COMMUNICATION / . Biochem. 98, 1723-1726 (1985)
Specific Cleavages of DNA by Ascorbate in the Presence
of Copper Ion or Copper Chelates
Shyh-Horng CfflOU,* Wen-Chang CHANG,*'** Yuh-Shan JOU,* Hui-Min M. CHUNG,** and Tung-Bin L O * * *
•Institute of Biochemical Sciences, National Taiwan University, Taipei, Taiwan, and "Institute of Biological Chemistry, Academia Sinica, Taipei, Taiwan
Received for publication, July 8, 1985
The DNA-cleavage specificity of ascorbate in the presence of copper ion is analyzed
with end-labeled pBR322 DNA fragments. The nonenzymatic reaction of Cu(n)/
ascorbate and DNA shows certain degrees of cleavage preference toward
purine-containing short segments in the labeled DNA under mild conditions (at 0°C and
10 min). The segments of pyrimidine clusters are least susceptible to cleavage.
The DNA scission cannot be detected in the absence of metal ions, and is greatly
diminished in the presence of EDTA and metal-chelating peptide. It is more specific
than the nuclease-like scission activity induced by cuprous-phenanthroline complex.
This scission activity in relation to the antiviral and antitumor activities of vitamin
C reported in the literature deserves a crucial consideration.
The controversy surrounding the claim of the
antitumor effect of vitamin C has attracted a lot
of public attentions (1-3). Despite reports on the
antiviral (4-6) and antitumor (7-9) activities of
ascorbic acid there is as yet no persuasive
explana-tions of these biological effects at the cellular and
molecular levels. Previous studies (9, 10) indicated
a preferential cytotoxicity of ascorbate and copper
ion against malignant melanoma cells with little
effect on the proliferation of normal cells. It is
also reported that several biological reductones
including ascorbic acid inhibit the growth of
sar-coma-180 implanted in mice and cause the
break-age of DNA molecules (11-13). However, there
is so far no direct evidence of the specific cleavages
of DNA by ascorbate and copper ion to account
for these observations. We have been interested
in the physiological effect of ascorbate and its
possible clinical applications in the prevention of
viral diseases and inhibition of tumor growth. The
impressive effectiveness of vitamin C, in
combina-tion with copper ion and copper chelates in in
vitro DNA-cleaving and protein-scission activities
(14, IS) has prompted us to study the
DNA-scission specificity.
DNA fragments of defined sequence were
ob-tained from pBR322 plasmid. pBR322 DNA were
prepared as described (16) and digested with
re-striction endonucleases Clal, BamHI, and Pstl to
produce three fragments with the molecular size
of 352, 777, and 3,233 base pairs, respectively. The
fragment mixture was added the [a-
lrP]dCTP (10
fid/ft]) and Klenow fragment of DNA polymerase
I (5 U//*I) to label the recessed 3' termini created
1724 COMMUNICATION by cleavage of D N A at Clal site {16). The
result-ing end-labeled fragments of 352 and 777 base pairs were purified on an 8% polyacrylamide gel and the fragments were extracted from the gel slices of the separated bands and precipitated three times with ethanol after centrifugation.
The reaction mixtures for the scission experi-ments contain the 3'-labeled DNA fragment (800-1,000 cpm/ng) of 777 base pairs in 2 mM Tris-HCl buffer (pH 7.5). The final concentrations of ascor-bate, copper ion, copper peptide or copper-1,10-phenanthroline are indicated in the legends of Figs. 1 and 2. The reactions were started by the addi-tion of a freshly prepared Na ascorbate at 0°C or 25°C, and terminated by the addition of 25 mM EDTA, pH 8.0 after 2 or 10 min. The stopped reaction mixtures were dried under vacuum and dissolved in the loading buffer for electrophoresis in 2 0 % polyacrylamide sequencing gels according to the procedure of Maxam and Gilbert (17). Autoradiography was done on a Fuji X-ray film at - 7 0 ° C . The cleaved oligonucleotides induced by ascorbate and copper ion were compared di-rectly with the four standard chemical reactions run concurrently on the same sequencing gel. The relative reactivities under various conditions were quant itated with a laser scanning densitometer (Biomed Instruments, Inc., California, U.S.A.) on the developed autoradiograms.
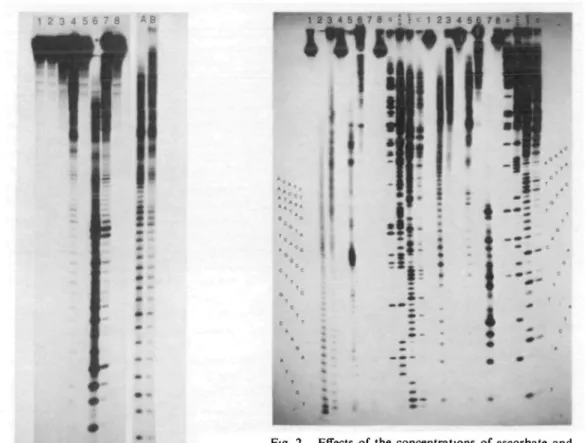
Figure 1 shows the cleavage patterns of the labeled D N A induced by copper/ascorbate (lane 4) and its comparison with that induced by copper-phenanthroline complex (lane 6). It clearly indi-cates that under the same conditions Cu(II)-ascor-bate has a much milder DNA-scission than that of cuprous-phenanthroline. It is noteworthy that D N A in the presence of ascorbate alone is not cleaved to a significant amount (lane 2 of Fig. 1 and lane 8 of Fig. 2). This is in contrast to the previous report which indicated the strand break-age of D N A with ascorbic acid in the presence of oxygen (18). We believe that the D N A cleavage of ascorbate observed in the absence of copper ion is probably caused by the trace metal contaminants present in the solutions; removal of these trace metal ions by 1-2 IHA of EDTA or 2,2'-biquinoline (cuproine) and use of deionized, double-distilled water effectively eliminate the minor cleavage pat-terns sometimes occurring in the ascorbate-treated D N A . It also occurs in the
phenanthroline-con-taining sample treated with ascorbate (lane 7 of Fig. 1) in the absence of copper ion; the obvious DNA scission could be ascribed to the presence of trace copper ions in the solution and the high cleavage activity of Cu(I)-phenanthroline complex thus formed. The scission reaction is temperature dependent; the cleavage can be controlled by low-ering the reaction temperature to 0°C (Fig. 1, lanes A and B). However the specific scission pat-terns are essentially the same at the two different temperatures albeit with the much lower reaction rate at the lower temperature, which is more ap-propriate for the study of this fast DNA-scission reaction.
We have investigated the cleavage specificity by comparing the patterns with those of the base-specific chemical cleavages of DNA-sequencing gel (Fig. 2). The relative intensity of each cleaved fragment should reflect the reactivities of the scis-sion reaction toward the different sequence seg-ments or different bases in the labeled DNA. Although the scission activity is not highly specific as those exhibited by the restriction enzymes, Jessee et al. (19) have shown an artificial micro-coccal nuclease-like activity associated with the cleavage reaction induced by 1,10-phenanthroline-cuprous complex (Fig. 2, lane 7). The scission reaction of ascorbate/copper is midler and more specific than that of phenanthroline/copper which shows the cleaved products mainly consisted of short segments of oligonucleotides. The quanti-tative densitometric scans of each individual lane reveal the relative susceptibilities of the different sequence segmenets (Fig. 3). D N A was cleaved more extensively at the 3' sides of deoxyguanosines and both 3' and 5' sides of deoxyadenosines than those of other bases, with the pyrimidine clusters the least susceptible to cleavage (segments of #21-25 (TTCTQ, #31-35 (TCTTC), and #3SM3 (CCCTT) numbered starting from the labeled 3' end at the Clal site). The DNA-cleavage reaction is greatly inhibited by the addition of the copper-chelating tripeptide, diglycyl-L-histidine (Fig. 1 and Refs. 14, 15). The reaction rate is also markedly decreased by the decrease in the concentrations of ascorbate and copper ions (Fig. 2), which suggests that the reaction is dependent on the oxidation products of ascorbate in the presence of copper and oxygen, including hydrogen peroxide and some unidentified, activated oxygen radicals. The
SPECIFIC DNA SCISSION BY ASCORBATE/COPPER 1725
1 2 3 4 5 6 7 8 « B
, U
• •-Fig. 1. DNA scissions of ascorbate in the presence of copper ion, copper tnpeptide and copper-l,10-phenan-throline. The 3'-end labeled DNA fragment of 777 base pairs was incubated with CuCl,, Cu-diglycyl-L-histidine, and Cu-l,10-phenanthrolme (final concen-trations in the reaction mixtures were 25 /<M in 2 mM Tris-HCI buffer, pH 7.5) at 0°C, followed by the addi-tion of freshJy-prepared Na ascorbate (1 mM after mix-ing) to start the reactions. The reactions were termi-nated by the addition of 25 mM EDTA (5 mM after mixing) after 10 mm at 0°C or 2 mm at 25°C (lane A shown on the right). Lane 1, DNA without treatment; lane 2, DNA plus ascorbate (the same pattern as that of DNA plus copper ion alone); lane 3, DNA plus Cu(II>phenanthroline in the absence of ascorbate; lane 4, DNA plus Cu(II) in the presence of ascorbate; lane 5, DNA plus Cu-tripeptide and ascorbate; lane 6, DNA plus Cu-phenanthroline and ascorbate; lane 7, DNA plus 1,10-phenanthroline (100/<M after mixing) and ascorbate without the addition of copper ion; lane 8, DNA in the presence of 1,10-phenanthroline (100 /<M) alone. Lanes A and B, DNA plus copper (50 /IM) and ascorbate (I mM) incubated for 2 min at 25°C (A) and 10 min at 0°C (B), respectively. Electrophoresis was run at 1,500 V for 2 h in the left panel and 3 h in the right panel (lanes A and B). The films were exposed for 96 h at -70°C.
- • • • « •
i
Fig. 2. Effects of the concentrations of ascorbate and copper ion on the DNA-scission and the comparison of cleavage sites with base-specific chemical reactions of Maxam and Gilbert (17). The conditions are the same as those described in Fig. 1 and the reactions were done at 0°C for 10 rrun. The four chemical cleavages of sequencing methods were as described (17), and the lanes on the left were the samples of first loading whereas those on the right corresponding to the same samples applied to the gel after 5 h electrophoresis (at 1,200 V) of first loading; the electrophoresis was con-tinued for another 3.5 h after second loading The autoradiography was done at —70°C for 44 h Lane 1, control DNA without treatment; lane 2, DNA plus ascorbate (1 mM) and copper (0.5 mM); lane 3, DNA plus ascorbate (0 1 ITIM) and copper (0 5 mM); lane 4, DNA plus ascorbate (0.01 mM) and copper (0.5 mM); lane 5, DNA plus ascorbate (1 mM) and copper (0.05 mM), lane 6, DNA plus ascorbate (1 mM) and copper (5 /IM); lane 7, DNA plus ascorbate (1 mM) and copper-1,10-phenanthroline (25 /IM); lane 8, DNA plus ascor-bate (1 mM) alone. Lanes G, A + G, C+T, and C correspond to the four base-specific sequencing reac-tions.
1726 COMMUNICATION
5' A^-T^-T^-??-T-G-T-T-T-C-A-C-A^?-C-7-T-A-7--C-A-T-<:* (3' >. 35 04
Fig. 3. Cleavage specificity of 3'-end-labeled DNA fragment of pBR322 plasmid induced by ascorbate/ copper. Arrows locate the most susceptible sites of scission from the densitometric scans of the autoradio-grams in the lane B of Fig. 1 and the lane 5 of Fig. 2. The numbers indicated below the arrows denote the relative cleavage intensities from the densitometric scans with the most preferred site of scission at nucleo-tide #19 from the 3' end rated as 100. Other minor cleavage sites with their corresponding cleavage inten-sites are as follows: #1-2 (32), #2-3 (30), #3^1 (39), #4-5 (45), #5-6 (38), #6-7 (35), #7-8 (25), #8-9 (32), #10-11 (65), #14-15 (68), #15-16 (38), #16-17 (50), and #17-18 (62).
nucleotides produced under this kind of mild
con-ditions have the same electrophoretic mobilities as
the markers of the four specific chemical reactions,
indicating that the phosphodiester bonds are the
main targets for the cleavage by ascorbate/copper
and the 5' termini of the breaks are probably
phosphorylated as those produced from the four
base-specific reactions (17). The detailed
charac-terization of the structures of the cleaved products
will be reported elsewhere.
The biological significance of the relatively
specific DNA-cleavage by ascorbate/copper is still
open to speculation. Attention is drawn to the
recent reports of the sequence-specific cleavages of
DNA by the antitumor drugs such as bleomycin
(20, 21) and mitomycin C (22), which indicated
that the dinucleotide sequences GT and GC are
two of the most specific sites of cleavage in both
cases. In our system, the most preferred sites
seem to locate at G-C, G-A, A-C, and A-T of
purine-contaming segments similar to the G-T and
G-C cleavage sites of these two drugs. This is
also reflected in the observation by Kasai and
Nishimura (23) that ascorbic acid in the presence
of oxygen specifically hydroxylated the C-8
posi-tion of deoxyguanosines in DNA. It is of great
interest to explore the possibility of utilizing this
DNA-scission activity as the basis for new
free-radical generating antiviral and antitumor drugs.
We thank the support of the National Science Council,
Taipei, Taiwan.
REFERENCES
1. Cameron, E. & Pauling, L. (1978) Proc. Natl. Acad.
Sci. U.S. 75, 4538-4542
2. Creagan, E.T., Moertel, C.G., O'Fallon, J.R., Schutt, A.J., O'Connell, M.J., Rubin, J , & Frytak, S. (1979) New Engl. J. Med. 301, 687-690
3. Moertel, C.G., Fleming, T.R., Creagan, E.T., Rubin, J., O'Connell, M.J., & Ames, M.M. (1985)
New Engl J. Med. 312, 137-141
4. Jungeblut, C.W. (1935) J Exp. Med. 62, 517-521 5. Jungeblut, C.W (1939) J. Exp. Med 70, 315-332 6. Murata, A , Kitagawa, K., & Saruno, R. (1971)
Agric. Biol. Chem. 35, 294-296
7. Park, C.H., Amare, M., Savin, M.A., & Hoog-straten, B. (1980) Cancer Res. 40, 1062-1065 8. Kimoto, E., Tanaka, H., Gyotoku, J., Monshige,
F., & Pauling, L. (1983) Cancer Res 43, 824-828 9. Bram, S., Froussard, P., Guichard, M., Jasmin, C , Augery, Y , Sinoussi-Barre, F., & Wray, W. (1980) Mjto* 284, 629-631
10. Lonn, U. & Lonn, S (1983) Carcinogenesis 4, 583-586
11. Yamafuji, K , Nakamura, Y., Omura, H., Soeda, T., & Gyotoku, K (1971) Z. Krebsforsch. 76, 1-7 12. Murata, A. & Kitagawa, K (1973) Agric. Biol.
Chem 37, 1145-1151
13. Omura, H., liyama, S., Tomita, Y., Narazaki, Y , Shinohara, K., & Murakami, H. (1975) J. Nulr.
Sa. Vilaminol. 21, 237-249
14. Chiou, S.-H. (1983) J. Bwchem. 94, 1259-1267 15. Chiou, S.-H. (1984) / . Bwchem. 96, 1307-1310 16. Maniatis, T., Fnstsch, E.F., & Sambrook, J. (1982)
Molecular Cloning pp 86-148, Cold Spring
Har-bor LaHar-boratory, Cold Spring HarHar-bor, New York 17. Maxam, A.M. & Gilbert, W. (1980) Methods
En-zymol 65, 499-560
18. Bode, V.C (1967)7 Mol Biol. 26, 125-129 19. Jessee, B , Gargiulo, G., Razvi, F., & Worcel, A.
(1982) Nucl. Acids Res. 10, 5823-5834
20. D'Andrea, A.D. & Haseltine, W A. (1978) Proc.
Nail. Acad. Sci. U.S. 75, 3608-3612
21. Takeshita, M., Grollman, A.P., Ohtsubo, E., & Ohtsubo, H. (1978) Proc Natl. Acad. Sci. U.S. 75, 5983-5987
22. Ueda, K., Morita, J., & Komano, T (1984)
Bio-chemistry 23, 1634-1640
23. Kasai, H. & Nishimura, S. (1984) Nucl. Acids Res. 12,2137-2145