New β-Caryophyllene-Derived Terpenoids from the Soft Coral
Sinularia nanolobata
Atallah F. Ahmed,†,‡Jui-Hsin Su,†Ru-Ting Shiue,†Xin-Jie Pan,†Chang-Feng Dai,§Yao-Haur Kuo,⊥and Jyh-Horng Sheu*,†
Department of Marine Resources, National Sun Yat-Sen University, Kaohsiung 804, Taiwan, Republic of China, Department of Pharmacognosy, Faculty of Pharmacy, Mansoura University, Mansoura 35516, Egypt,
Institute of Oceanography, National Taiwan University, Taipei 106, Taiwan, Republic of China, and National Research Institute of Chinese Medicine, Taipei 112, Taiwan, Republic of China
Received June 26, 2003
Two new norsesquiterpenoids, nanonorcaryophyllenes A (1) and B (2), two new diterpenoids, nanolobatins A (3) and B (4), and a novel norditerpenoid, nanolobatin C (5), were isolated from the n-hexane extract of the Taiwanese soft coral Sinularia nanolobata. Also, two new furanone derivatives, 6 and 7, were isolated for the first time from natural sources. The structures of 1-5 were elucidated on the basis of extensive spectroscopic analyses and by comparison of the spectral data with those of the related metabolites. Nanonorcaryophyllenes A (1) and B (2) were characterized as 13-norcaryophyllenes that lack a methyl group at C-11, while nanolobatin C (5) represents the first example of a xeniaphyllane-based 17-norditerpenoid. The cytotoxicity of 1-6 against the growth of a limited panel of cancer cell lines is also described.
Soft corals belonging to genus Sinularia (Alcyoniidae) have been found to be a rich source of structurally unique and biologically active diterpenoids1and norditerpenoids.1,2 A previous study on a Japanese sample of Sinularia nanolobata Verseveldt resulted in the isolation of several cytotoxic amphilectane-type diterpenoids, sinulobatins.3 During the course of our screening of bioactive metabolites from marine organisms,2,4-7an investigation on the chemi-cal constituents of S. nanolobata from Taiwanese waters has afforded two xeniaphyllane-type8diterpenoids, lobatins A (3) and B (4). A novel norditerpenoid, nano-lobatin C (5), has also been isolated and identified as the first 17-norxeniaphyllane. Although xeniaphyllanes have been known as the secondary metabolites of soft corals of Xenia,9 Nephthea,8 and Cespitularia,10 this is the first report on metabolites of this type from the genus Sinularia. Furthermore, two norsesquiterpenes, nanonorcaryophyl-lenes A (1) and B (2), and two furanone derivatives, 6 and
7, were also isolated in the present study. The structures
of 1-5 were elucidated on the basis of extensive spectro-scopic analyses, and their relative stereochemistries were deduced by the assistance of NOESY experiments and by comparison of the NMR data with those of related xenia-phyllene and β-caryoxenia-phyllene-derived metabolites.8-16
In addition to the isolation and structural elucidation of these compounds, in vitro cytotoxic activities of metabolites
1-6 against KB (human oral epidermoid carcinoma),
Hepa59T/VGH (human liver carcinoma), NCI-H661 (hu-man lung large cell carcinoma), Hela (hu(hu-man cervical epitheloid carcinoma, DLD-1 (human colon adenocarci-noma), and Med (human medulloblastoma) cell lines also have been evaluated.
Results and Discussion
The minced tissues of S. nanolobata were successively extracted with n-hexane and dichloromethane. The residue
of the dichloromethane layer was further extracted with n-hexane. The combined n-hexane extract was concen-* To whom correspondence should be addressed. Tel: 886-7-5252000, ext.
5030. Fax: 886-7-5255020. E-mail: sheu@mail.nsysu.edu.tw.
†National Sun Yat-Sen University. ‡Mansoura University.
§National Taiwan University.
⊥National Research Institute of Chinese Medicine.
10.1021/np030286w CCC: $27.50 © 2004 American Chemical Society and American Society of Pharmacognosy Published on Web 03/06/2004
Downloaded by NATIONAL TAIWAN UNIV on November 2, 2009 | http://pubs.acs.org
trated, and the residue was subjected to column chroma-tography over silica gel. Five fractions obtained were chosen for further purification by normal-phase HPLC to yield nanonorcaryophyllenes A (1) and B (2), nanolobatins A-C (3-5), and the furanone derivatives 6 and 7 (see Experimental Section).
Nanonorcaryophyllene A (1) possesses the molecular formula C14H22O2as revealed by its HREIMS (m/z 222.1617, [M]+) and NMR spectral data (Tables 1 and 2). Thus, 1 possesses four degrees of unsaturation. The infrared spec-trum and MS data revealed the presence of a hydroxyl group in 1 (νmax3371 cm-1and m/z 204 [M - H2O]+). From the 14 carbon signals appearing in the13C NMR spectrum of 1, two methyls (δ 17.1 and 21.5), one trisubstituted epoxide (δ 59.9, C and 63.9, CH), one double bond contain-ing an exomethylene group (δ 113.9, CH2 and 150.5, C), and two methines (δ 44.9 and 53.7) could be assigned. Due
to the presence of two sp2carbon resonances in the13C NMR spectrum of 1 for one carbon-carbon double bond, 1 was considered to be a tricyclic compound. By the as-sistance of extensive 2D NMR study (COSY, HMQC, and HMBC), the norcaryophyllene-based skeleton of 1 was proposed (Figure 1). Also, comparison of the 1H and 13C NMR spectral data of 1 with those of 811(Tables 1 and 2) revealed that a methyl substituent at C-11 in 8 was replaced by a hydroxyl group in 1. Thus, the norsesqui-terpenoid nature of 1 was fully established. The relative stereochemistry of 1 (Figure 2) was proposed on the basis of the key NOE correlations observed in the NOESY spectrum. Furthermore, it was found that the spectral data of (1R,4R,5R,9S,11R)-4,5-epoxy-12-nor-8(14)-caryophyllen-11-ol (12),12obtained previously by a microbial biotrans-formation of β-caryophyllene, were almost identical in all aspects with those of 1 except the sign of optical rotation. Nanocaryophyllene A (1) ([R]D+42.6°) was thus enantio-meric with 12 ([R]D-36.3°), and the absolute structure of
1 was established as
(1S,4S,5S,9R,11S)-4,5-epoxy-12-nor-8(14)-caryophyllen-11-ol.
Nanonorcaryophyllene B (2) has the same molecular formula, C14H22O2, as that of 1, as revealed from the HREIMS (m/z 222.1618, [M]+) and NMR spectral data (Tables 1 and 2). The existence of a ketone functional group (νmax1700 cm-1) and a hydroxyl moiety (νmax3422 cm-1 and m/z 204 [M - H2O]+) in 2 could be observed. The13C NMR spectrum also revealed that 2 is a norcaryophylene-based metabolite. Comparison of13C NMR spectral data of 2 and 1 (Table 1) indicated that the two carbons of the trisubstituted epoxide (δ 59.9, C and 63.9, CH) in 1 were replaced by a methine (δ 48.0) and a ketone carbonyl (δ 217.0, C) carbon. The proton resonating at δ 2.54 (1H, m) that showed COSY correlations (Figure 1) with the signals of a secondary methyl at δ 1.04 (3H, d, J ) 6.9 Hz) and H2-3 (δ 1.65, m; 1.80, t, J ) 9.3 Hz) was designated as H-4. Furthermore, the HMBC correlations of H-4, H3-15, H2-3, H2-6, and H2-7 with the carbon signal at δ 217.0 ppm revealed the ketone functionality of C-5. On the basis of the above findings and key COSY and HMBC correlations observed (Figure 1), the skeleton of 2 could be established unambiguously.
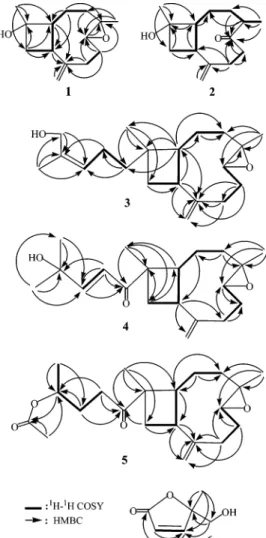
Table 1. 13C NMR Chemical Shifts of Compounds 1-5, 8,11and 1412
no. 1a 2a 3a 4a 5b 8c 14d,e 1 53.7 (CH)f 56.1 (CH) 49.8 (CH) 45.3 (CH) 45.3 (CH) 50.8 (CH) 47.7* (CH) 2 26.5 (CH2) 26.5 (CH2) 27.9 (CH2) 28.2 (CH2) 28.0 (CH2) 27.2 (CH2) 29.8** (CH2) 3 38.9 (CH2) 30.8 (CH2) 39.1 (CH2) 38.6 (CH2) 38.5 (CH2) 39.1 (CH2) 38.5 (CH2) 4 59.9 (C) 48.0 (CH) 59.9 (C) 59.6 (C) 59.4 (C) 59.8 (C) 59.5 (C) 5 63.9 (CH) 217.0 (C) 63.9 (CH) 63.7 (CH) 63.6 (CH) 63.7 (CH) 63.5 (CH) 6 30.3 (CH2) 42.2 (CH2) 30.2 (CH2) 30.1 (CH2) 30.0 (CH2) 30.2 (CH2) 29.7** (CH2) 7 29.2 (CH2) 32.1 (CH2) 29.8 (CH2) 29.7 (CH2) 29.5 (CH2) 29.8 (CH2) 35.1** (CH2) 8 150.5 (C) 151.7 (C) 151.8 (C) 150.8 (C) 150.6 (C) 151.8 (C) 150.4 (C) 9 44.9 (CH) 39.7 (CH) 48.8 (CH) 47.3 (CH) 47.2 (CH) 48.7 (CH) 47.3* (CH) 10 42.6 (CH2) 41.9 (CH2) 38.6 (CH2) 35.3 (CH2) 35.1 (CH2) 39.8 (CH2) 27.5** (CH2) 11 71.1 (C) 71.0 (C) 36.0 (C) 47.8 (C) 48.2 (C) 34.0 (C) 41.8 (C) 12 21.5 (CH3) 21.3 (CH3) 44.4 (CH2) 203.7 (C) 213.1 (C) 29.9 (CH3) 182.8 (C) 13 22.4 (CH2) 120.2 (CH) 32.5 (CH2) 21.6 (CH3) 16.8*** (CH3) 14 113.9 (CH2) 112.2 (CH2) 128.9 (CH) 153.6 (CH) 29.6 (CH2) 112.7 (CH2) 114.0 (CH2) 15 17.1 (CH3) 16.7 (CH3) 134.1 (C) 71.2 (C) 70.3 (CH) 17.0 (CH3) 16.4*** (CH3) 16 61.6 (CH2) 29.6(CH3) 20.2 (CH3) 17 21.3 (CH3) 29.6 (CH3) 18 18.9 (CH3) 17.0 (CH3) 16.8 (CH3) 19 113.0 (CH2) 115.0 (CH2) 114.1 (CH2) 20 17.1 (CH3) 17.1 (CH3) 17.0 (CH3) Ac 170.7 (C) 21.3 (CH3) aSpectra recorded at 75 MHz in CDCl 3at 25 °C.b125 MHz in CDCl3at 25 °C.c100 MHz in CDCl3, see ref 11.d75.5 MHz in CDCl3.
e*,**,***Assignments of chemical shifts may be exchanged between shifts indicated by the same superscript, see ref 12.fMultiplicity
deduced by DEPT and indicated by usual symbols. The values are in ppm downfield from TMS.
Table 2. 1H NMR Chemical Shifts of Compounds 1, 2, and 8
1a 2a 8b,11 H-1 2.07 t (10.0)c 1.68 m 1.76 t (10.0) H-2R 1.88 m 1.24 dd (15.0, 8.4) 1.32 m H-2β 1.43 m 1.82 d (15.0) 1.55-1.72 m H-3R 1.04 dt (4.8, 13.0) 1.80 t (9.3) 0.95 m H-3β 2.10 m 1.65 m 2.10 dt (3.5, 12.5) H-4 2.54 m H-5 2.89 dd (10.5, 3.6) 2.87 dd (10.6, 4.1) H-6R 2.25 dt (12.0, 3.5) 2.50-2.54 m 1.42 m H-6β 1.34 m 2.50-2.54 m 2.25 ddd (16.4, 7.8, 4.1) H-7R 2.35 ddd(12.3, 6.0, 3.3) 2.42-2.50 m 2.11 t (12.4) H-7β 2.14 m 2.42-2.50 m 2.34 ddd (12.4, 8.1, 4.4) H-9 2.15 m 1.89 dt (7.5, 9.3) 2.61 q (10.0) H-10R 2.02 dd (10.2, 7.5) 1.77 t (9.6) 1.55-1.72 m H-10β 1.88 m 2.07 dd (9.6,7.5) 1.55-1.72 m H-12 1.25 3H, s 1.16 3H, s 0.98 3H, s H-13 1.00 3H, s H-14 4.90 1H, s 4.93 2H,s 4.85 s 5.00 1H, s 4.97 s H-15 1.19 3H, s 1.04 3H, d (6.9) 1.20 3H, s aSpectra recorded at 300 MHz in CDCl 3at 25 °C.b400 MHz
in CDCl3, see ref 11.cThe J values (in parentheses) are in Hz.
Downloaded by NATIONAL TAIWAN UNIV on November 2, 2009 | http://pubs.acs.org
The relative stereochemistry of 2 (Figure 2) was deduced from the NOE interactions observed in the NOESY spec-trum and by comparison of its1H and 13C spectral data with those of the related compounds (9 and 11).13-16 Assuming the β-orientation of H-1, it was found that H-1 exhibited a weak but meaningful NOE correlation with H-4 (δ 2.54, m). This observation is consistent with H-4 being located on the β face. Also, H-1 exhibited NOE correlation with the β-proton at C-2 (δ 1.82, d, J ) 15.0 Hz), which in turn showed interaction with H-3β (δ 1.65, m), while the
latter further correlated with H-4. Thus, the β-orientation of H-4 could be established unambiguously. H-1 was also found to exhibit significant NOE interactions with H3-12 (δ 1.16, 3H, s) and H-9 (δ 1.89, dt, J ) 7.5, 9.3 Hz), implying the β-orientations of H-9 and the methyl substituent at C-11. On the basis of the above findings and other key NOE interactions observed, the cis ring junction at C-1 and C-9 in 2 was established. Since only a few 9-epi-β-caryophyl-lenes have been isolated from nature,13-17further confir-mation of the configuration at the ring junction was sought. It was found that a cis configuration of the ring junction (as in 9 and 11) induces an upfield shift at C-9 by almost more than 5.0 ppm relative to the corresponding trans isomers (as in the cases of 8 and 10).13Because the C-9 of
2 showed a signal at δ 39.7 ppm, which is found to be
upfield shifted by 5.2 and 6.0 ppm, respectively, in com-parison to those of the related norsesquiterpenoids 1 (δC-9 ) 44.9 ppm) and 13 (δC-9 ) 45.7 ppm),18 the cis ring junction in 2 was established unambiguously. Although at this stage we do not have sufficient data for determination of the absolute configuration, the relative stereochemistry of nanonorcaryophyllene B (2) could be fully assigned as (1R*,4S*,9R*,11S*)-5-oxo-13-nor-8(14)-caryophyllen-11-ol on the basis of the above findings.
The new metabolite nanolobatin A (3) was obtained as a colorless oil. The molecular formula of 3 was established as C20H32O2by HREIMS (m/z 304.2404, [M]+), thus requir-ing five degrees of unsaturation. The presence of a hydroxyl group in 3 was revealed from the absorption band at 3437 cm-1and the ion peak at m/z 286 [M - H2O]+in the IR and EIMS spectra, respectively. The13C NMR spectrum of 3 showed signals of 20 carbons (Table 1), among which 11 carbons have very similar chemical shifts in comparison with those of the ring carbons (C-1 to C-11) of 8, suggesting that 3 could be a caryophyllene-related diterpenoid. De-tailed analyses of 2D NMR spectra of 3, focusing in particular on the interpretation of1H-1H COSY, HMQC, and HMBC correlations, illustrated the gross structure of
3 as shown in Figure 1. Thus, the structure of 3 was
determined to be consistent with a metabolite possessing a 4,5-epoxyxeniaphyllane skeleton.
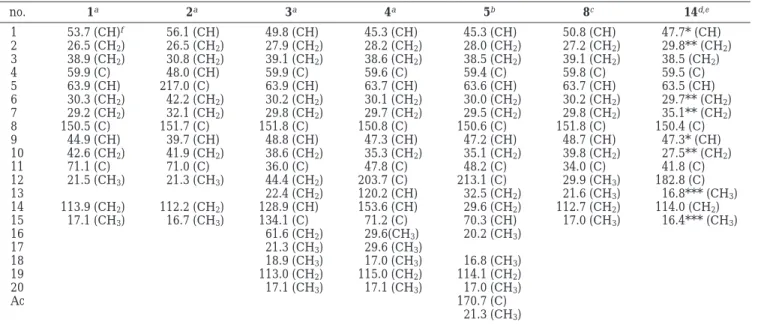
The relative stereochemistry of 3 was determined on the basis of the NOE correlations observed in a NOESY spectrum (Figure 3). Assuming the R-orientation of H-1, it was found that H-1 exhibited strong NOE interaction with Figure 1. 1H-1H COSY and HMBC correlations for 1-6.
Figure 2. Key NOESY correlations of 1 and 2.
Figure 3. Key NOESY correlations of 3 and 5.
Downloaded by NATIONAL TAIWAN UNIV on November 2, 2009 | http://pubs.acs.org
H-5, indicating the R-orientation of H-5. One proton at-tached at C-3 and resonating at δ 0.94 (dt, J ) 6.0, 14.4 Hz) showed NOE correlation with H-5 and was assigned as H-3R. The other proton attached at C-3, H-3β (δ 2.06, m), displayed NOE correlation with H3-20, which further interacted with H-9, revealing the β-orientations of H-9 and H3-20. H3-18 showed NOE interaction with H-9, but not with H-1, and should be positioned on the β face. Further-more, the NOE correlations displayed between the H-14 and H3-17 revealed the Z geometry of the 14,15-double bond. Therefore, the relative structure of nanolobatin A (3) was unambiguously established as in formula 3. As com-pound 3 ([R]D+15.0°) is the prenylated derivative of 1 ([R]D +42.6°) and both are isolated from the same organism, it is reasonable to assign the same absolute configuration at C-1, C-4, C-5, C-9, and C-11 for 3 as that of 1. The structure of nanolobatin A (3) was thus determined as (1S,4S,5S, 9R,11S,14Z )-4,5-epoxyxeniaphylla-8(19),14-dien-16-ol.
The second related diterpenoid metabolite, nanolobatin B (4), was also obtained as a colorless oil. Its HREIMS spectral data (m/z 318.2394) suggested the molecular formula C20H30O3, requiring six degrees of unsaturation. The infrared absorption band at 3640 cm-1indicated the presence of a hydroxyl group. Comparison of 13C NMR spectral data of 4 with those of 3 (Table 1) revealed that 4 should be another xeniaphyllene-type diterpenoid and differs from 3 only in the isoprene tail (C-12 to C-18) portion of the molecule. It was found that 4 possesses a ketone carbonyl (δ 203.7, C), a 1,2-disubstituted carbon-carbon double bond (δ 120.2, CH and 153.6, CH), and a carbon containing a tertiary hydroxyl group (δ 71.2, C). The downfield shift exerted at C-11 (δ 47.8, C) of 4 relative to that of 3 (δ 36.0, C) allowed C-12 to be assigned to the ketone carbonyl, as the similar magnitude of this downfield shift also was observed on comparison of13C NMR data of the known compounds 811and 1412(Table 1). The E-1,2-disubstituted double bond was revealed by the signals of two vicinal protons (δ 6.41, 1H, d, J ) 15.3 Hz; δ 6.97, 1H, d, J ) 15.3 Hz). On the basis of the above observations and other key COSY and HMBC correlations (Figure 1), the molecular framework of 4 was established. Comparison of chemical shifts and J values of H-1, H-5, and H-9 and chemical shifts of C-1, C-4, C-5, C-9, and C-11 of 4 with those of 3 and 5 (discussed later) indicated that 3-5 possess the same relative stereochemistries at C-1, C-4, C-5, C-9, and C-11. By comparison with one of the fermen-tation products of β-caryophyllene, (1R,4R,5R,9S,11R)-4,5-epoxycaryophyll-8(14)-en-12-oic acid (14)12([R]
D -55.5°), the prenylated derivative nanolobatin B (4) ([R]D+66.7°) was finally defined as (1S,4S,5S,9R,11S,13E)-4,5-epoxy-xeniaphylla-8(19),13-dien-12-on-15-ol, on the basis of the signs of optical rotations of these two compounds.
The novel norditerpene nanolobatin A (5) was isolated from a less polar fraction of the hexane extract. A molecular formula of C21H32O4for 5 was suggested by the FABMS spectrum of 5, which exhibited a peak at m/z 349 [M + H]+, and13C and1H NMR spectral data (Tables 1 and 3). The HREIMS spectrum showed a strong ion peak at m/z 288.2084, which corresponds to a fragment with a molec-ular formula C19H28O2arising from the elimination of an equivalent of acetic acid. Infrared absorption bands at 1734 and 1700 cm-1indicated that 5 possesses ester and ketone functionalities. Comparison of the13C NMR spectral data of 5 with those of 4 (Table 1) indicated that 5 has the same carbon skeleton as that of 4 from C-1 to C-12, while the 13,14-double bond in 4 was reduced. In addition to the presence of an acetate methyl group which showed a signal
at δ 2.02 (3H, s), it was found that there is only one secondary methyl attached at C-15 (δ 1.23, d, J ) 6.5 Hz), thus indicating the norditerpenoid nature of 5. The mo-lecular framework of 5 was further confirmed by the observed COSY and HMBC correlations (Figure 1).
The relative stereochemistries at C-1, C-4, C-5, C-9, and C-11 in 5 were found to be the same as those of 3 on the basis of the NOE correlations observed in a NOESY spectrum (Figure 3). The chemical shifts of both C-1 protons in 4 (δ 2.46, t, J ) 9.7 Hz) and 5 (δ 2.41, t, J ) 10.0 Hz) were found to be close to that of 14 (δ 2.52, dd, J ) 10.0, 9.7 Hz)12and are significantly downfield shifted in comparison with those of 3 (δ 1.83, t, J ) 9.6 Hz) and 16 (δ 1.76, t, J ) 10.0 Hz).11These data revealed that both C-1 protons of 4 and 5 are syn oriented relative to the carbonyl-containing substituents at C-10. From the above observations and other NOE interactions (Figure 3), the structure of nanolobatin C (5) ([R]D +21.0°), which is produced together with 3 and 4 from the same organism, is thus elucidated as (1S,4S,5S,9R,11S)-4,5-epoxy-15-ac-etoxy-17-norxeniaphyll-8(19)-en-12-one.
In addition to the discovery of the above caryophyllene-based metabolites, two furanones, 6 and 7, were also isolated. The structures of these two compounds were established by 1D and 2D NMR experiments. Compound
6 was obtained as a colorless oil, [R]25
D +9.8° (c 1.02,
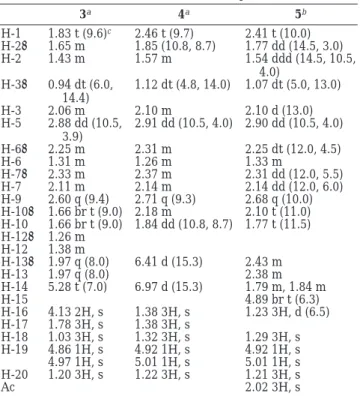
Table 3. 1H NMR Chemical Shifts of Compounds 3-5
3a 4a 5b H-1 1.83 t (9.6)c 2.46 t (9.7) 2.41 t (10.0) H-2R 1.65 m 1.85 (10.8, 8.7) 1.77 dd (14.5, 3.0) H-2β 1.43 m 1.57 m 1.54 ddd (14.5, 10.5, 4.0) H-3R 0.94 dt (6.0, 14.4) 1.12 dt (4.8, 14.0) 1.07 dt (5.0, 13.0) H-3β 2.06 m 2.10 m 2.10 d (13.0) H-5 2.88 dd (10.5, 3.9) 2.91 dd (10.5, 4.0) 2.90 dd (10.5, 4.0) H-6R 2.25 m 2.31 m 2.25 dt (12.0, 4.5) H-6β 1.31 m 1.26 m 1.33 m H-7R 2.33 m 2.37 m 2.31 dd (12.0, 5.5) H-7β 2.11 m 2.14 m 2.14 dd (12.0, 6.0) H-9 2.60 q (9.4) 2.71 q (9.3) 2.68 q (10.0) H-10R 1.66 br t (9.0) 2.18 m 2.10 t (11.0) H-10β 1.66 br t (9.0) 1.84 dd (10.8, 8.7) 1.77 t (11.5) H-12R 1.26 m H-12β 1.38 m H-13R 1.97 q (8.0) 6.41 d (15.3) 2.43 m H-13β 1.97 q (8.0) 2.38 m H-14 5.28 t (7.0) 6.97 d (15.3) 1.79 m, 1.84 m H-15 4.89 br t (6.3) H-16 4.13 2H, s 1.38 3H, s 1.23 3H, d (6.5) H-17 1.78 3H, s 1.38 3H, s H-18 1.03 3H, s 1.32 3H, s 1.29 3H, s H-19 4.86 1H, s 4.92 1H, s 4.92 1H, s 4.97 1H, s 5.01 1H, s 5.01 1H, s H-20 1.20 3H, s 1.22 3H, s 1.21 3H, s Ac 2.02 3H, s aSpectra recorded at 300 MHz in CDCl 3at 25 °C.b500 MHz
in CDCl3at 25 °C.cThe J values (in parentheses) are in Hz.
Table 4. Cytotoxicity of Terpenoid Metabolites 1-6a
cell lines ED50(µg/mL)
compd Hepa59T/VGH KB NCI-H661 Hela Med DLD-1
1 >20.0 >20.0 >20.0 >20.0 >20.0 >20.0 2 18.5 >20.0 >20.0 >20.0 >20.0 16.9 3 4.6 7.3 -b - - -4 8.3 7.6 - - - -5 >20.0 19.5 19.0 15.9 >20.0 -6 >20.0 >20.0 15.7 >20.0 >20.0 >20.0
aStandard is doxorubicin with ED
50values of 0.47, 0.25, 0.29,
0.41, 0.57, and 0.22 µg/mL against Hepa59T/VGH, KB, NCI-H661, Hela, Med, and DLD-1, respectively.bNot tested.
Downloaded by NATIONAL TAIWAN UNIV on November 2, 2009 | http://pubs.acs.org
CHCl3). Its EIMS (m/z 128, [M]+) together with NMR data revealed the molecular formula C6H8O3. Compound 6 was found to possess hydroxyl (IR νmax3422 cm-1; EIMS m/z 110 [M - H2O]+) and ester carbonyl (IR νmax1746 cm-1; 13C NMR δ 172.5, C) moieties. A conjugated cis-1,2-disubstituted double bond was also assigned (13C NMR δ 122.1 (CH) and 158.1 (CH);1H NMR δ 6.12 and 7.38, each 1H, d, J ) 5.7 Hz for both protons). Moreover, the1H NMR data of 6 showed two 1H doublet signals of an oxymeth-ylene at δ 3.70 and 3.80 (each d, J ) 12.0 Hz) and one 3H singlet of a tertiary methyl at δ 1.48, which were found to be correlated with the signals of the fully substituted oxycarbon at δ 89.3 (C) and the olefinic methine carbon at δ 158.1 in the HMBC spectrum (Figure 1). Comparison of NMR spectral data of 6 (see Experimental Section) with those of the known synthesized compound 1519established the identity of 6 and 15. Therefore, on the basis of the sign of its optical activity, compound 6 was established as (+)-5-hydroxymethyl-5-methylfuran-2-one.
Furthermore, 7, a less polar compound relative to 6, was also obtained as a colorless oil, [R]25
D+21.4 (c 0.28, CHCl3). Its IR spectrum indicated the absence of the hydroxyl and the presence of the ester carbonyl (νmax1740 cm-1). NMR spectral data (see Experimental Section) indicated that 7 is simply an acetylated derivative of 6, as shown by the presence of additional carbon signals at δ 20.6 (CH3) and 170.4 (CdO of ester) and a proton signal at δ 2.05 (3H, s, OCOCH3). According to the sign of the optical rotation, the structure of 7 was thus established as (+)-5-acetoxymethyl-5-methylfuran-2-one.
It is noteworthy to mention that the novel metabolite 2 represents the first example of a 13-nor-9-epi-β-caryophyl-lene compound, and 5 is the first 17-norxeniaphyllane-based derivative reported. In contrast to the trans ring fused β-caryophyllenes, which are common natural me-tabolites, the caryophyllenes with a cis ring junction are rarely found.13-17 To the best of our knowledge, the norsesquiterpenoid 2 is the fourth natural product of this series and is the first one isolated from a marine organism. Moreover, we report the isolation of furanones 6 and 7 for the first time from natural sources.
The new terpenoid metabolites (1-5) and the furanone derivative 6 were evaluated for their in vitro cytotoxic activity against a limited panel of cancer cell lines. The results showed that the xeniaphyllane-type diterpenoids
3 and 4 exhibited moderate cytotoxicities against KB and
Hepa59T/VGH cell lines (ED50values of 7.3 and 7.6 µg/ mL against the growth of KB cells and 4.5 and 8.3 µg/mL against the growth of Hepa59T/VGH cells, respectively). Norditerpenoid 5 exhibited weak activity against Hela, NCI-H661, and KB cells (ED50values of 15.9, 19.0, and 19.5 µg/mL, respectively) and was inactive toward Hepa59T/ VGH and Med cell lines. It was also found that the cis ring fused β-caryophyllene-type norsesquiterpenoid 2 exhibited only weak cytotoxicity against DLD-1 and Hepa59T/VGH (ED50values of 16.9 and 18.5 µg/mL, respectively), while the trans ring fused related metabolite 1 showed no activity against the above six cancer cell lines. Moreover, furanone
6 was found to show only weak activity against NC-H661
cancer cells, with a ED50value of 15.7 µg/mL.
Experimental Section
General Experimental Procedures. Optical rotations were measured on a Jasco DIP-1000 digital polarimeter. IR spectra were recorded on a Jasco FT-5300 infrared spectro-photometer. NMR spectra were recorded on a Bruker Avance
DPX300 FT-NMR at 300 MHz for1H and 75 MHz for13C or
on a Varian Unity INOVA 500 FT-NMR at 500 MHz for1H
and 125 MHz for13C, respectively, in CDCl
3using TMS as
internal standard. EIMS was obtained with a VG Quattro GC/ MS spectrometer. HRMS spectra were recorded on a Finnigan MAT-95XL mass spectrometer. Silica gel (Merck, 230-400 mesh) was used for column chromatography. Precoated silica gel plates (Merck, Kieselgel 60 F-254, 0.2 mm) were used for analytical TLC.
Organism. S. nanolobata was collected by hand via scuba off the coast of southern Pingtung, Taiwan, in February 2001, at a depth of 15-20 m, and stored in a freezer until extraction. A voucher sample was deposited at the Department of Marine Resources, National Sun Yat-Sen University (specimen no. SC003).
Extraction and Isolation. The lyophilized bodies of S.
nanolobata (1.2 kg, wet wt) were minced and extracted
exhaustively with n-hexane (1 L× 5) and then with dichlo-romethane (0.4 L× 3). The residue of dichloromethane layer was further extracted with n-hexane. The combined n-hexane extract was evaporated under vacuum to yield an oily residue (9.6 g), which was chromatographed over silica gel, using
n-hexane, an n-hexane and EtOAc mixture of increasing
polarity, then EtOAc-MeOH (stepwise, 100:0 to 50:50) to yield 28 fractions. Fraction 6, eluting with n-hexane-EtOAc (9:1),
was chromatographed by normal-phase HPLC using a CH2
-Cl2-MeOH gradient (99:1 to 97:3) to yield 5 (2.0 mg). Fraction
12, eluting with n-hexane-EtOAc (7:3), was purified by normal-phase HPLC using n-hexane-EtOAc (4:1) to afford 7 (2.5 mg). Fractions 13 and 14, eluting with n-hexane-EtOAc (1:1), were separately purified by normal-phase HPLC using
n-hexane-EtOAc (3:1) to afford 4 (11.4 mg) and 3 (5.8 mg),
respectively. Fraction 21, eluting with EtOAc, was further separated by normal-phase HPLC using n-hexane-acetone (4:1 to 3:1) to give 2 (10.2 mg), 1 (18.5 mg), and 6 (9.6 mg).
Nanonorcaryophyllene A (1): colorless oil; [R]25
D+42.6°
(c 1.48, CHCl3); IR (neat) νmax3371, 2968, 2928, 2878, 1541,
1456, 1299, 1219 cm-1;1H NMR (CDCl
3, 300 MHz) and13C
NMR (CDCl3, 75 MHz), see Tables 1 and 3, respectively; EIMS m/z 222 (0.2, [M]+), 204 (0.3, [M - H2O]+), 189 (0.8, [M - Me
- H2O]+), 149 (14.9), 121 (31.0); HREIMS m/z 222.1617 (calcd
for C14H22O2, 222.1622).
Nanonorcaryophyllene B (2): colorless oil; [R]25
D -7.6°
(c 0.92, CHCl3); IR (neat) νmax3422, 2948, 2928, 2878, 1700,
1647, 1541, 1456 cm-1;1H NMR (CDCl
3, 300 MHz) and13C
NMR (CDCl3, 75 MHz), see Tables 1 and 3, respectively; EIMS m/z 222 (0.2, [M]+), 204 (1.1, [M - H2O]+), 189 (2.2, [M - Me
- H2O]+), 149 (14.9), 161 (11.3), 146 (12.6), 131 (14.5);
HREIMS m/z 222.1618 (calcd for C14H22O2, 222.1622).
Nanolobatin A (3): colorless oil; [R]25
D +15.0° (c 1.60,
CHCl3); IR (neat) νmax 3437, 3011, 2981, 2934, 2874, 1632,
1454, 1381, 1373, 997, 896 cm-1;1H NMR (CDCl
3, 300 MHz)
and13C NMR (CDCl
3, 75 MHz), see Tables 1 and 2,
respec-tively; EIMS (30 eV) m/z 304 (0.2, [M]+
), 286 (0.4, [M-H2O]+),
271 (1.0, [M - Me - H2O]+), 256 (0.8), 241 (0.6), 205 (2.7),
149 (11.7), 135 (12.4); HREIMS m/z 304.2404 (calcd for C20H32O2, 304.2404).
Nanolobatin B (4): colorless oil; [R]25
D +66.7° (c 0.60,
CHCl3); IR (neat) νmax 3640, 3025, 2965, 2935, 2875, 1709,
1689, 1630, 1452, 1379, 1037, 902 cm-1;1H NMR (CDCl 3, 300
MHz) and13C NMR (CDCl
3, 75 MHz), see Tables 1 and 2,
respectively; FABMS m/z 341 (0.4, [M + Na]+), 319 (0.2, [M +
H]+), 304 (0.5, [M - Me + H]+), 301 (0.5, [M - H2O + H]+),
286 (0.5, [M - Me - H2O + H]+); HREIMS m/z 318.2394 (calcd
for C20H30O3, 318.2196).
Nanolobatin C (5): colorless oil; [R]25
D +21.0° (c 0.92,
CHCl3); IR (neat) νmax 2970, 2936, 2875, 1734, 1700, 1635,
1541, 1456, 1375, 1244, 1039, 960 cm-1;1H NMR (CDCl 3, 500
MHz) and13C NMR (CDCl
3, 100 MHz), see Tables 1 and 2,
respectively; FABMS m/z 371 (2.0, [M + Na]+), 349 (5.5, [M +
H]+), 289 (15.8, [M - AcOH + H]+), 154 (71.1); HREIMS m/z 288.2083 (calcd for C21H32O4- AcOH, 288.2084).
(+)-5-Hydroxymethyl-5-methylfuran-2-one (6): colorless oil; [R]25 D+9.8° (c 1.02, CHCl3); IR (neat) νmax3422, 2924, 2852, 1746, 1647, 1458, 1219, 1119, 1055, 956 cm-1;1H NMR (CDCl 3, 300 MHz) 7.38 (1H, d, J ) 5.7, H-4), 6.12 (1H, d, J ) 5.7, H-3),
Downloaded by NATIONAL TAIWAN UNIV on November 2, 2009 | http://pubs.acs.org
3.80 (1H, d, J ) 12.0, H-7), 3.70 (1H, d, J ) 12.0, H-7), 1.48 (3H, s, H3-6);13C NMR (CDCl3, 75 MHz) 172.5 (C, C-5), 158.1 (CH, C-4), 122.1 (CH, C-3), 89.3 (C, C-5), 66.5 (CH2, C-7), 20.1 (CH3, C-6); EIMS m/z 128 (2.3, [M]+), 113 (0.5, [M - Me]+), 110 (1.8, [M - H2O]+), 98 (100.0), 97 (83.4); HREIMS m/z 129.0549 (calcd for C6H9O4, 129.0546). (+)-5-Acetoxymethyl-5-methylfuran-2-one (7): colorless oil; [R]25 D +21.5° (c 0.28, CHCl3); IR (neat) νmax2955, 2926, 2866, 1740, 1659, 1611, 1458, 1375, 1165, 1020, 885, 814 cm-1; 1H NMR (CDCl 3, 300 MHz) 7.33 (1H, d, J ) 5.7, H-4), 6.10 (1H, d, J ) 5.7, H-3), 4.34 (1H, d, J ) 11.7, H-7), 4.17 (1H, d, J ) 11.7, H-7), 2.05 (3H, s, OCOCH3), 1.15 (3H, s, H3-6);13C NMR (CDCl3, 75 MHz) 171.8 (C, C-2), 170.4 (C, Ac-carbonyl), 156.9 (CH, C-4), 122.2 (CH, C-3), 86.8 (C, C-5), 65.8 (CH2, C-7), 20.8 (CH3, C-6), 20.6 (CH3, Ac-Me); EIMS m/z 170 (0.1, [M]+), 155 (1.5, [M - Μe]+), 134 (58.1), 110 (3.4, [M - AcOH]+), 98 (100.0).
Cytotoxicity Testing. Cell lines were purchased from the American Type Culture Collection (ATCC). Cytotoxicity assays of the test compounds 1-5 and 7 were performed using the MTT [3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bro-mide] colorimetric method.20,21
Acknowledgment. This work was supported by a grant from the National Science Council of the Republic of China (Contract No. NSC-90-2323-B-110-003) awarded to J.-H.S. References and Notes
(1) Faulkner, D. J. Nat. Prod. Rep. 2002, 19, 1-48, and previous reports in this series.
(2) Sheu, J.-H.; Ahmed, A. F.; Shiue, R.-T.; Dai, C.-F.; Kuo, Y.-H. J. Nat.
Prod. 2002, 65, 1904-1908, and references therein.
(3) Yamada, K.; Ujie, T.; Yoshida, K.; Miyamoto, T.; Higuchi, R.
Tetrahedron 1997, 53, 4569-4578.
(4) Sheu, J.-H.; Sung, P.-J.; Su, J.-H.; Duh, C.-Y.; Chiang, M. Y.
Tetrahedron 1999, 55, 14555-14564.
(5) Sheu, J.-H.; Chen S.-P.; Sung, P.-J.; Chiang, M. Y.; Dai, C.-F.
Tetrahedron Lett. 2000, 41, 7885-7888.
(6) Sung, P.-J.; Su, J.-H.; Duh, C.-Y.; Chiang, M. Y.; Sheu, J.-H. J. Nat.
Prod. 2001, 64, 318-323.
(7) Wang, G.-H.; Ahmed, A. F.; Sheu J.-H.; Duh C.-Y.; Shen Y.-C.; Wang, L.-T. J. Nat. Prod. 2002, 65, 887-891.
(8) Ahond, A.; Bowden, B. F.; Coll, J. C.; Fourneron, J.-D.; Mitchell, S. J. Aust. J. Chem. 1981, 34, 2657-2664.
(9) Groweiss, A.; Kashman, Y. Tetrahedron 1983, 39, 3385-3396, and references therein.
(10) Ko¨nig, G. M.; Wright, A. D. J. Nat. Prod. 1993, 56, 2198-2200. (11) Thebtaranonth, C.; Thebtaranonth, Y.; Wanauppathamkul, S.;
Yuthavong, Y. Phytochemistry 1995, 40, 125-128.
(12) Abraham, W.-R.; Ernst, L.; Stumpf, B. Phytochemistry 1990, 29, 115-120.
(13) Bohlmann, F.; Zdero, C. Phytochemistry 1978, 17, 1135-1153. (14) Bohlmann, F.; Ziesche, J. Phytochemistry 1981, 20, 469-472. (15) Berry, K. M.; Perry, N. B.; Weavers, R. T. Phytochemistry 1985, 24,
2893-2898.
(16) Hinkley, S. F. R.; Perry, N. B.; Weavers, R. T. Phytochemistry 1994,
35, 1489-1494.
(17) Barrero, A. F.; Sa´nchez, J. F.; Ferrol, N. Tetrahedron Lett. 1989, 30, 247-250.
(18) Demirci, B.; Baser, K. H. C.; Demirci, F.; Hamann, M. T. J. Nat. Prod.
2000, 63, 902-904.
(19) Ohe, K.; Takahashi, H.; Uemura, S.; Sugita, N. J. Org. Chem. 1987,
52, 4859-4863.
(20) Alley, M. C.; Scudiero, D. A.; Monks, A.; Hursey, M. L.; Czerwinski, M. J.; Fine, D. L.; Abbott, B. J.; Mayo, J. G.; Shoemaker, R. H.; Boyd, M. R. Cancer Res. 1988, 48, 589-601.
(21) Scudiero, D. A.; Shoemaker, R. H.; Paull, K. D.; Monks, A.; Tierney, S.; Nofziger, T. H.; Currens, M. J.; Seniff, D.; Boyd, M. R. Cancer
Res. 1988, 48, 4827-4833. NP030286W
Downloaded by NATIONAL TAIWAN UNIV on November 2, 2009 | http://pubs.acs.org