Effects of concentrated ambient particles on heart rate variability in spontaneously hypertensive rats
全文
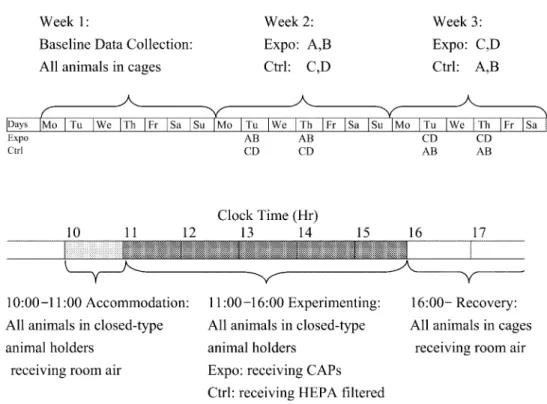
(2) 472. J Occup Health, Vol. 47, 2005. have not yet been used to evaluate heart rate variability effects. Additionally, some epidemiological studies using root mean square of successive differences of adjacent normal-to-normal intervals (RMSSD) as an indicator of parasympathetic activity have shown inconsistent results for PM toxicity3, 15). Thus, the effect of CAPs on RMSSD was also examined in this study. A large number of animals were required to delineate the actual effects of ambient particles, because of the variations among diseased animals 20). Furthermore, variations in the circadian cycle of hemodynamic parameters also make such studies more difficult. To overcome these problems, we used each animal as its own control by exposing the individual animals repeatedly to concentrated particulate matter in air and filtered air, and successfully detected the effects of particles on the heart rate and blood pressure in three pulmonary hypertensive Sprague-Dawley (SD) rats32). This methodology has also been proved effective for analyzing cardiac contractility in SH rats exposed to CAPs33). In this report, the tactic was applied to test its applicability to delineating the heart rate variability effects of CAPs.. Methods Experimental design Male SH rats, weighing around 200 g, were obtained from the National Laboratory Animal Breeding and Research Center in Taiwan. They were housed individually on Aspen chip bedding and provided with Lab Diet 5001 and water ad libitum. A 12-h light/dark cycle, a constant room temperature, and a constant relative humidity were maintained in the animal room during the study. A systolic pressure over 150 mmHg developed spontaneously in the population by the age of 5 weeks, and was maintained at even higher levels throughout their lives. Four SH rats were implanted with radiotelemetry transmitters (model TL11M2-C50-PXT, Data Sciences International, St. Paul, MN, USA) at the age of 10 weeks. Starting from the 18th post-operation day, ECG signals were collected continuously throughout the experimental period. The one week long data collected on unanesthetized, unrestrained animals, immediately before the experiment were defined as the baseline data. Ambient particles in the Chung-Li area, a suburb of Taipei, were concentrated using an ultra-fine particle concentrator (UFPC) 34) modified for better automatic. Fig. 1. Schematic representation of the experimental procedure. Upper panel shows the experimental designs for data collection. Lower panel shows the procedures during each experimental day. See text for detailed information. Expo= Exposure group. Ctrl= Control group. CAPs= Concentrated ambient particles. A–D: SH rats A–D, respectively..
(3) Chuen-Chau CHANG, et al.: Particle Effects on Heart Rate Variability in SH Rats. control by computer algorithms. The theory of virtual impaction was utilized to concentrate ambient particles of 0.1–2.5 µm. CAPs remained in suspension without physical or chemical alteration for inhalation exposures or for collection onto filters for mass concentration analyses. A condensation particle counter (CPC, model 3022A, TSITM, Shoreview, MN, USA), located on both input and output arms, was used to analyze the particle concentrations before and after the UFPC every 30 s. Placed in one of the two output arms, polycarbonate filter paper was used to collect the particles for mass concentration calculation. As shown in Fig. 1, there were totally 4 d of exposure in the experiment, specifically, on Tuesdays and Thursdays in the second and third weeks, following baseline data collection in the first week. On the days of experimentation, the animals were accommodated for about 1 h (10:00 to 11:00) in closed type animal holders (model CH-2500, CH Technologies (USA), Westwood, NJ, USA) before the animals were fitted to the connector cones of nose-only exposure systems (12 Port Nose-Only Modular, CH Technologies) at 11:00. Two SH rats were then exposed to CAPs (exposure group), while the other two were exposed to HEPA filtered air (control group) for 5 h (11:00 to 16:00). SH rats serving as an exposure group in the second week were switched to the control group in the third week, and vice versa. RR interval measurement All signals from the telemetry system throughout the study were collected continuously. The sampling rate for ECG signals was set at 1,000 points per second (1,000 Hz) for better temporal discrimination. Time intervals between adjacent R waves in the ECG channel (RR) were calculated on a beat-to-beat basis using the computer package of Dataquest A.R.T.TM Analysis, version 2.20 (Data Sciences International, St. Paul, MN, USA). In this typical cardiac data acquisition system, signals from ECG were fed through a threshold detector, which triggered if it detected voltage level and/or slope change characteristics of the R-waves in ECG. Under this working system, two types of errors may occur. Errors resulting from a prematurely triggered Rwave detector may subdivide an RR interval into two successive intervals, which are much shorter compared with other normally recorded RR intervals (Type A errors), and errors resulting from trigger failure of the Rwave detector may merge consecutive RR intervals into intervals that are exceptionally long (Type B errors). In order to obtain normal-to-normal (NN) intervals, a computer algorithm based on the recommendation by Cheung was used to eliminate type A and type B errors in the NN calculation35). Basically the NN calculation followed a two-step procedure. The increase or decrease of any RR compared with the previous RR was limited. 473. to 33% in a first step correction. Data points with distances to the median greater than 1.5 standard deviation on Lorenz plots were eliminated in the second step for every 30 min. On average, 5.9 ± 1.7 % (Mean ± SD) of the original RR intervals were eliminated during the error correction. Standard deviation of NN (SDNN), and root mean square of successive differences of adjacent NN (RMSSD) were then calculated from NN at 5 min intervals. Statistic analysis The series of 5-min measurements of SDNN and RMSSD were transformed to natural logarithm scale and denoted as LnSDNN and LnRMSSD, respectively, so that distributions of the new response variables were symmetric. The measurements were classified into three categories using experimental conditions as baseline, control and exposure group data. The circadian baselines were estimated using medians of hourly measurements obtained from the baseline data. The circadian baselines were then subtracted from the LnSDNN and LnRMSSD data for the corresponding hours to obtain crude effects in log scale for each rat. To examine the PM effects, the crude effects on LnSDNN and the crude effects on LnRMSSD during the experimental period, i.e. from 10:00 to 16:00, were analyzed with a linear mixed-effects model. Chamber effects were modeled using a quadratic polynomial, while the PM effects were modeled using a line. If Yijkt is the crude effect for the ith SH rat in the jth condition (0 for baseline group, 1 for control group, 2 for exposure group) during the kth experiment at time t (1 to 72 for each 5min section during the 6 h of experiment), the mixedeffects model is given by Yijkt = (α1+a1i) • (Iin)ijkt + (α2+a2i) • (tin)ijkt + (α3+a3i) • [(tin)ijkt]2 + (β1+b1i) • (IPM)ijkt + (β2+b2i) • (tPM)ijkt + εijkt where (Iin)ijkt =1 and (tin)ijkt =t-1, when the ith rat was in the chamber during the kth experiment and 0 otherwise; (IPM)ijkt =1 and (tPM)ijkt =t-13, when the ith rat during the kth experiment was receiving CAPs and 0 otherwise. The error term εijkt was chosen to be an autoregressive process with second order to model time dependence. In the control group, when the fixed chamber effects were assumed to be the only stress source during the 6 h of experiments, the mean LnSDNN and/or LnRMSSD behaved like a quadratic curve of α1 + a2 • (tin) + α3 • (tin)2, where (tin) denoted the time staying in the closed type animal holders. For the exposure group on the other hand, after adjusting for the chambering effects, the mean LnSDNN and/or LnRMSSD shifted β1 units away from original levels at first, and changed linearly with a slop.
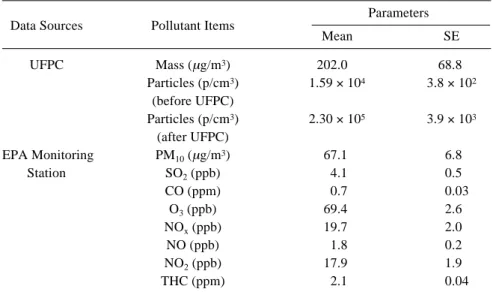
(4) 474. J Occup Health, Vol. 47, 2005. Table 1. Characterization of PM and Co-pollutants during Exposure. PM data were calculated according to the UFPC recordings. Data of co-pollutants were retrieved from an EPA monitoring station nearest to the experimental site. All data were expressed as time-weighted averages during the 5 × 4 h of experimenting Parameters Data Sources. Pollutant Items. UFPC. Mass (µg/m3) Particles (p/cm3) (before UFPC) Particles (p/cm3) (after UFPC) PM10 (µg/m3) SO2 (ppb) CO (ppm) O3 (ppb) NOx (ppb) NO (ppb) NO2 (ppb) THC (ppm). EPA Monitoring Station. Mean. SE. 202.0 1.59 × 104. 68.8 3.8 × 102. 2.30 × 105. 3.9 × 103. 67.1 4.1 0.7 69.4 19.7 1.8 17.9 2.1. 6.8 0.5 0.03 2.6 2.0 0.2 1.9 0.04. UFPC: Ultrafine Particle Concentrator, THC: Total Hydrocarbons. of β2 during the 5 h of exposure, behaving like a line of β 1 + β 2 • (t PM), where (t PM) denotes the time of PM exposure. Since the 4 SH rats were randomly selected from a population, random components a1i, a 2i, a3i, b1i and b2i were added to show the rat-to-rat variation of these effects. All of the random coefficients were assumed to be normally distributed with a mean of 0 and some constant variances. Statistical software package, S-PLUS 2000 (MathSoft Inc., Cambridge, MA, USA) was used to estimate the parameters and standard errors of the estimates in the model.. Results During exposure, condensation particle counter (CPC) readings showed an average pre-concentration particle concentration of 1.59 × 104 (range, 5.28 × 103 to 5.08 × 104) p/cm3 and a post-concentration level of 2.30 × 105 (range, 7.12 × 103 to 8.26 × 105) p/cm3, with an average concentration factor of around 14. Gravimetric analysis revealed the post-concentration mass concentration of particles during the 5 h of exposure as 202.0 ± 68.8 (Mean ± SE) µg/m3. Ambient gaseous co-pollutant data were derived from the nearest monitoring station of the Environmental Protection Agency, Taiwan (Table 1). The exposure temperature and humidity of this modified UFPC were shown to be consistently physiological, with temperature ranging from 16.9°C to 22.4°C, and relative humidity from 59% to 65%. As shown in Fig. 2, during the inhalation stage (11:00 to 16:00), crude effects of both LnSDNN and LnRMSSD for the exposure and control groups decreased from the. Fig. 2. The averages of crude effects across all 4 SH rats plotted against clock hours. LnSDNN is in the upper panel and LnRMSSD is in the lower panel. Dashed vertical lines at 10:00 denote the entrance of SH rats into closed-type animal holders, and at 11:00 and 16:00 denote the beginning and conclusion of experiments, respectively. Baseline data are in thin black lines ( ), the control group is in thick gray lines ( ), and the exposure group is in thick black lines ( )..
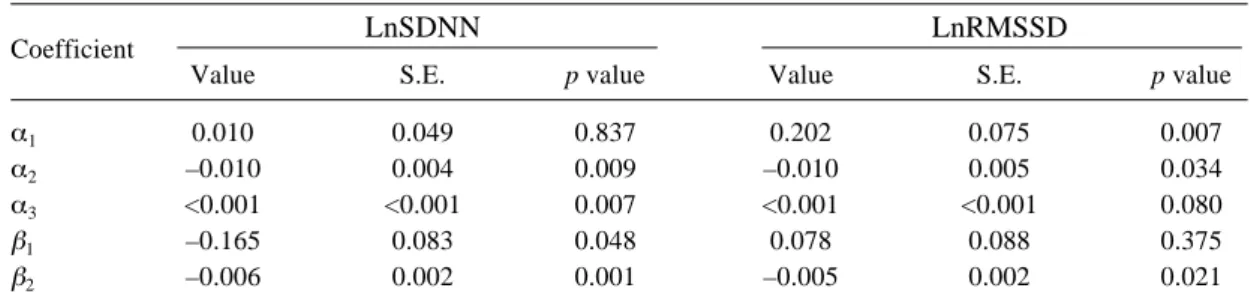
(5) Chuen-Chau CHANG, et al.: Particle Effects on Heart Rate Variability in SH Rats. 475. Table 2. Regression Results of Fixed Effects on LnSDNN and LnRMSSD Using a Linear Mixed-effect Model. LnSDNN. Coefficient α1 α2 α3 β1 β2. LnRMSSD. Value. S.E.. p value. Value. S.E.. p value. 0.010 –0.010 <0.001 –0.165 –0.006. 0.049 0.004 <0.001 0.083 0.002. 0.837 0.009 0.007 0.048 0.001. 0.202 –0.010 <0.001 0.078 –0.005. 0.075 0.005 <0.001 0.088 0.002. 0.007 0.034 0.080 0.375 0.021. The chamber effects were fitted to a binomial curve with intercept α1, time constants of α2 and α3. The PM effects were fitted to a linear model with intercept β1 and slope β2.. Fig. 3. The averages of PM effects across all 4 SH rats plotted against clock hours. LnSDNN is in the upper panel and LnRMSSD is in the lower panel. Dashed vertical lines at 11:00 and 16:00 denote the beginning and conclusion of experiments, respectively. Thin solid lines represent the crude averaged PM effects. Thick solid lines during the inhalation stage (11:00 to 16:00) represent the mean PM effects estimated from linear mixed-effect models. Thick solid lines after the experiments (after 16:00) represent the smoothed curve of log-transformed heart rate variability parameters estimated by robust local linear fits.. baseline values. Immediately after the experiments, both LnSDNN and LnRMSSD decreased due to stresses produced by release from the exposure system, then returned to the baseline values. The data were further analyzed using a linear mixedeffects model (Table 2). The chambering effects fitted. Fig. 4. The estimated mean PM effects during exposure with 95% CI in their original scales plotted against clock hours. SDNN is in the upper panel and RMSSD is in the lower panel.. quadratic curves well, with significant coefficients of α2 and α3. After controlling for the chambering effects, decreased or decreasing LnSDNN and LnRMSSD (significantly negative β1, or β2, or both) was observed during the PM exposure. The regression lines in Fig. 3 were calculated from Table 2 (shown in solid lines between 11:00 and 16:00) and revealed a decreased or decreasing trend during the PM exposure. After the experiments (after 16:00), the PM effects diminished and the heart rate variability parameters returned to their baseline level. This recovery course was better manifested using a smoothing technique utilizing robust local linear fits (shown in solid lines after.
(6) 476. 16:00). For better illustration, the linear regressions of LnSDNN and LnRMSSD for the PM effects were transformed back to their original scales, and plotted against the clock hours in Fig. 4, with 95% confidence intervals (CI). It appears that, during CAPs exposure, SDNN decreased from 85% to 60% of the base line. All of the decrements were statistically significant at an α level of 5%. In contrast, the CAPs effects on RMSSD were not significant though the trend of RMSSD changes with time was significant.. Discussion In the current study, our data demonstrated that CAPs exposure decreased SDNN of experimental animals. However, the effects of CAPs on RMSSD were less prominent. Heart rate variability has recently been used in epidemiologic air pollution studies to explore cardiovascular pathogenesis. As a time domain parameter of heart rate variability reflecting global heart rate variability, SDNN has been used the most extensively. In elderly populations, consistent decreases of SDNN have been observed with exposures to PM1036, 37), to PM 2.53, 11, 15, 38), to ambient submicrometer particles with a size range of 0.02–1 microns (NC0.02–1)39), and to CAPs10). Decreased SDNN have also been observed in young and healthy subjects exposed to environmental and occupational ambient PM12, 13, 40), and to ambient NC0.02–139). Nevertheless, heatrt rate responses inconsistent with increased PM levels were also noted, which precluded the conclusion of an overall decrease in vagal tone or increase in sympathetic tone accompanying a high pollution episode3). In a human trial, a modest increase in parasympathetic stimulation of heart rate variability was demonstrated with CAPs exposures41). Lately, heart rate variability has also been used to demonstrate the PM effects on experimental animals. In an animal study, high dose ROFA exposure decreased SDNN, but did not change the heart rate significantly, in sedated SD rats with acute myocardial infarction (MI)23). On the other hand, exposure to CB decreased heart rate and increased SDNN significantly in both healthy and terminally senescent conscious mice25). However, short term oral exposure to oxides and sulfates of transitional metals in old conscious beagle dogs did not present significant heart rate variability changes24). Thus, no firm conclusion could be drawn because of the short duration of the observations, pharmacological sedation, inadequate control for individual variation and circadian cycles, differences in animal species, and inconsistent findings. On the other hand, although these PM substitutes are more homogenous in composition and result in more reproducible responses, they are different from “real world” ambient particles and pose another challenge for extrapolating to human observations in natural. J Occup Health, Vol. 47, 2005. environments. Compared with frequency domain counterparts, time domain measurements are considered as simple and practical tools for assessing autonomic function. Though coefficient of variance (CV R-R) is sometimes calculated to detect overall variability independent of changes in mean NN intervals, it is strongly correlated with SDNN and usually adds little additional information42). Thus SDNN and RMSSD were chosen for this study. With careful design and analysis, we demonstrated that CAPs exposure decreased SDNN in the present study. In our previous study, CAPs generated under the same conditions increased heart rate, blood pressure, and cardiac contractility during the exposure hours 33) . These findings, altogether, are consistent and compatible with a picture of autonomic nervous system activation43). Conventionally, decreased SDNN has been associated with increased morbidity and mortality, and is commonly used as a tool for health risks stratification. Cardiac deaths in patients with recent MI44), and deaths due to progressive heart failure in congestive heart failure (CHF) patients 45–47) are both reasonably predictable through decreased SDNN. SDNN is also indicative of long term cardiovascular events in young populations with structural heart diseases 48, 49) . Decreased SDNN in subjects who were generally young and healthy were demonstrated to predict the development of hypertension50), coronary arterial heart disease and cardiac mortality51). Decreased SDNN is even predictive of death from all causes in a middle-aged general population52). Our current findings are consistent with epidemiological and toxicological air pollution studies, and may show a link between PM air pollution and increased cardiovascular risks. In contrast, the risk predictive value of RMSSD, a time domain heart rate variability parameter that stands for short-cycled variability reflecting parasympathetic activity, has been inconsistent in recent studies. One researcher concluded that RMSSD was unsuitable for evaluating the autonomic nervous system function in autoneuropathy because of low reproducibility 53) . Furthermore, RMSSD was also unsatisfactory in the predictive value of cardiac death due to CHF54) and acute MI 55). However, Rapenne et al.56) demonstrated that increased RMSSD was the most powerful heart rate variability parameter predicting the deterioration to brain death of patients with severe head trauma. Application of RMSSD in air pollution studies has shown inconsistent results. While Pope III et al.15) observed an increase in RMSSD during PM air pollution, the opposite responses were also demonstrated in epidemiological studies3, 39, 57). Though increased RMSSD was observed in an animal study using CB exposed senescent mice as the subjective model25), our study showed a less prominent decrease of RMSSD to CAPs during the exposure hours. Hence, the.
(7) Chuen-Chau CHANG, et al.: Particle Effects on Heart Rate Variability in SH Rats. role of RMSSD in CAP-related cardiac toxicity needs further assessment. Various types of receptors and neurons in mammalian airways have been associated with autonomic nervous system functioning. Vagal pulmonary C fibers58) and the olfactory bulb as a whole59) have been demonstrated in anesthetized SD rats as an important afferent port and integral center for cardiovascular sympathoexcitatory reflexes. It was speculated that CAPs might influence the autonomic nervous system function via these pathways, and thereby link to its cardiovascular pathogenesis. Epidemiological studies have documented the health effects of PM air pollution mostly in susceptible subjects while its effects on healthy groups are generally of limited scale. Diseased animals have therefore been used in PM toxicity studies to investigate the credibility of the epidemiological findings. However, selections are limited for susceptible animals in this relatively new field. SH rats have been proven to be an effective model of cardiovascular diseases (CVD) because they develop heart failure 60, 61) and artherosclerosis 62), which may contribute to the PM-related cardiovascular toxicity. Although representative of only a portion of human counterparts, SH rats have been widely used as rat models in studies of PM-related CVD. SH rats have been demonstrated as susceptible to air pollutants in the field of pulmonary outcomes 26). Aside from their airway sensitivity to PM and pathologic ANS dysfunction, SH rats also possess a higher level of oxidative stress than their cogenic controls63). All of these characteristics make SH rats prone to adverse cardiovascular outcomes induced by PM inhalation16). Therefore, SH rats have been used in the most recent toxicological studies which have explored the relationship between air pollution and cardiovascular effects27–29, 32, 64). Thus we chose this widely used rodent model not only for its biological relevance, but also for comparability with the results of other researches. However, wide variation among diseased inbred subjects still presents difficulties in data analysis. To overcome the common problems of wide variation among diseased subjects, each animal was used as its own control in our study by exposing the individual animals repeatedly to CAPs and filtered air. The proposed mixedeffects model is a commonly used approach for analyzing these kinds of repeated measurement data3, 12, 14, 65). Our model also included random components for animal-toanimal variation and autocorrelations of each animal’s repeated measurements besides the fixed effects for CAPs exposure. Owing to the varying concentrations of CAPs, and differences in animal responses, day-to-day variations of responses were wide. The wide day-to-day variation blurred the dose-response relationship, and justified the pooling of data so as to treat exposure as a binary descriptor in this study, instead of regressing heart rate. 477. variability measurements on concurrent CAPs levels. Our results demonstrated that exposure to CAPs induced heart rate variability alterations during the period of exposure. To the best of our knowledge, this is the first study to demonstrate the heart rate variability effects of CAPs on unanaesthetized experimental animals. This may have some implications for explaining the epidemiological findings. The results of the study also showed that SDNN was more sensitive to PM effects than RMSSD. Acknowledgments: The authors are grateful to Mr. Ming-Chih Chen and Ms. Yu-Chen Lei for their technical assistance. The authors also thank the National Institute of Environmental Analysis, EPA, and National Science Council (NSC) Taiwan for their helpful assistance on this project. This study was funded by the EPA of Taiwan (EPA-91-FA11-03-91DF02). A portion of the data included in this paper was presented at the 2003 AAAR PM Meeting, “Particulate Matter: Atmospheric Sciences, Exposure and the Fourth Colloquium on PM and Human Health”, Pittsburgh, PA, March 31–April 4, 2003.. References 1). 2). 3). 4). 5). 6). 7). 8). Pope III CA, Dockery DW. In: Holgate ST, Samet JM, Koren HS, Maynard RL, eds. Epidemiology of particle effects. In: Air Pollution and Health. London: Academic Press, 1999: 673–705. JM Samet, F Dominici, FC Curriero, I Coursac and SL Zeger: Fine particulate air pollution and mortality in 20 U.S. cities, 1987–1994. N Engl J Med 343, 1742– 1749 (2000) DR Gold, A Litonjua, J Schwartz, E Lovett, A Larson, B Nearing, G Allen, M Verrier, R Cherry and R Verrier: Ambient pollution and heart rate variability. Circulation 101, 1267–1273 (2000) A Ibald-Mulli, J Stieber, H Wichmann, W Koenig and A Peters: Effects of air pollution on blood pressure: a population-based approach. Am J Public Health 91, 571–577 (2001) A Peters, S Perz, A Doring, J Stieber , W Koenig and HE Wichmann: Increases in heart rate during an air pollution episode. Am J Epidemiol 150, 1094–1098 (1999) CA Pope III, DW Dockery, RE Kanner, M Villegas and J Schwartz: Oxygen saturation, pulse rate, and particulate air pollution. Am J Respir Crit Care Med 159, 365–372 (1999) A Peters, E Liu , RL Verrier, J Schwartz, DR Gold, M Mittleman, J Baliff, JA Oh, G Allen, K Monahan and DW Dockery: Air pollution and incidence of cardiac arrhythmia. Epidemiology 11, 11–17 (2000) PH Stone and JJ Godleski: First step toward understanding the pathophysiological link between air pollution and cardiac mortality. Am Heart J 138, 804– 807 (1999).
(8) 478. 9). 10). 11). 12). 13). 14). 15). 16). 17). 18). 19). 20). 21). J Occup Health, Vol. 47, 2005. J Creason, L Neas, D Walsh, R Williams, L Sheldon, D Liao and C Shy: Particulate matter and heart rate variability among elderly retirees: the Baltimore 1998 PM study. J Expo Anal Environ Epidemiol 11, 116– 122 (2001) RB Devlin, AJ Ghio, H Kehrl, G Sanders and W Cascio: Elderly humans exposed to concentrated air pollution particles have decreased heart rate variability. Eur Respir J Suppl 40, 76s–80s (2003) D Liao, J Creason, C Shy, R Williams, R Watts and R Zweidinger: Daily variation of particulate air pollution and poor cardiac autonomic control in the elderly. Environ Health Perspect 107, 521–525 (1999) SR Magari, R Hauser, J Schwartz, PL Williams, TJ Smith and DC Christiani: Association of heart rate variability with occupational and environmental exposure to particulate air pollution. Circulation 104, 986–991 (2001) SR Magari, J Schwartz, PL Williams, R Hauser, TJ Smith and DC Christiani: The association between personal measurements of environmental exposure to particulates and heart rate variability. Epidemiology 13, 305–310 (2002) SR Magari, J Schwartz, PL Williams, R Hauser, TJ Smith and DC Christiani: The association of particulate air metal concentrations with heart rate variability. Environ Health Perspect 110, 875–880 (2002) CA Pope III, RL Verrier, EG Lovett, AC Larson, ME Raizenne, RE Kanner, J Schwartz, GM Villegas, DR Gold and DW Dockery: Heart rate variability associated with particulate air pollution. Am Heart J 138, 890–899 (1999) MJ Campen, JP Nolan, MC Schladweiler, UP Kodavanti, DL Costa and WP Watkinson: Cardiac and thermoregulatory effects of instilled particulate matterassociated transition metals in healthy and cardiopulmonary-compromised rats. J Toxicol Environ Health A 65, 1615–1631 (2002) LC Chen, PD Miller, MO Amdur and T Gordon: Airway hyperresponsiveness in guinea pigs exposed to acid-coated ultrafine particles. J Toxicol Environ Health 35, 165–174 (1992) T Gordon, C Nadziejko, R Schlesinger and LC Chen: Pulmonary and cardiovascular effects of acute exposure to concentrated ambient particulate matter in rats. Toxicol Lett 96–97, 285–288 (1998) T Gordon, C Nadziejko, LC Chen and R Schlesinger: Effects of concentrated ambient particles in rats and hamsters: an exploratory study. Res Rep Health Eff Inst 93, 5–34 (2000) UP Kodavanti, DL Costa and PA Bromberg: Rodent model of cardiopulmonary disease: their potential applicability in studies of air pollutant susceptibility. Environ Health Perspect 106, 111–130 (1998) UP Kodavanti, MC Schladweiler, AD Ledbetter, R Hauser, DC Christiani, J McGee, JR Richards and DL Costa: Temporal association between pulmonary and systemic effects of particulate matter in healthy and cardiovascular compromised rats. J Toxicol Environ Health A 65, 1545–1569 (2002). 22) MM Ulrich, GM Alink, P Kumarathasan, R Vincent, AJ Boere and FR Cassee: Health effects and time course of particulate matter on the cardiopulmonary system in rats with lung inflammation. J Toxicol Environ Health A 65, 1571–1595 (2002) 23) GA Wellenius, PH Saldiva, JR Batalha, GG Krishna Murthy, BA Coull, RL Verrier and JJ Godleski: Electrocardiographic changes during exposure to residual oil fly ash (ROFA) particles in a rat model of myocardial infarction. Toxicol Sci 66, 327–335 (2002) 24) BA Muggenburg, JM Benson, EB Barr, J Kubatko and LP Tilley: Short-term inhalation of particulate transition metals has little effect on the electrocardiograms of dogs having preexisting cardiac abnormalities. Inhal Toxicol 15, 357–371 (2003) 25) CG Tankersley, M Campen, A Bierman, SE Flanders, KW Broman and R Rabold: Particle effects on heartrate regulation in senescent mice. Inhal Toxicol 16, 381–390 (2004) 26) UP Kodavanti, MC Schladweiler, AD Ledbetter, WP Watkinson, MJ Campen, DW Winsett, JR Richards, KM Crissman, GE Hatch and DL Costa: The spontaneously hypertensive rat as a model of human cardiovascular disease: evidence of exacerbated cardiopulmonary injury and oxidative stress from inhaled emission particulate matter. Toxicol Appl Pharmacol 164, 250–263 (2000) 27) MJ Campen, JD McDonald, AP Gigliotti, SK Seilkop, MD Reed and JM Benson: Cardiovascular effects of inhaled diesel exhaust in spontaneously hypertensive rats. Cardiovasc Toxicol 3, 353–361 (2003) 28) UP Kodavanti, CF Moyer, AD Ledbetter, MC Schladweiler, DL Costa, R Hauser, DC Christiani and A Nyska: Inhaled environmental combustion particles cause myocardial injury in the Wistar Kyoto rat. Toxicol Sci 71, 237–245 (2003) 29) C Nadziejko, K Fang, E Nadziejko, SP Narciso, M Zhong and LC Chen: Immediate effects of particulate air pollutants on heart rate and respiratory rate in hypertensive rats. Cardiovasc Toxicol 2, 245–252 (2002) 30) CA Murphy, RP Sloan and MM Myers: Pharmacologic responses and spectral analyses of spontaneous fluctuations in heart rate and blood pressure in SHR rats. J Auton Nerv Syst 36, 237–250 (1991) 31) D Dias, P Viana, M de, R Jr Fazan, TG Ruscone, A Porta, A Malliani, HC Salgado and N Montano: Intravenous amiodarone modifies autonomic balance and increases baroreflex sensitivity in conscious rats. Auton Neurosci 95, 88–96 (2002) 32) TJ Cheng, JS Hwang, PY Wang, CF Tsai, CY Chen, SH Lin and CC Chan: Effects of concentrated ambient particles on heart rate and blood pressure in pulmonary hypertensive rats. Environ Health Perspect 111, 147– 150 (2003) 33) CC Chang, JS Hwang, CC Chan, PY Wang, TH Hu and TJ Cheng: Effects of concentrated ambient particles on heart rate, blood pressure, and cardiac contractility in spontaneously hypertensive rats. Inhal Toxicol 16, 421–429 (2004).
(9) Chuen-Chau CHANG, et al.: Particle Effects on Heart Rate Variability in SH Rats. 34) C Sioutas, S Kim and M Chang: Development and evaluation of a prototype ultra-fine particle concentrator. J Aerosol Med 30, 1001–1007 (1999) 35) MN Cheung: Detection of and recovery from errors in cardiac interbeat intervals. Psychophysiology 18, 341– 346 (1981) 36) H Jr Gong, WS Linn, SL Terrell, KW Clark, MD Geller, KR Anderson, WE Cascio and C Sioutas: Altered heartrate variability in asthmatic and healthy volunteers exposed to concentrated ambient coarse particles. Inhal Toxicol 16, 335–343 (2004) 37) D Liao, Y Duan, EA Whitsel, ZJ Zheng, G Heiss, VM Chinchilli and HM Lin: Association of higher levels of ambient criteria pollutants with impaired cardiac autonomic control: a population-based study. Am J Epidemiol 159, 768–777 (2004) 38) CA Pope III, ML Hansen, RW Long, KR Nielsen, NL Eatough, WE Wilson and DJ Eatough: Ambient particulate air pollution, heart rate variability, and blood markers of inflammation in a panel of elderly subjects. Environ Health Perspect 112, 339–345 (2004) 39) CC Chan, KJ Chuang, GM Shiao and LY Lin: Personal exposure to submicrometer particles and heart rate variability in human subjects. Environ Health Perspect 112, 1063–1067 (2004) 40) M Riediker, WE Cascio, TR Griggs, MC Herbst, PA Bromberg, L Neas, RW Williams and RB Devlin: Particulate matter exposure in cars is associated with cardiovascular effects in healthy young men. Am J Respir Crit Care Med 169, 934–940 (2004) 41) H Jr Gong, WS Linn, C Sioutas, SL Terrell, KW Clark, KR Anderson and LL Terrell: Controlled exposures of healthy and asthmatic volunteers to concentrated ambient fine particles in Los Angeles. Inhal Toxicol 15, 305–325 (2003) 42) Malik M, Camm AJ. Time-Domain Measurements of Heart Rate Variability. In: Heart Rate Variability. New York, Futura Publishing Company, Inc., 1995: 33–45. 43) L Mangin, A Kobeissi, D Lelouche, TY Dherouville, P Mansier, B Swynghedauw and I Macquin-Mavier: Simultaneous analysis of heart rate variability and myocardial contractility during head-up tilt in patients with vasovagal syncope. J Cardiovasc Electrophysiol 12, 639–644 (2001) 44) MT La Rovere, JT Jr Bigger, FI Marcus, A Mortara and PJ Schwartz: Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet 351, 478–484 (1998) 45) KC Bilchick, B Fetics, R Djoukeng, SG Fisher, RD Fletcher, SN Singh, E Nevo and RD Berger: Prognostic value of heart rate variability in chronic congestive heart failure (Veterans Affairs’ Survival Trial of Antiarrhythmic Therapy in Congestive Heart Failure). Am J Cardiol 90, 24–28 (2002) 46) J Nolan, PD Batin, R Andrews, SJ Lindsay, P Brooksby, M Mullen, W Baig, AD Flapan, A Cowley, RJ Prescott, JM Neilson and KA Fox: Prospective study of heart rate variability and mortality in chronic heart failure:. 47). 48). 49). 50). 51). 52). 53). 54). 55). 56). 57). 479. results of the United Kingdom heart failure evaluation and assessment of risk trial (UK-heart). Circulation 98, 1510–1516 (1998) MT Kearney, J Nolan, AJ Lee, PW Brooksby, R Prescott, AM Shah, AG Zaman, DL Eckberg, HS Lindsay, PD Batin, R Andrews and KA Fox: A prognostic index to predict long-term mortality in patients with mild to moderate chronic heart failure stabilised on angiotensin converting enzyme inhibitors. Eur J Heart Fail 5, 489–497 (2003) L Fauchier, D Babuty, P Cosnay, P Poret, P Rouesnel and JP Fauchier: Long-term prognostic value of time domain analysis of signal-averaged electrocardiography in idiopathic dilated cardiomyopathy. Am J Cardiol 85, 618–623 (2000) M Karcz, L Chojnowska, W Zareba and W Ruzyllo: Prognostic significance of heart rate variability in dilated cardiomyopathy. Int J Cardiol 87, 75–81 (2003) JP Singh, MG Larson, H Tsuji, JC Evans, CJ O’Donnell and D Levy: Reduced heart rate variability and newonset hypertension: insights into pathogenesis of hypertension: the Framingham Heart Study. Hypertension 32, 293–297 (1998) JM Dekker, RS Crow, AR Folsom, PJ Hannan, D Liao, CA Swenne and EG Schouten: Low heart rate variability in a 2-minute rhythm strip predicts risk of coronary heart disease and mortality from several causes: the ARIC Study. Atherosclerosis Risk In Communities. Circulation 102, 1239–1244 (2000) JM Dekker, EG Schouten, P Klootwijk, J Pool, CA Swenne and D Kromhout: Heart rate variability from short electrocardiographic recordings predicts mortality from all causes in middle-aged and elderly men. The Zutphen Study. Am J Epidemiol 145, 899–908 (1997) D Ziegler, G Laux, K Dannehl, M Spuler, H Muhlen, P Mayer and FA Gries: Assessment of cardiovascular autonomic function: age-related normal ranges and reproducibility of spectral analysis, vector analysis, and standard tests of heart rate variation and blood pressure responses. Diabet Med 9, 166–175 (1992) P Ponikowski, SD Anker, TP Chua, R Szelemej, M Piepoli, S Adamopoulos, K Webb-Peploe, D Harrington, W Banasiak, K Wrabec and AJ Coats: Depressed heart rate variability as an independent predictor of death in chronic congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol 79, 1645–1650 (1997) AD Doulalas, MD Flather, A Pipilis, S Campbell, F Studart, IK Rizos, IH Gialafos, PK Toutouzas and P Sleight: Evolutionary pattern and prognostic importance of heart rate variability during the early phase of acute myocardial infarction. Int J Cardiol 77, 169–179 (2001) T Rapenne, D Moreau, F Lenfant, M Vernet, V Boggio, Y Cottin and M Freysz: Could heart rate variability predict outcome in patients with severe head injury? A pilot study. J Neurosurg Anesthesiol 13, 260–268 (2001) CA Pope III, ML Hansen, RW Long, KR Nielsen, NL Eatough, WE Wilson and DJ Eatough: Ambient.
(10) 480. 58). 59). 60). 61). J Occup Health, Vol. 47, 2005. particulate air pollution, heart rate variability, and blood markers of inflammation in a panel of elderly subjects. Environ Health Perspect 112, 339–345 (2004) CJ Lai and YR Kou: Stimulation of vagal pulmonary C fibers by inhaled wood smoke in rats. J Appl Physiol 84, 30–36 (1998) JA Moffitt, AJ Grippo, PV Holmes and AK Johnson: Olfactory bulbectomy attenuates cardiovascular sympathoexcitatory reflexes in rats. Am J Physiol Heart Circ Physiol 283, H2575–H2583 (2002) CH Conrad, WW Brooks, KG Robinson and OH Bing: Impaired myocardial function in spontaneously hypertensive rats with heart failure. Am J Physiol 260, H136–H145 (1991) JM Pfeffer, MA Pfeffer, MC Fishbein and ED Frohlich: Cardiac function and morphology with aging in the. 62). 63). 64). 65). spontaneously hypertensive rat. Am J Physiol 237, H461–H468 (1979) M Baudouin-Legros and P Meyer: Hypertension and atherosclerosis. J Cardiovasc Pharmacol 15 Suppl 1, S1–S6 (1990) FA DeLano, R Balete and GW Schmid-Schonbein: Control of oxidative stress in microcirculation of spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 288, H805–812 (2005) I Kooter, J Pennings, A Opperhuizen and F Cassee: Gene expression pattern in spontaneously hypertensive rats exposed to urban particulate matter (EHC-93). Inhal Toxicol 17, 53–65 (2005) McCulloch CE, Searle SR: Generalized, Linear, and Mixed Models. New York: John Wiley & Sons, 2001..
(11)
數據
相關文件
Despite significant increase in the price index of air passenger transport (+16.97%), the index of Transport registered a slow down in year-on-year growth from +12.70% in July to
The increasing charges of medical services, rising prices in jewellery and air tickets pushed the indices of Health; Other goods and services; and Transport and communications up
On the other hand, lower prices in hairdressing services, outbound package tours and air tickets after the Lunar New Year, as well as continuous price reduction in winter clothing
In these lessons, students will evaluate the impacts of genetic engineering on our daily life, and analyze the moral issues raised in its development, especially those related
*Teachers need not cover all the verbal and non-verbal cues in the list. They might like to trim the list to cover only the most commonly used expressions. Instead of exposing
conglomerates and religious bodies have to consult these high-level stipulations when they settle on their own constitutions. Worldly law developed in this way step by step. The
The quantity of landscape planting in campus was evaluated and used as an indicator to divide the campus into different landscape zones where the air anions
In order to detect each individual target in the crowded scenes and analyze the crowd moving trajectories, we propose two methods to detect and track the individual target in