual reproduction of the scleractinian coral Merulina ampliata in southern Taiwan. Bull. Mar. Sci. 62: 897–904.
Giese, A. C. and J. S. Pearse. 1974. Introduction: general principles. Pages 1–49 in A. C. Giese and J. S. Pearse, eds. Reproduction of marine invertebrates. I. Acoelomate and pseudocoe-lomate metazoans. Academic Press, New York. 546 p.
Harrison, P. L. and C. C. Wallace. 1990. Reproduction, dispersal and recruitment of sclerac-tinian corals. Pages 133–207 in Z. Dubinsky, ed. Ecosystems of the world. 25. Coral reefs. Elsevier, Amsterdam. 550 p.
McFadden, C. S. 1986. Colony fi ssion increases particle capture rates of a soft coral: advantages of being a small colony. J. Exp. Mar. Biol. Ecol. 103: 1–20.
Mendes, J. M. and J. D. Woodley. 2002. Timing of reproduction in Montastraea annularis: relationship to environmental variables. Mar. Ecol. Prog. Ser. 227: 241–251.
Michalek-Wagner, K. and B. L. Willis. 2001. Impacts of bleaching on the soft coral Lobophytum
compactum. I. Fecundity, fertilization and off spring viability. Coral Reefs 19: 231–239.
Shinkarenko, L. 1981. Th e natural history of fi ve species of octocorals (Alcyonacea), with spe-cial reference to reproduction, at Heron Island Reef, Great Barrier Reef. P.h.D Th esis, Uni-versity of Queensland, Australia.
Shlesinger, Y. and Y. Loya. 1985. Coral community reproductive patterns: Red Sea versus the Great Barrier Reef. Science 228: 1333–1335.
Sier, C. J. S. and P. J. W. Olive. 1994. Reproduction and reproductive variability in the coral
Pocil-lopora verrucosa from the Republic of Maldives. Mar. Biol. 118: 713–722.
Tursch, B. and A. Tursch. 1982. Th e soft coral community on a sheltered reef quadrat at Laing Island (Papua New Guinea). Mar. Biol. 68: 321–332.
Verseveldt, J. 1983. A revision of the genus Lobophytum Von Marenzeller (Octocorallia, Alcyo-nacea). Zool. Verh. Leiden 200: 1–103.
Wu, C. S. 1994. Sexual reproduction and population structure of three Sinularia species in southern Taiwan. M.S. Th esis, National Taiwan University, Taipei. 44 p.
Yamazato, K., M. Sato, and H. Yamashiro. 1981. Reproductive biology of an alcyonarian coral,
Lobophytum crassum Marenzeller. Proc. 4th Int. Coral Reef Symp., Manila 2: 671–678.
DATE SUBMITTED: 21 November, 2003. DATE ACCEPTED: 21 May, 2004.
ADDRESSES: (T.-Y.F.) National Museum of Marine Biology and Aquarium, Pingtung 944,
Tai-wan, Republic of China. (Y.-H.C., C.-F.D.) Institute of Oceanography, National Taiwan Univer-sity, Taipei 106, Taiwan, Republic of China. CORRESPONDING AUTHOR: (T.-Y.F.) Telephone: 886-8-8825001 ext. 2109, Fax: 886-8-8825085, E-mail: <tyfan@nmmba.gov.tw>.