Pharmacokinetics and Tissue Distribution of Resveratrol,
Emodin and Their Metabolites after Intake of Polygonum
cuspidatum in Rats
Shiuan-Pey Lin a,1, Pei-MingChu b,1, Shang-YuanTsai a, Meng-HaoWuc,
Yu-ChiHou a, d, *
a School of Pharmacy,ChinaMedicalUniversity,Taichung,Taiwan b Department of Anatomy, College of Medicine, China Medical
University, Taichung, Taiwan
c Institute of Chinese Pharmaceutical Sciences, China Medical University,
Taichung, Taiwan.
d Department of Medical Research, China Medical University Hospital,
Taichung, Taiwan.
1 These twoauthorscontributedequally.
* Corresponding author at: School of Pharmacy, China Medical
University, No.91 Hsueh-Shih Road, Taichung 40402, Taiwan, ROC Tel.: +886 422 031 028; fax: +886 422 031 028
E-mail address: hou5133@gmail.com (Y.-C. Hou)
Abbreviations
PC, Polygonum cuspidatum; S/G, sulfates/glucuronides; LLOQ
,
lower limit of quantitation; LOD, limit of detection; Cmax, the peak plasmaconcentration; tmax, the time to peak concentration; AUC0-t, the areas
under the plasma concentration-time curves; MRT, mean residence time.
Abstract
Aim of the study: The rhizome of Polygonum cuspidatum SIEB. et ZUCC.
(PC) is a widely used Chinese herb. PC contains various polyphenols including stilbenes, anthraquinones and flavonoids. This study
investigated the pharmacokinetics and tissue distribution of emodin and resveratrol in PC.
Material and Methods: Male Sprague-Dawley rats were orally
administered PC (2 and 4 g/kg) and blood samples were withdrawn at the designed time points via cardiopuncture. Moreover, after 7-dose
administrations of PC (4 g/kg), brain, liver, lung, kidney and heart were collected. The concentrations of resveratrol and emodin in the plasma and tissues were assayed by HPLC before and after hydrolysis with
β-glucuronidase and sulfatase.
Results: The glucuronides/sulfates of emodin and resveratrol were
exclusively present in the plasma. In liver, kidney, lung and heart, the glucuronides/sulfates of resveratrol were the major forms. For emodin, its glucuronides/sulfates were the major forms in kidney and lung, whereas considerable concentration of emodin free form was found in liver. Neither free forms nor conjugated metabolites of resveratrol and emodin were detected in brain.
Conclusion: The sulfates/glucuronides of resveratrol and emodin were the
major forms in circulation and most assayed organs after oral intake of PC. However, the free form of emodin was predominant in liver.
Keywords: Polygonum cuspidatum; resveratrol; emodin;
pharmacokinetics; metabolites; tissue distribution
1. Introduction
The rhizome of Polygonum cuspidatum SIEB. et ZUCC. (PC), a widely
used Chinese medicine, is commonly prescribed for the treatments of amenorrhea, arthralgia, jaundice, abscess, scald and bruises (Chinese Pharmacopoeia Committee, 2000). Pharmacological studies have
reported a variety of beneficial bioactivities of the extract or constituents of PC such as antioxidation (Huang et al., 2008), liver protection (Kimura et al., 1983), anti-cancer (Yeh et al., 1988) and wound healing effects (Wu et al., 2012).
PC contains various polyphenols including
anthraquinones, stilbenes and flavonoids (Huang et al., 2008).Among the polyphenolic constituents, resveratrol and emodin (chemical structures shown in Figure 1) were of great interest to
researchers.Resveratrol, also existing in grapes, peanuts and cranberry (Baur and Sinclair, 2006), has been reported to show antioxidation, anti-inflammation, anti-diabetic, cardiovascular protection,
cancer-chemoprevention and neuroprotection activities (Baolin et al., 2004; Bastianetto et al., 2000; Han et al., 2004; Jang and Surh, 2003; Jang et al., 1997; Kumerz et al., 2011; Smoliga et al., 2011). Emodin, also present in rhubarb, has been shown with cancer, hepatoprotection,
anti-inflammation, anti-virus and neuroprotective effects (Gu et al., 2005; Hsu et al., 2010; Kitano et al., 2007; Lin et al., 1996; Xiong et al., 2011). However, the reported bioactivities of resveratrol and emodin were mostly based on in vitro studies. Therefore, whether the in vitro
bioactivities of resveratrol and emodin can be demonstrated in vivo remains unanswered. Figure 1. O O OH OH H3C OH Emodin HO OH OH Resveratrol
Figure 1. Chemical structures of emodin and resveratrol.
Notwithstanding several studies have reported the pharmacokinetics and tissue distribution of resveratrol and emodin in liver, kidney, lung and heart (Shia et al., 2010; Shia et al., 2011; Wenzel and Somoza, 2005), however, the jigsaw puzzle particularly the plasma pharmacokinetics and tissue distribution of the major metabolites of resveratrol and emodin has not yet been accounted for in a satisfying manner. Therefore, this study set out to investigate the dose-dependent pharmacokinetics and tissue distribution of resveratrol and emodin after oral administration of PC in
rats.
2. Material and methods
2.1. Chemicals
Acetonitrile, ethyl acetate and methanol were products of J.T. Baker,
Inc. (Philipsburg, NJ, USA)
.
Emodin (purity ≧ 99 %) was purchased from Carl Roth(Karlsruhe, Germany). Butyl paraben, indomethacin, propyl paraben, β-glucuronidase (type B-1, from bovine liver), resveratrol and sulfatase (type H-1 from Helix pomatia) were obtained from Sigma(St. Louis, MO, USA). L(+)-ascorbic acid and ortho-phosphoric
acid were obtained from Riedel-deHaën AG (Seelze, Germany).
2.2. Plant material
The rhizome of Polygonum cuspidatum was obtained from a Chinese drugstore in Taichung, Taiwan. Dr. Yu-Chi Hou identified the origin of PC with microscopic examination and a voucher specimen
(CMU-P-1905-10) was deposited in School of Chinese Pharmaceutical Sciences and Chinese Medicine Resources, China Medical University, Taichung, Taiwan.
2.3. Hhigh performance liquid chromatography (HPLC)
An HPLC apparatus (LC-2010C, Shimazdu, Japan) included two pumps, an autoinjector and an UV detector set at 280 nm was used for analysis. The column used was Apollo C18 (5 μm, 250×4.6 mm, Alltech Associates, Inc., USA).
2.4. Preparation and characterization of PC extract
Water (5 L) was added to 250 g crude drug of PC and heating on a gas stove. After boiling, gentle heating was continued until the volume reduced to about 2.5 L and then the mixture was filtered while hot. The filtrate was gently boiled until the volume less than 500 mL and sufficient water was added to make 500 mL, then frozen at -20℃ for later use. In order to quantitate the glycosides of resveratrol and emodin, PC extracts was hydrolyzed with hydrochloric acid. Briefly, 1 mL of HCl (1.2 N) and 25 mg of ascorbic acid was added into 1 mL of PC extract. After vortex
mixing, the mixture was incubated at 80℃ for 2 h in water bath. The PC extract before and after acid hydrolysis was diluted with 4-fold water, and then was added 4-fold methanol. After vortex and centrifugation, the supernatant (100 µL) was added butyl paraben solution (100 µL, 10 µg/mL in methanol), and 20 µL was subject to HPLC analysis.
The mobile phase comprised 0.1 % phosphoric acid (A) and
acetonitrile (B) using gradient elution program : A/B = 64/36 (0-8 min), 30/70 (11-16 min), 64/36 (19-25 min). The flow rate was 1.0 mL/min.
2.5. Animals
Male Sprague-Dawley rats (300 – 450 g) were obtained from BioLASCO (Taipei, Taiwan, ROC) and kept at least 2 week under conditioned environment with free access to food and water. Before experiment, rats were fasted overnight but drinking water was allowed ad
libitum. Three hours after administration of PC extract, food was supplied
to the rats. All animal experiments adhered to ‘‘The Guidebook for the Care and Use of Laboratory Animals’’ published by the Chinese Society of Animal Science, Taiwan, ROC). The animal protocol was approved by the Institutional Animal Care and Use Committee of China Medical
University, Taiwan (CMU-92-16).
2.6. Drug administration and sample collection
For pharmacokinetic study, twelve rats were divided into two groups and PC extract was given at doses of 2 and 4 g/kg via gastric gavage. Blood samples were withdrawn via cardiopuncture at 0, 10, 30, 60, 120, 180, 300, 480, 720, 1440, 2160 and 2880 min after dosing. Blood
samples were collected into microtube containing heparin. All blood samples were centrifuged at 10,000 g for 15 min to obtain plasma. For tissue distribution studies, three rats were orally given 4 g/kg of PC twice daily for seven doses prior to tissue collection. The rats were sacrificed at 30 min after the 7th dose by using CO
2 and systemically perfused with
cool normal saline. Then, the brain, liver, lung, heart and kidney were collected, blotted dry with filter paper and weighed. The tissues were homogenized with normal saline (700 mg/mL). The plasma and tissue homogenates were stored at -30 °C until analysis.
2.7. Quantitation of resveratrol, emodin and their conjugated metabolites in plasma
The concentrations of resveratrol and emodin in plasma were determined prior to and after treatments with β-glucuronidase and sulfatase, individually. Plasma was divided into three aliquots. For glucuronides (G) quantation, the plasma (100 µL) was mixed with 200 µL of glucuronidase (500 units/mL in pH 5 acetate buffer), 50 µL of ascorbic acid (50 mg/mL) and incubated at 37℃ for 4 h. For
sulfates/glucuronides (S/G) quantation, plasma (100 µL) was mixed with 200 µL of sulfatase (containing 25 unit of sulfatase and 525 unit of β-glucuronidase in pH 5.0 acetate buffer), 50 µL of ascorbic acid (50 mg/mL) and incubated at 37℃ for 1 h.
After incubation, the mixture was added with 50 µL of 0.1N HCl and partitioned with 400 µL of ethyl acetate (containing 1.0 µg/mL of propyl paraben). The ethyl acetate layer was evaporated under N2 gas to dryness
and reconstituted with an appropriate volume of methanol, then 20 µL was subjected to HPLC analysis. For free form determination, the plasma was mixed with pH 5 acetate buffer without incubation with
glucuronidase or sulfatase and processed as the procedure described above.
acetonitrile (B) and eluted in a gradient program : A/B = 71/29 (0-8 min), 30/70 (11-16 min), 13/87 (19-20 min), 71/29 (21-25 min). The flow rate was 1.0 mL/min.
2.8. Quantitation of resveratrol, emodin and their conjugated metabolites in tissues
To determine the concentrations of resveratrol and emodin in tissue homogenates, samples were analyzed prior to and after treatments with β-glucuronidase and sulfatase, individually. For the quantation of
resveratrol G, the homogenates (500 µL) was mixed with 100 µL of glucuronidase solution (1000 units/mL in pH 5 acetate buffer), 100 µL of ascorbic acid (50 mg/mL) and incubated at 37℃ for 4 h. For the
quantation of resveratrol S/G, tissue homogenates (500 µL) was mixed with 100 µL of sulfatase solution (250 units/mL in pH 5 acetate buffer), 100 µL of ascorbic acid (50 mg/mL) and incubated at 37℃ for 1 h.
After incubation, the mixture was added with 20 µL of 0.1N HCl and partitioned with 720 µL of ethyl acetate (containing 0.5 µg/mL of
indomethacin). For free form determination, the homogenate was mixed with pH 5 acetate buffer without incubation with glucuronidase or
sulfatase and processed as the procedure described above. The ethyl acetate layer was evaporated under N2 gas to dryness and reconstituted
with an appropriate volume of methanol then 20 µL was subjected to HPLC analysis.
The mobile phase of HPLC comprised 0.1 % phosphoric acid (A) and acetonitrile (B) and eluted in a gradient program : A/B = 67/33 (0-15 min), 25/75 (20-35 min), 67/33 (4045 min). The flow rate was 1.0
mL/min.
The method to assay emodin and its conjugated metabolites in tissue homogenates followed that reported in a previous study with little
modification (Shia et al., 2011). Briefly, the quantation of emodin G or emodin S/G, the homogenates (300 µL) was mixed with 150 µL of
glucuronidase or sulfatase (1000 units/mL in pH 5 acetate buffer), 150 µL of ascorbic acid (100 mg/mL) and incubated at 37℃ for 10 min.
After incubation, the mixture was added with 150 µL of 0.1N HCl and partitioned with 750 µL of ethyl acetate (containing 2.0 µg/mL of butyl paraben).. For the quantitation of free form of emodin, the
homogenates was mixed with pH 5 acetate buffer without incubation with glucuronidase or sulfatase and processed as the procedure described
above. The ethyl acetate layer was evaporated under N2 gas to dryness
and reconstituted with an appropriate volume of methanol, then 20 µL was subjected to HPLC analysis. The mobile phase of HPLC consisted of methanol/0.1 % ortho-phosphoric acid (80:20) and eluted isocratically.
2.9. Validation of analysis methods
The accuracy and precision of the analytical methods were estimated by intra-day and inter-day analysis of triplicates at different
concentrations of resveratrol and emodin over a period of three days. Lower limit of quantitation (LLOQ) represents the lowest concentration of analyte in a sample that can be determined with acceptable precision and accuracy. The limit of detection (LOD) represents the lowest
concentration of analyte in a sample that can be detected (with S/N > 3).
2.10. Data analysis
The peak plasma concentration (Cmax) and the time to peak
concentration (Tmax) were observed from experimental data. The areas
under the plasma concentration-time curves (AUC0-t) were calculated
Statistical Consulting, Inc., Apex, NC, USA). Paired Student’s t-test was used for statistical comparisons.
3. Results
3.1. Characterization of PC
Quantitation of the PC decoction indicated that the concentrations of resveratrol and emodin in the PC extract were 138 and 282 μg/mL,
respectively. After acid hydrolysis, the concentrations of resveratrol and emodin were 186 and 1410 μg/mL, which had been increased by 35 and 398 %, respectively, indicating that resveratrol mainly presented as
aglycon and emodin predominately existed as glycoside in the PC extract.
3.2. Quantitation of resveratrol, emodin and their conjugated metabolites in plasma
The concentrations of resveratrol and emodin in plasma were determined by an HPLC method before and after hydrolysis with sulfatase or β-glucuronidase. Before enzymolysis, the free forms of resveratrol and emodin were not detected in the plasma. After hydrolysis
with glucuronidase or sulfatase, the free forms of resveratrol and emodin emerged. The calibration curves of resveratrol and emodin in plasma showed good linearities (r>0.99) in the ranges of 0.16-20.0 and 0.31-20.0 μg/mL, respectively. The precision evaluation showed that all coefficients of variation were below 9.9 % and the accuracy analysis showed that the relative errors to the true concentrations were below 9.6 %. The recoveries of resveratrol and emodin from plasma were 65-98 %. The LLOQ of resveratrol and emodin were 0.16 and 0.31 μg/mL, and the LOD were 0.08 and 0.16 μg/mL, respectively.
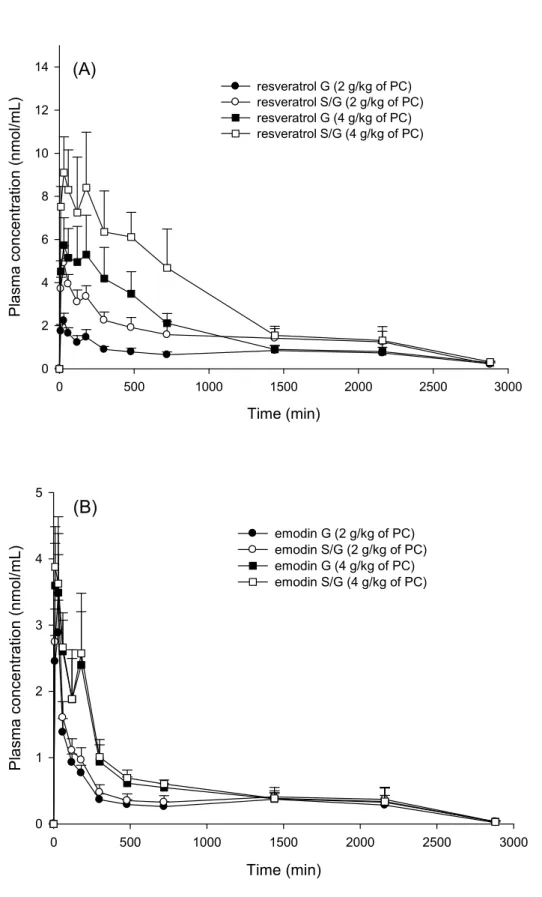
The mean plasma concentration - time profiles of the conjugated metabolites of resveratrol and emodin are shown in Figure 2. The profiles of emodin S/G and emodin G were largely superposed. However, the profiles of resveratrol S/G were well above the correspondent profiles of resveratrol G. The pharmacokinetic parameters are shown in Table 1. The AUC0-2880 of resveratrol S/G following both doses was significantly
greater than those of resveratrol G by about 1-fold, whereas the AUC0-2880
Time (min) 0 500 1000 1500 2000 2500 3000 P la sm a co n ce n tr at io n ( n m o l/m L) 0 2 4 6 8 10 12 14 resveratrol G (2 g/kg of PC) resveratrol S/G (2 g/kg of PC) resveratrol G (4 g/kg of PC) resveratrol S/G (4 g/kg of PC) (A) Time (min) 0 500 1000 1500 2000 2500 3000 P la sm a co nc en tr at io n (n m ol /m L) 0 1 2 3 4 5 emodin G (2 g/kg of PC) emodin S/G (2 g/kg of PC) emodin G (4 g/kg of PC) emodin S/G (4 g/kg of PC) (B)
Figure 2. Mean (± S.E.) plasma concentration-time profiles of
emodin (B) after oral administration of PC (2 and 4 g/kg) to six rats.
Table 1
Pharmacokinetic parameters of resveratrol glucuronides (RG), resveratrol sulfates/glucuronides (R S/G), emodin glucuronides (EG) and emodin sulfates/glucuronides (E S/G) after oral administration of 2 and 4 g/kg of PC extracts to rats. Parameters Treatment Tmax (min) Cmax (nmol/mL) AUC0-2880 (nmol‧min/mL) MRT (min) 2 g/kg RG R S/G EG E S/G 4 g/kg RG R S/G EG E S/G 31.7 31.7 23.3 20.0 60.0 70.0 60.0 70.0 ± ± ± ± ± ± ± ± 6.5 6.5 4.2 4.5 30.0 35.0 29.6 35.0 2.4 5.8 3.2 3.8 7.0 11.2 4.7 5.0 ± ± ± ± ± ± ± ± 0.2 0.3*** 0.3 0.4 1.5 2.0* 0.7 0.8 2176.9 4370.0 1017.1 1224.1 4912.4 8561.0 1638.1 1732.2 ± ± ± ± ± ± ± ± 363.5 524.2** 210.8 258.0 799.2 1376.2* 223.9 245.7 1162.5 1039.5 901.6 927.3 887.9 854.1 799.9 803.4 ± ± ± ± ± ± ± ± 114.6 133.8 147.9 147.3 161.9 144.0 148.2 156.3
Values are means ± S.E.
Cmax: the maximum plasma concentration
AUC0-2880: the area under plasma concentration - time curve to the last time (480 min)
MRT0-2880: the mean residence time
*P<0.05, **P<0.01, ***P<0.001, compared to correspondent glucuronides
3.3. Quantitation of resveratrol, emodin and their conjugated metabolites
in tissues
The concentrations of resveratrol and emodin in tissues were determined by an HPLC method before and after hydrolysis with
sulfatase or β-glucuronidase. The calibration curves of resveratrol and emodin in various tissue homogenates showed good linearities (r>0.99) in the ranges of 0.08-2.5 μg/mL and 0.16-20.0 μg/m, respectively. The precision evaluation showed that all coefficients of variation were below 14.3 % and the accuracy analysis showed that the relative errors to the true concentrations were below 14.0 %. The recoveries of resveratrol and emodin from various tissues were 88-112 %. The LLOQ of resveratrol and emodin were 0.08 and 0.16 μg/mL, and the LOD were 0.04 and 0.08 μg/mL, respectively.
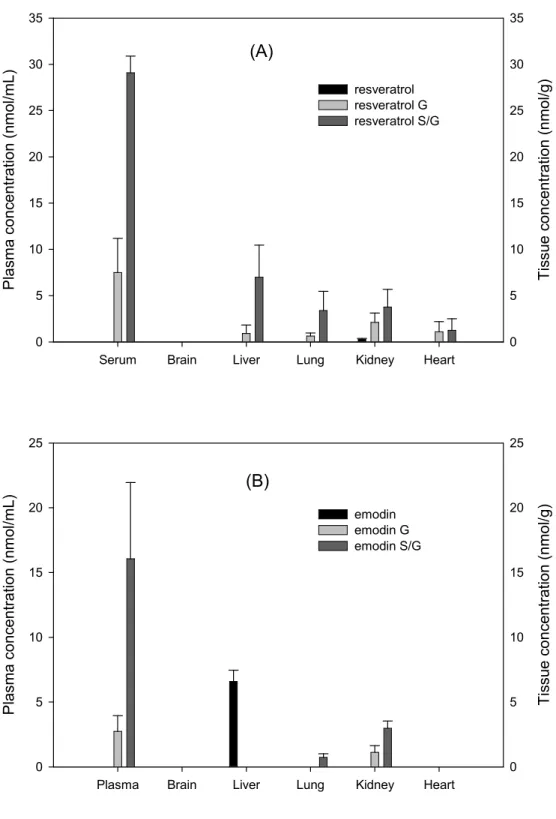
The concentrations of resveratrol, emodin and their conjugated metabolites in various tissues after the 7th dose of PC are shown in Figure
3. Resveratrol S/G were the major forms in liver, lung, kidney and heart, and the relative concentration was ranked as liver > kidney, lung > heart. Only trace of free form resveratrol was detected in kidney. In regard to emodin, emodin S/G were the major forms in kidney and lung, and the concentration in kidney was higher than lung. In liver, considerable concentration of free form emodin was detected. In brain, neither free forms nor the conjugated metabolites of resveratrol and emodin were detected.
Serum Brain Liver Lung Kidney Heart P la sm a c o n ce n tr a tio n (n m ol /m L ) 0 5 10 15 20 25 30 35 T is su e c o n ce n tr a tio n ( n m o l/g ) 0 5 10 15 20 25 30 35 resveratrol resveratrol G resveratrol S/G (A)
Plasma Brain Liver Lung Kidney Heart
P la sm a c o n ce n tr a tio n (n m ol /m L ) 0 5 10 15 20 25 T is su e c o n ce n tr a tio n ( n m o l/g ) 0 5 10 15 20 25 emodin emodin G emodin S/G (B)
Figure 3. Mean (± S.E.) concentration of (A) resveratrol, resveratrol glucuronides (G) and resveratrol sulfates/glucuronides (S/G) and (B)
emodin, emodin glucuronides and emodin sulfates/glucuronides in plasma and various tissues after oral administration of 7 doses of
Polygonum cuspidatum (4 g/kg).
4. Discussions
The quantitation methods of resveratrol and emodin in plasma and various tissue homogenates were established in this study. Validation of the analytical method indicated that the accuracy, precision and recovery were quite satisfactory. Owing to the unavailability of the authentic standards of conjugated metabolites, the concentrations of the conjugated metabolites of resveratrol and emodin were determined indirectly after hydrolyzed by β-glucuronidase and sulfatase. Because the sulfatase used in this study contained sulfatase and β-glucuronidase, hydrolysis with sulfatase resulted in simultaneous hydrolysis of both S and G.
After oral administration of PC extract, the highest concentration of resveratrol S/G was detected at the first sampling time (10 min),
suggesting that the absorbed resveratrol was rapidly and extensively converted into resveratrol S/G, which was in good agreement with
previous reports (Azorin-Ortuno et al., 2010; Azorin-Ortuno et al., 2011; Marier et al., 2002; Vitaglione et al., 2005; Wenzel and Somoza, 2005). Comparison of the Cmax and AUC0-2880 between resveratrol S/G and
resveratrol G showed marked differences, which clearly indicated that the conjugated metabolites included not only resveratrol G but also
resveratrol S. The AUC0-2880 of resveratrol S/G was around 2-fold of
resveratrol G, implying comparable systemic exposure between resveratrol G and resveratrol S in the circulation. This finding was consistent with previous studies pointing out that glucuronidation and sulfation were the main metabolic pathways of resveratrol (de Santi et al., 2000a; De Santi et al., 2000b). Furthermore, there was a second peak in each profile in Fig. 2(A), demonstrating the existence of enterohepatic recirculation of resveratrol metabolites, which echoes the finding of a previous report (Marier et al., 2002).
Like resveratrol, the highest concentration of emodin S/G was detected at the first sampling time (10 min), indicating that emodin was also absorbed rapidly and extensively metabolized. The profiles of emodin G and emodin S/G were superposable, indicating that the
S in the circulation, corroborating the results of several reports (Shia et al., 2009a; Shia et al., 2010; Shia et al., 2009b; Shia et al., 2011).
Comparison of the AUC0-2880 of resveratrol S/G with those of emodin
S/G listed in Table 1 revealed that the systemic exposure of resveratrol S/G was significantly greater than that of emodin S/G by 2-3 folds
although the concentration of emodin was 7.6-fold higher than resveratrol in the acid hydrolysate of PC extract. This phenomenon may stem from a much poorer bioavailability of emodin than resveratrol. The log P
(octanol-water partition coefficient) of resveratrol and emodin are 1.87 and 2.91, respectively (Fabris et al., 2008; Shang and Yuan, 2003). Emodin is much more lipophilic and thus less soluble in gastrointestinal juice than resveratrol, which can account for the poorer bioavailability of emodin.
For tissue distribution analysis, before organ collection a systemic perfusion with cool normal saline was performed to avoid the interference of residual blood. Our results showing that the analyzed organs contained resveratrol S/G as the major forms without any free form was identical with the finding in plasma. This results was quite different from a
organs except brain after giving an alcoholic extract of PC to rats (Wang et al., 2008). In contrast, the alcoholic extract of PC given in the previous study contained a higher dose of resveratrol (19.2 mg/kg) that was 13 fold as much as the dose (1.5 mg/kg) used in this study. The dose of
resveratrol used in the previous study well exceeded that commonly consumed through drinking red wine, in which the concentration of resveratrol was only 2-40 μM (0.5-9.2 mg/L) (McMurtrey, 1996). In brain, neither resveratrol nor resveratrol S/G were detected, which was consistent with a previous study (Wang et al., 2008). In regard to the tissue distribution of emodin and its metabolites, the free form of emodin was only detected in liver, whereas emodin S/G were predominant in lung and kidney, but not detectable in brain and heart, which was essentially similar with our previous study reporting the tissue distribution of anthraquinones after administration of the rhizome of Rheum palmatum (Shia et al., 2011).
Both resveratrol and emodin have been reported to exhibit in vitro neuroprotective effects (Bastianetto et al., 2000; Gu et al., 2005; Han et al., 2004; Jang and Surh, 2003). However, the present study showing that neither the free forms nor the sulfates/glucuronides of resveratrol and
emodin were detected in brain provided strong indication that resveratrol and emodin can not exert neuroprotection activity in the central nerve system. On the other hand, emodin has been reported to possess in vivo hepatoprotective effects which could be attributed to the activity of free form emodin in the liver (Bhadauria, 2010; Lee et al., 2012; Zhan et al., 2000). We suggest that the in vitro bioactivity of emodin can predict the
in vivo effect in liver rather than other organs, given that the effective
concentration of emodin is achievable in liver.
In recent years, many natural polyphenol products are widely used by general public as dietary supplement to prevent chronic diseases. Among the supplements, resveratrol is one of the well known compounds because of its multiple benefits originated from “French Paradox”. The commercially available products of resveratrol include extracts of PC and grapes. Whether the ingestion of these supplements is helpful for health improvement remains unknown. From pharmacokinetic point of view, all the claimed benefits of resveratrol based solely on in vitro studies should be questioned.
Generally, the conjugated metabolites were recognized as the
showing that the conjugated metabolites of polyphenols such as emodin, baicalein, wogonin, aloe-emodin, rhein, chrysophanol, quercetin and morin demonstrated various bioactivities (Fang et al., 2003; Shia et al., 2009a; Shia et al., 2010; Shia et al., 2009b; Shirai et al., 2006; Yang et al., 2006; Yoshino et al., 2011). Based on this study showing that resveratrol and emodin mainly existed as conjugated metabolites in the circulation and most organs, the bioactivities of the conjugated
metabolites of resveratrol and emodin are worthy of future investigations in order to understand the clinical implications of PC..
In conclusion, resveratrol S/G and emodin S/G were the major forms in circulation and most organs, whereas emodin free form was the major form in liver after oral administration of PC.
Conflict of interest
The authors declare that there are no conflicts of interest.
Acknowledgements
Number NSC99-2320-B-039-009-MY3, NSC99-2320-B-039-017-MY3) and China Medical University (Grant Number 100-S-05, CMU-100-S-17).
References
Azorin-Ortuno, M., Yanez-Gascon, M.J., Pallares, F.J., Vallejo, F., Larrosa, M., Garcia-Conesa, M.T., Tomas-Barberan, F., Espin, J.C., 2010. Pharmacokinetic study of trans-resveratrol in adult pigs. Journal of Agricultural Food Chemistry 58, 11165-11171. Azorin-Ortuno, M., Yanez-Gascon, M.J., Vallejo, F., Pallares, F.J.,
Larrosa, M., Lucas, R., Morales, J.C., Tomas-Barberan, F.A., Garcia-Conesa, M.T., Espin, J.C., 2011. Metabolites and tissue distribution of resveratrol in the pig. Molecular Nutrition & Food Research 55, 1154-1168.
Baolin, L., Inami, Y., Tanaka, H., Inagaki, N., Iinuma, M., Nagai, H., 2004. Resveratrol inhibits the release of mediators from bone marrow-derived mouse mast cells in vitro. Planta Medica 70, 305-309.
of resveratrol and other red wine constituents against nitric oxide-related toxicity in cultured hippocampal neurons. British Journal of Pharmacology 131, 711-720.
Baur, J.A., Sinclair, D.A., 2006. Therapeutic potential of resveratrol: the
in vivo evidence. Nature Reviews Drug Discovery 5, 493-506.
Bhadauria, M., 2010. Dose-dependent hepatoprotective effect of emodin against acetaminophen-induced acute damage in rats. Experimental and Toxicologic Pathology 62, 627-635.
Chinese Pharmacopoeia Committee (Ed.), 2000. The Pharmacopoeia of the People's Republic of China. Chemical Industry Press, Beijing. De Santi, C., Pietrabissa, A., Mosca, F., Pacifici, G.M., 2000a.
Glucuronidation of resveratrol, a natural product present in grape and wine, in the human liver. Xenobiotica 30, 1047-1054.
De Santi, C., Pietrabissa, A., Spisni, R., Mosca, F., Pacifici, G.M., 2000b. Sulphation of resveratrol, a natural product present in grapes and wine, in the human liver and duodenum. Xenobiotica 30, 609-617. Fabris, S., Momo, F., Ravagnan, G., Stevanato, R., 2008. Antioxidant
properties of resveratrol and piceid on lipid peroxidation in micelles and monolamellar liposomes. Biophysical Chemistry 135, 76-83.
Fang, S.H., Hou, Y.C., Chang, W.C., Hsiu, S.L., Chao, P.D., Chiang, B.L., 2003. Morin sulfates/glucuronides exert anti-inflammatory activity on activated macrophages and decreased the incidence of septic shock. Life Sciences 74, 743-756.
Gu, J.W., Hasuo, H., Takeya, M., Akasu, T., 2005. Effects of emodin on synaptic transmission in rat hippocampal CA1 pyramidal neurons in
vitro. Neuropharmacology 49, 103-111.
Han, Y.S., Zheng, W.H., Bastianetto, S., Chabot, J.G., Quirion, R., 2004. Neuroprotective effects of resveratrol against beta-amyloid-induced neurotoxicity in rat hippocampal neurons: involvement of protein kinase C. British Journal of Pharmacology 141, 997-1005.
Hsu, C.M., Hsu, Y.A., Tsai, Y., Shieh, F.K., Huang, S.H., Wan, L., Tsai, F.J., 2010. Emodin inhibits the growth of hepatoma cells: finding the common anti-cancer pathway using Huh7, Hep3B, and HepG2 cells. Biochemical and Biophysical Research Communications 392, 473-478.
Huang, W.Y., Cai, Y.Z., Xing, J., Corke, H., Sun, M., 2008. Comparative analysis of bioactivities of four Polygonum species. Planta Medica 74, 43-49.
Jang, J.H., Surh, Y.J., 2003. Protective effect of resveratrol on beta-amyloid-induced oxidative PC12 cell death. Free Radical Biology & Medicine 34, 1100-1110.
Jang, M., Cai, L., Udeani, G.O., Slowing, K.V., Thomas, C.F., Beecher, C.W., Fong, H.H., Farnsworth, N.R., Kinghorn, A.D., Mehta, R.G., Moon, R.C., Pezzuto, J.M., 1997. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 275, 218-220.
Kimura, Y., Ohminami, H., Okuda, H., Baba, K., Kozawa, M., Arichi, S., 1983. Effects of stilbene components of roots of Polygonum ssp. on liver injury in peroxidized oil-fed rats. Planta Medica 49, 51-54. Kitano, A., Saika, S., Yamanaka, O., Ikeda, K., Okada, Y., Shirai, K.,
Reinach, P.S., 2007. Emodin suppression of ocular surface inflammatory reaction. Investigative Ophthalmology & Visual Science 48, 5013-5022.
Kumerz, M., Heiss, E.H., Schachner, D., Atanasov, A.G., Dirsch, V.M., 2011. Resveratrol inhibits migration and Rac1 activation in EGF- but not PDGF-activated vascular smooth muscle cells. Molecular Nutrition & Food Research 55, 1230-1236.
Lee, B.H., Huang, Y.Y., Duh, P.D., Wu, S.C., 2012. Hepatoprotection of emodin and Polygonum multiflorum against CCl(4)-induced liver injury. Pharmaceutical Biology 50, 351-359.
Lin, C.C., Chang, C.H., Yang, J.J., Namba, T., Hattori, M., 1996. Hepatoprotective effects of emodin from Ventilago leiocarpa. Journal of Ethnopharmacology 52, 107-111.
Marier, J.F., Vachon, P., Gritsas, A., Zhang, J., Moreau, J.P., Ducharme, M.P., 2002. Metabolism and disposition of resveratrol in rats: extent of absorption, glucuronidation, and enterohepatic recirculation evidenced by a linked-rat model. The Journal of Pharmacology Experimental Therapeutics 302, 369-373.
McMurtrey, K.D., 1996. Resveratrol in wine, in: Watkins, T.R. (Ed.), Wine: Nutritional and therapeutic benefits. American Chemical Society, Washington, DC.
Shang, X., Yuan, Z., 2003. Determination of active components in
rhubarb and study of their hydrophobicity by micellar electrokinetic chromatography. Bioorganic & Medicinal Chemistry Letters 13, 617-622.
Chao, P.D., 2009a. Metabolism and pharmacokinetics of
San-Huang-Xie-Xin-Tang, a polyphenol-rich Chinese medicine formula, in rats and ex-vivo antioxidant activity. Evidence Based
Complementary and Alternative Medicine 2011, 721293.
Shia, C.S., Hou, Y.C., Tsai, S.Y., Huieh, P.H., Leu, Y.L., Chao, P.D., 2010. Differences in pharmacokinetics and ex vivo antioxidant activity following intravenous and oral administrations of emodin to rats. Journal of Pharmaceutical Sciences 99, 2185-2195.
Shia, C.S., Juang, S.H., Tsai, S.Y., Chang, P.H., Kuo, S.C., Hou, Y.C., Chao, P.D., 2009b. Metabolism and pharmacokinetics of
anthraquinones in Rheum palmatum in rats and ex vivo antioxidant activity. Planta Medica 75, 1386-1392.
Shia, C.S., Tsai, S.Y., Lin, J.C., Li, M.L., Ko, M.H., Chao, P.D., Huang, Y.C., Hou, Y.C., 2011. Steady-state pharmacokinetics and tissue distribution of anthraquinones of Rhei Rhizoma in rats. Journal of Ethnopharmacology 137, 1388-1394.
Shirai, M., Kawai, Y., Yamanishi, R., Kinoshita, T., Chuman, H., Terao, J., 2006. Effect of a conjugated quercetin metabolite, quercetin 3-glucuronide, on lipid hydroperoxide-dependent formation of
reactive oxygen species in differentiated PC-12 cells. Free Radical Research 40, 1047-1053.
Smoliga, J.M., Baur, J.A., Hausenblas, H.A., 2011. Resveratrol and health--a comprehensive review of human clinical trials. Molecular Nutrition & Food Research 55, 1129-1141.
Vitaglione, P., Sforza, S., Galaverna, G., Ghidini, C., Caporaso, N., Vescovi, P.P., Fogliano, V., Marchelli, R., 2005. Bioavailability of trans-resveratrol from red wine in humans. Molecular Nutrition & Food Research 49, 495-504.
Wang, D., Xu, Y., Liu, W., 2008. Tissue distribution and excretion of resveratrol in rat after oral administration of Polygonum cuspidatum extract (PCE). Phytomedicine 15, 859-866.
Wenzel, E., Somoza, V., 2005. Metabolism and bioavailability of trans-resveratrol. Molecular Nutrition & Food Research 49, 472-481. Wu, X.B., Luo, X.Q., Gu, S.Y., Xu, J.H., 2012. The effects of
Polygonum cuspidatum extract on wound healing in rats. Journal of
Ethnopharmacology 141, 934-937.
Xiong, H.R., Luo, J., Hou, W., Xiao, H., Yang, Z.Q., 2011. The effect of emodin, an anthraquinone derivative extracted from the roots of
Rheum tanguticum, against herpes simplex virus in vitro and in vivo. Journal of Ethnopharmacology 133, 718-723.
Yang, J.H., Hsia, T.C., Kuo, H.M., Chao, P.D., Chou, C.C., Wei, Y.H., Chung, J.G., 2006. Inhibition of lung cancer cell growth by
quercetin glucuronides via G2/M arrest and induction of apoptosis. Drug Metabolism and Disposition 34, 296-304.
Yeh, S.F., Chou, T.C., Liu, T.S., 1988. Effects of anthraquinones of
Polygonum cuspidatum on HL-60 cells. Planta Medica 54, 413-414.
Yoshino, S., Hara, A., Sakakibara, H., Kawabata, K., Tokumura, A., Ishisaka, A., Kawai, Y., Terao, J., 2011. Effect of quercetin and glucuronide metabolites on the monoamine oxidase-A reaction in mouse brain mitochondria. Nutrition 27, 847-852.
Zhan, Y., Li, D., Wei, H., Wang, Z., Huang, X., Xu, Q., Lu, H., 2000. Emodin on hepatic fibrosis in rats. Chinese Medical Journal 113, 599-601.