Ref.: Ms. No. RHEI-D-12-00685
Diabetes mellitus and accompany hyperlipidemia are independent risk factors of adhesive capsulitis: A
nationwide population-based cohort study
Running Head: Diabetes mellitus predispose to adhesive capsulitis
Sui-Foon Lo
1,2, Ssu-Wei Chu
3,4, Chih-Hsin Muo, MS
3,4,Nai-Hsin Meng
1,5, Li-Wei Chou
1,2, Wei-Cheng Huang
1Chung-Ming Huang
6,7, Fung-Chang Sung
3,41 Department of Physical Medicine and Rehabilitation, 2 Department of Chinese Medicine, 3Management Office for Health
Data, 4Department of Public Health, 5Department of Medicine, 6 Division of Immunology and Rheumatology, 7Graduate
Institute of Integrated Medicine; China Medical University and Hospital, Taichung 404, Taiwan.
Correspondence:
Fung-Chang Sung, PhD, MPH,Professor
Department of Public Health, China Medical University 91 Hsueh Shih Road,
Taichung 404, Taiwan
Phone: 886-4-2206-2295; fax: 886-4-2201-9901
E-mail: tw.mohd@gmail.com; fcsung@mail.cmu.edu.tw,
Number of words: 250 in the abstract, 2707 in the text, 3 Tables and 1 Figure, and 32 references
ABSTRACT
diabetes mellitus (DM). To further investigated the risk of AC in subjects with DM in an Asian population, we performed the present cohort study featured the analyses of a randomly selected sub-dataset of one million individuals insured by the Taiwan National Health Insurance for the period spanning 1996 to 2008. The study and comparison cohorts consisted of 5,109 newly diagnosed diabetic patients and 20,473 randomly selected non-diabetic subjects aged ≥20 years in the year 2000. Both cohorts were followed up until December 2008 to measure AC incidence. We found the incidence density of AC in the DM cohort was 3.08 times that of the comparison cohort (146.9 vs. 47.7 per 10,000 person-years), and rate ratios varied from 1.23 to 4.98 by categorized sociodemographic factors and co-morbidity. The hazard ratio (HR) of AC for DM subjects remained significantly higher than that for non-DM subjects (p<0.001) in all models. The HR increased in older age groups (p<0.001) and females (p<0.001). Hyperlipidemia consistently increase the risk of AC in both univariate (HR = 2.67, 95% confidence interval (CI): 2.36-4.06) and multivariate analyses (HR = 1.29, 95% CI: 1.11-1.49).In this eight-year study period, we found DM and accompany hyperlipidemia were independent risk factors of AC. The risks are higher for older age women. Findings in the present study help to identify high-risk patient groups to exercise early prevention of AC, and enhance comprehensive care quality of DM subjects.
Introduction
Diabetes mellitus (DM) is a complex metabolic disorder of multiple etiologies characterized by chronic hyperglycemia arising from defects in insulin secretion, insulin
action, or both [
1
]. DM triggers the chronic damage, dysfunction, and failure of variousorgans. The combination of microangiopathy and macroangiopathy frequently leads to complications, such as retinopathy, nephropathy, neuropathy, and increased risks for cardiovascular, peripheral vascular, and cerebral vascular diseases.
Musculoskeletal complications, which are less adequately described and understood, occur quite frequently in patients with DM. One of the common musculoskeletal
complications is adhesive capsulitis (AC), also known as frozen shoulder [
2
]. Thiscomplication is characterized by progressive, painful restrictions of the shoulder’s range of motions due to a thickened joint capsule adherent to the humeral head, resulting in a reduction of the glenohumeral joint volume. Although it’s clinical manifestations are characterized by three phases — painful, adhesive, and resolution — its exact etiology remains unknown.
A number of studies have described an elevated prevalence of AC in DM patients.
Thomas et al. [
3
] reported that frozen shoulder had a prevalence rate of 4.3% in DM patientsand 0.5% in a control group. Another study conducted by Bridgman [
4
],found that 10.8% ofDM patients and 2.3% of controls were afflicted with AC. Meanwhile, Pal et al., who
controls. The variance of AC prevalence in people with DM and normal controls in these
studies [
3-5
], may be attributed to differences in the definition of AC and the studypopulations enrolled.
Furthermore, a high frequency of limited joint mobility has been observed among DM
patients with AC [
6
]. Although the association of microvascular complications of DM withlimited joint mobility has been thoroughly documented, the association of AC with microvascular or macrovascular complications of DM remains less defined. In view of the above findings, a retrospective cohort study was conducted using population-based universal insurance data. The goal of the study is to delineate the risk of AC in DM subjects, as well as its relationship with other medical co-morbidity.
METHODS
Data source and study population
The Taiwan National Health Insurance, a universal health program reformed from several public insurance systems in 1995, covers over 96% of Taiwan’s population of 23 million and has forged contracts with 97% of country’s hospitals and practitioners since 1996. For this study, claims data of the longitudinal health insurance database, established by the National Health Research Institute, were obtained. The data included a registry of one million randomly selected insured individuals for the period covering 1996 to 2000. Ambulatory and inpatient claims, as well as an updated registry of beneficiaries from 1996 to 2008, were
of the insured population.
Study subjects
Data bases used in this study including both outpatient files with two diagnosis fields for each visit and inpatient files with 5 diagnosis fields for each discharge. All the seven fields were used for diagnoses identification. The study cohort consisted of new diabetic patients aged ≥ 20 years with at least three diagnostic records of DM in the year 2000 based on the
International Classification of Disease, 9th Revision, Clinical Modification (ICD-9CM) codes,
including 250 and A-code A181. A-code and ICD-9-CM code were used to define the disease before 2000 in Taiwan, but 9-CM was used starting 2000. “A-code” covered wide ICD-9-CM and if there was no specific A-code as ICD-ICD-9-CM we just used ICD-ICD-9-CM to define the disease. The comparison cohort was composed of randomly selected nearly 4 folds of insured individuals without DM, aged ≥20 years in the year 2000. We excluded 92 and 137 subjects who manifested AC (ICD-9CM code 726.0) development before the baseline period from the study cohort and the comparison cohort, respectively. Subjects who developed DM after the baseline in the comparison cohort were excluded as well. Finally, a total of 5,109 DM cases and 20,473 references were included for the data analyses in this study. In the DM cohort,
296 (5.79%) patients were diagnosed as type 1 DM.
Subjects with baseline co-morbidity, including stroke (ICD-9-CM 430-438 and A-codes A290-A294 and A299), hypertension (ICD-9-CM 401-405 and codes A260 and A269), hyperlipidemia (ICD-9-CM 272 andA-code A182), obesity (ICD-9-CM 278 and A-A-code A183) and chronic obstructive pulmonary disease (COPD, ICD-9-CM 491.2 and 496) were also identified with at least three diagnostic records of the respective comorbidity.
Statistical analyses
Categorical distributions of age, gender, occupation, urbanization level of subject’s residential area, premium based income, and co-morbidity between the study cohort (subjects with DM) and comparison cohort (subjects without DM) were compared and examined using Chi-square tests. The urbanization levels of living areas were grouped into three levels based on the population density (persons/km2) for each area (Department of Statistics, Ministry of the Interior, Executive Yuan of the Republic of China). The high level was those living in areas in upper two quartiles and the low level was the lowest quartile. The level for premium based income was grouped into three levels: less than 15,840, 15,840-19,200 and more than 19,200 NTD. The incidence density rates of AC of the two groups were estimated using these variables, and their rate ratios were compared. To calculate the incidence density, the study subjects’ observational person-years were summed until AC was diagnosed; for those without AC, person-years were summed until 31 December 2008 or until the respective dates when the subjects left the NHI because of reasons such as death, emigration, and so on. Because few DM patients were type 1 patients, data analysis would not stratify by the type of DM.
Cox hazards proportional regression analysis for subjects with DM compared with those without DM. Univariate analysis was performed initially. Multivariate analysis included socio-demographic variables and medical comorbidity, which were significant in the
univariate analysis. The Cox model was further used to estimate sex-specific aHRs using men without DM as the reference group, and age-specific aHRs using 20-29 years old subjects without DM as the reference group. A plot of the Kaplan-Meier survival analysis was used to demonstrate the probability of remaining individuals free from AC, and the logrank test was used to test the difference between the two cohorts. All analyses were accomplished using SAS statistical software (Version 9.1 for Windows; SAS Institute, Inc., Cary, NC, USA). Hazard ratios are presented with 95% confidence intervals, and p-values are two-tailed.
RESULTS
Subjects characteristics, incidence of adhesive capsulitis
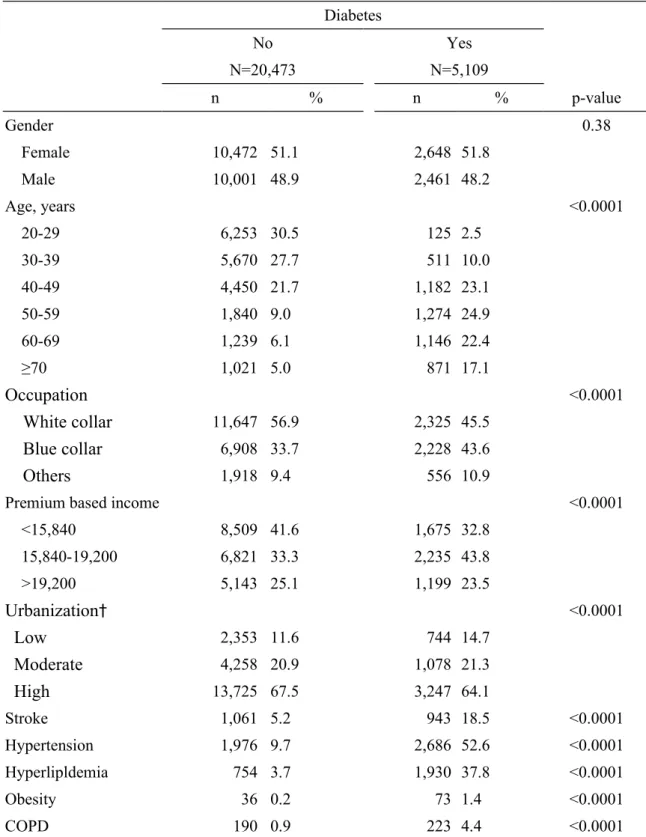
Proportional distributions revealed more male subjects, but the difference was
insignificant (Table 1). Compared to comparisons, DM patients were older, less white collar (45.5% vs. 56.9%) and living in urban area (64.1% vs. 67.5%), had lower income (32.8% vs. 41.6%) and more prevalent with comorbidities including stroke (18.5% vs, 5.2%),
hypertension (52.6% vs. 9.7%), hyperlipidemia (37.8% vs. 3.7%), obesity (1.4% vs. 0.2%) and COPD (4.4% vs. 0.9%),
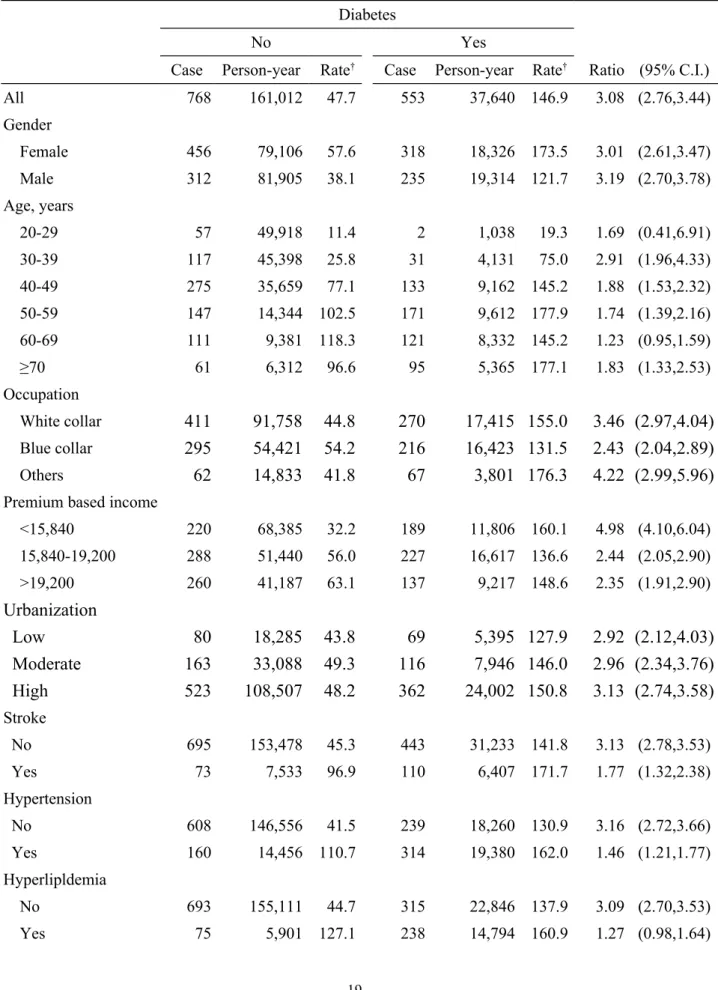
Table 2 presents the incidence density of frozen shoulder in the cohorts, as well as their comparison with respect to socio-demographic characteristics and comorbidity. From 2001 to
2008, cumulative incidence rates of AC were 10.8% (553/5,109) and 3.75% (768/20,473) for the study and comparison cohorts, respectively. The corresponding incidence density of AC in the study cohort was 3.08 times higher than that in the comparison cohort (146.9 vs. 47.7 per 10,000 person-years). The incidence was higher in females than in males and increased with age in both cohorts. But, the age-specific diabetes cohort to comparison cohort incidence rate ratio was the highest in subjects of 30-39 years old. The risk ratio was greater in low insurance payers. Table 2 also shows the incidence rate ratio was higher in those without comorbidity than those with comorbidity.
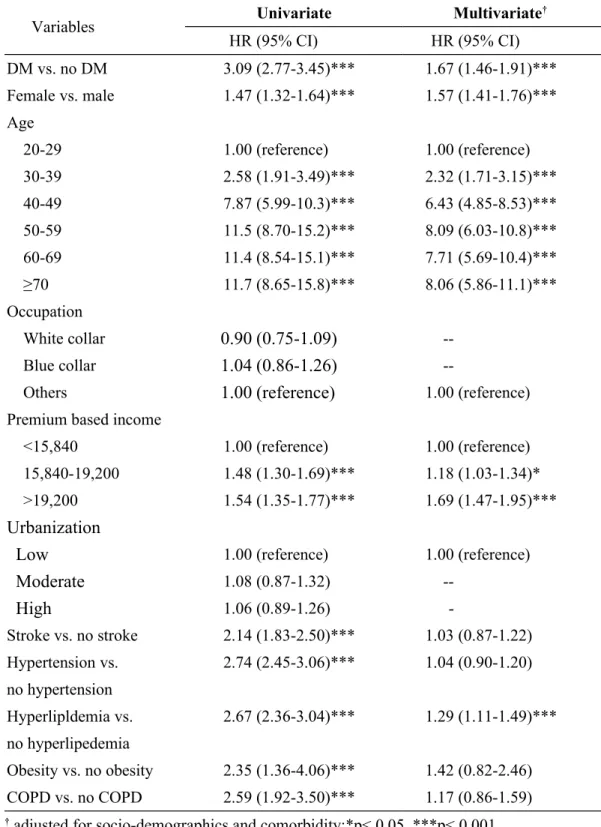
Hazards of adhesive capsulitis
Table 3 shows the hazard ratio (HR) of AC in association with DM in univariate and multivariate models. The HR of AC for patients with DM was significantly high in the univariate analysis. The HR of AC was 1.67 (95% CI: 1.46-1.91, p <0.001) in the multivariate analysis, after adjusting for age, premium based income, stroke, hypertension,
hyperlipidemia, obesity and COPD. The HR of AC increased dramatically to comparatively high levels from the 40-49 years old group onward in circumstances with and without adjustments of socio-demographic characteristics and comorbidity. Persistently higher risks of AC in female subjects (HR = 1.57, 95% CI: 1.41-1.76, p <0.001), as well as those with high incomes (HR = 1.69, 95% CI: 1.47-1.95, p <0.001), were observed even after adjustment of all socio-demographic characteristics and comorbidity in the multivariate analysis. This
model also reveals that only hyperlipidemia was a significant independent risk factor for AC (HR = 1.29, 95% CI: 1.11-1.49, p <0.001). Stroke, hypertension, obesity and COPD were associated with significantly higher HRs of AC only in the univariate analysis.
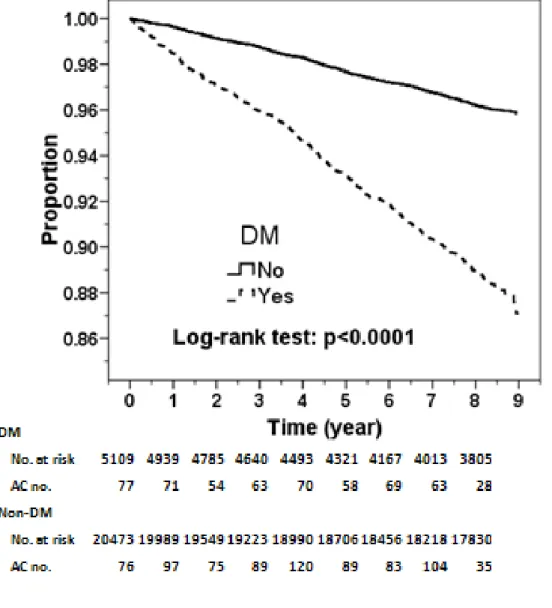
A plot of the Kaplan-Meier analysis reveals that the AC-free probability progressively decreased over time in the study cohort relative to the comparison cohort, 9 percent lower for the diabetes cohort after 9-year follow-up (Log-rank test: p<0.0001) (Figure 1).
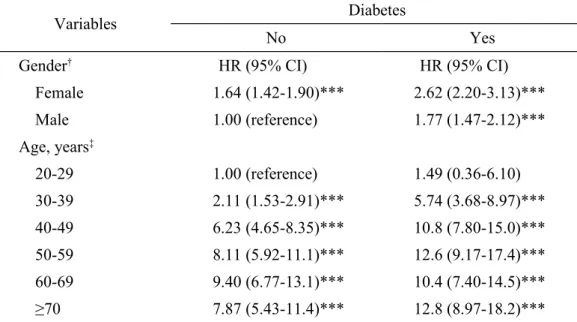
After the adjustment of other sociodemographic characteristics and comorbidity, the risk of AC for female DM subjects was observed to be 2.62-fold higher than that for male non-DM subjects (Table 4). Compared with non-DM subjects aged 20 to 29 years, the HR of AC for both cohorts increased with increasing age. The adjusted HR of AC in DM subjects increased to 5.74 (95% CI: 3.68-8.97) and 12.8 (95% CI: 8.97-18.2) for the age groups ages 30 to 39 years and > 70 years, respectively. DM subjects generally recorded higher HRs of AC compared to non-DM subjects in all age groups.
DISCUSSION
The present study, which featured population-based cohort analyses, found that the incidence of AC in DM patients is considerably higher than in the non-DM cohort. The prevalence of AC (12.4%) in our DM subjects is between those found in Type 1 (10.3%) and Type 2 DM (22.4%) subjects reported by Arkkila et al in a study involving 291 Type 1 and 134 Type 2 DM subjects [7], similar to that (14.7%) reported by Aydeniz et al. in a study of
102 Type 2 DM patients [8], and more close to that (10.8%) of a study involving 800 DM
patients (23% insulin dependent) reported by Bridgman [4].
Further data analysis by type
of DM in our study showed that the incidence of AC was higher in type 1 DM
patients than in type 2 DM patients (164.4 vs. 145.8 per 10,000 person-years) (data
not shown).
The similarities of the incidence and prevalence of AC in DM patients between our present Oriental study and past Western studies, may suggest similar global trend of AC risk in DM patients. The risk of AC consistently increases in DM patients despite different socio-demographic status and co-morbidity, as demonstrated by the persistent increase in corresponding incidence rate ratios for all these variables.In the uncontrolled analysis, stroke, hypertension, and hyperlipidemia, obesity and COPD were all observed to be risk factors for AC. These preliminary findings are compatible with a previous report which found adhesive capsulitis as a cause of hemiplegic shoulder pain in patients with stroke [9], and agree with another study which found the association of obesity and smoking with shoulder pain and/or stiffness in univariate analysis [10]. However, only hyperlipidemia remained a significant risk following adjustments for sociodemographic status and co-morbidity. Hyperlipidemia is suggested to be an underlying risk factor for stroke and hypertension [11, 12]. Elevated serum triglyceride and cholesterol levels were
found in patients with frozen shoulder [
13
]. Our study suggests that hyperlipidemia is anthe vascular complications of DM. Cumulative detrimental effects of hyperlipidemia on tendon properties were found [14]. Increased risk of rotator cuff disease in patients with hypercholesterolemia is possible [15] and lead to secondary frozen shoulder [16]. Furthermore, patients taking hydroxymethylglutaryl coenzyme A (HMG-CoA) reductase inhibitors have an increased risk of shoulder stiffness [17] that may predispose to AC.
The pathology of AC was also observed to involve a process of fibrosis, increased vascularity, and low-grade inflammation, similar to those observed in Dupuytren’s diseases of
the hand [18].Subsequent study also observed significant associations between AC and
Dupuytren’s diseases, and AC and limited joint mobility in DM patients [
19
].Although the pathogenesis of AC in DM patients is not clearly understood yet, the
induction of fibrosis by the proliferation of several cell types [20, 21],as well as alterations in
quality and quantity of extracellular matrix in a variety of DM patient’s tissues were found
[22].The increased expression of vascular endothelial growth factor and angiogenesis in the
diabetic AC has been observed as well [
23
].Previous reports also suggested theimmunological pathogenesis of AC [
24
], and DM [25-27]. It is possible that these twoconditions are interrelated. The risk association is significantly greater in all women, as demonstrated by their persistently higher AC hazard ratios, regardless of whether or not adjustments to their socio-demographic status and co-morbidity are made. This finding is compatible with a previous study that found that females are associated with a number of
hand or shoulder syndromes [
28
].DM and the female gender as risk factors for AC are again confirmed by the attainment of the highest hazard ratio difference when female diabetic subjects were compared to male non-diabetic subjects (Table4). The risk for AC increases with age in circumstances with and without adjustments to socio-demographic status and comorbidity (Table 3). A plot of the Kaplan-Meier analysis shows that the AC-free probability markedly decreased with time in the DM cohort in comparison to the comparison cohort. This suggests that the risk of AC increases remarkably with time in DM subjects. These findings are in accordance with previous studies that determined the increased risk of AC in DM patients with older ages [7,
19].Compared with non-DM subjects aged 20 to 29 years, the HR of AC of both cohorts
increased with age, with DM subjects attaining a higher risk in all age groups. The rate of
increase HR of AC in DM subjects was more abrupt from the 3rd decade to 5th decade and
then remains at a relatively high level. These findings suggest that DM subjects generally
possess higher risks of AC development from adulthood to their 5th decade then remain at
relative high risk status with aging. Another report from Arkkila et al [29]further supports the
findings of the present study. The higher risks of AC observed in subjects with higher incomes cannot be clearly explained as of yet. It is speculated that income-related changes in dietary intake may play a role in the increase risk of DM and AC as well. Further
The present study includes a number of strengths. The dataset is composed of
nationwide population-based claims with little selection bias, lower loss follow-up rates, and convenient access to longitudinal records of different geographically distributed subjects. Such a large sample size of study also renders stratification analyses possible and effective.
However, several limitations are present in the study as well. First, misclassification biases may occur because of the study’s exclusive reliance on registry-based data for
diagnoses. Information on factors
associating with DM and risk of AC is not available in
the retrospective design and biases may affect the selection of comparisons.
The likelihood of DM misclassification was reduced by enrolling subjects with at least three diagnostic records of DM in the year 2000. AC is a common disease of the general population, posing little difficulty in diagnosis and coding. Further, the insurance bureau randomly samples a fixed percentage of claims from each hospital annually to verify the validity of diagnoses and the quality of care through chart reviews conducted by an independent peer review group. Any discrepancies are subjected to heavy penalties by the bureau. Second, although the existence of unclear differentiations between Type 1 and Type 2 DM subjects has been explained in the study, a previous study reported that Type 1 DM patients account for merely 1.8% of all DM patients in Taiwan [30]. Consequently, the risks of AC estimation for DM patients in the present study may more appropriately describe the risks of Type 2 DM subjects in Taiwan. Third, variables that may modify the risk of AC, suchas cigarette smoking, body mass index, physical activity level, blood sugar levels, and HbA1c values were unavailable in the database. We make up for this inadequacy by including obesity and COPD in the baseline comorbidity. The associations of these variables to the risk of AC in DM subjects require further studies.
In summary, an eight-year study period revealed that DM subjects in Taiwan are at an increased risk of AC; the risk increases particularly for female subjects and older age groups. The presence of hyperlipidemia, which often accompanies DM, independently intensifies this risk. Disturbed glucose and lipid metabolism may be the underlying mechanisms for the increase of risk. Adequate DM and glycemic control may reduce the incidence of AC, although it cannot be confirmed in our present study. These findings have important implications for the comprehensive management of DM subjects, as AC causes profound upper limb disability-related impairment of daily activities and decreases the working ability and quality of lives of DM subjects. With this in mind, health education, proper therapeutic
exercise [31], avoidance of unfavorable shoulder postures and prolong immobility [
32
],mayprevent high-risk patient groups to develop AC and its consequent adverse health and economic sequelae. Such measures are very important in the comprehensive care of DM subjects.
(grant number
NSC 100-2621-M-039-001), the Department of Health (grant nos.
DOH100-TD-B-111-004 and DOH100-TD-C-111-005), and the China Medical
University Hospital (grant no. 1MS1 and DMR-100-187).
Competing interests: None.
Ethic approval: This study used scrambled identification numbers to link data sets and to
ensure privacy of the insured population. Additional IRB approval is exempted.
Patient consent: Not need.
REFERENCES
1. Alberti KG, Zimmet PZ (1998) Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabetic Med 15:539-553
2. Reeves B (1975) The natural history of the frozen shoulder syndrome. Scand J Rheumatol 4:193-196
3. Thomas SJ, McDougall C, Brown ID et al (2007) Prevalence of symptoms and signs of shoulder problems in people with diabetes mellitus. J Shoulder Elb Surg 16:748-751 4. Bridgman JF (1972) Periarthritis of the shoulder and diabetes mellitus. Ann Rheum Dis
31:69-71
5. Pal B, Anderson J, Dick WC, Griffiths ID (1986) Limitation of joint mobility and shoulder capsulitis in insulin- and non-insulin-dependent diabetes mellitus. Br J Rheumatol 25:147-151
6. Fisher L, Kurtz A, Shipley M (1986) Association between cheiroarthropathy and frozen shoulder in patients with insulin-dependent diabetes mellitus. Br J Rheumatol 25:141-146 7. Arkkila PE, Kantola IM, Viikari JS, Ronnemaa T (1996) Shoulder capsulitis in type I and
II diabetic patients: association with diabetic complications and related diseases. Ann Rheum Dis 55:907-914
8. Aydeniz A, Gursoy S, Guney E (2008) Which musculoskeletal complications are most frequently seen in type 2 diabetes mellitus? J Int Med Res 36:505-511
9. Lo SF, Chen SY, Lin HC, Jim YF, Meng NH, Kao MJ (2003) Arthrographic and clinical findings in patients with hemiplegic shoulder pain. Arch Phys Med Rehabil 84:1786-1791
10. Cole A, Gill TK, Shanahan EM, Phillips P, Taylor AW, Hill CL (2009) Is diabetes associated with shoulder pain or stiffness? Results from a population based study. J Rheumatol 36:371-377
11. Amarenco P, Bogousslavsky J, Callahan A et al (2006) High-dose atorvastatin after stroke or transient ischemic attack. New Engl J Med 355:549-559
12. Halperin RO, Sesso HD, Ma J, Buring JE, Stampfer MJ, Gaziano JM (2006) Dyslipidemia and the risk of incident hypertension in men. Hypertension 47:45-50
13. Bunker TD, Esler CN (1995) Frozen shoulder and lipids. J Bone Joint Surg Br
77:684-686
14. Beason DP, Abboud JA, Kuntz AF, Bassora R, Soslowsky LJ (2011)
Cumulative effects of hypercholesterolemia on tendon biomechanics in a mouse model. J Orthop Res 9:380-383
15. Abboud JA, Kim JS (2010) The effect of hypercholesterolemia on rotator cuff disease. Clin Orthop Relat Res 468:1493-1497
16. Zuckerman JD, Rokito A (2010) Frozen shoulder: a consensus definition. J Shoulder Elb Surg 20:322-325
17. Harada K, Tsuruoka S, Fujimura A (2001) Shoulder stiffness:
a common adverse effect of HMG-CoA reductase inhibitors in women? Intern Med 40:817-818
18. Bunker TD, Anthony PP (1995) The pathology of frozen shoulder. A Dupuytren-like disease. J Bone Joint Surg Br 77:677-683
19. Balci N, Balci MK, Tuzuner S (1999) Shoulder adhesive capsulitis and shoulder range of motion in type II diabetes mellitus: association with diabetic complications. J Diabetes Complicat 13:135-140
20. Rowe DW, Starman BJ, Fujimoto WY, Williams RH (1977) Abnormalities in
proliferation and protein synthesis in skin fibroblast cultures from patients with diabetes mellitus. Diabetes 26:284-290
21. Oikawa S, Hayasaka K, Hashizume E et al (1996) Human arterial smooth muscle cell proliferation in diabetes. Diabete;45:114-116
22. Brownlee M (1992) Glycation products and the pathogenesis of diabetic complications. Diabetes Care 15:1835-1843
23. Ryu JD, Kirpalani PA, Kim JM, Nam KH, Han CW, Han SH (2006) Expression of vascular endothelial growth factor and angiogenesis in the diabetic frozen shoulder. J Shoulder Elb Surg 15:679-685
24. Bulgen D, Hazleman B, Ward M, McCallum M (1978) Immunological studies in frozen shoulder. Ann Rheum Dis 37:135-138
26. Leslie RD, Williams R, Pozzilli P (2006) Clinical review: Type 1 diabetes and latent
autoimmune diabetes in adults: one end of the rainbow. J Clin Endocrinol Metabol
91:1654-1659
27. Dworacka M, Winiarska H, Borowska M, Abramczyk M, Bobkiewicz-Kozlowska T, Dworacki G (2007) Pro-atherogenic alterations in T-lymphocyte subpopulations related to acute hyperglycaemia in type 2 diabetic patients. Circ J 71:962-967
28. Cagliero E, Apruzzese W, Perlmutter GS, Nathan DM (2002) Musculoskeletal disorders of the hand and shoulder in patients with diabetes mellitus. Am J Med 112:487-490 29. Arkkila PE, Gautier JF (2003) Musculoskeletal disorders in diabetes mellitus: an update.
Best Pract Res Clin Rheumatol 17:945-970
30. Chuang LM, Tsai ST, Huang BY, Tai TY (2001) The current state of diabetes management in Taiwan. Diabetes Res Clin Pr 54:55-65
31. Kelley MJ, McClure PW, Leggin BG (2009) Frozen shoulder: evidence and a proposed model guiding rehabilitation. J Orthop Sports Phys Ther 39:135-148
32. Tanishima T, Yoshimasu N (1997) Development and prevention of frozen shoulder after acute aneurysm surgery. Surg Neurol 48:19-22
Table 1. Distribution of socidemographic characteristics and baseline comorbidity between DM and non-DM cohort
Diabetes No N=20,473 Yes N=5,109 n % n % p-value Gender 0.38 Female 10,472 51.1 2,648 51.8 Male 10,001 48.9 2,461 48.2 Age, years <0.0001 20-29 6,253 30.5 125 2.5 30-39 5,670 27.7 511 10.0 40-49 4,450 21.7 1,182 23.1 50-59 1,840 9.0 1,274 24.9 60-69 1,239 6.1 1,146 22.4 ≥70 1,021 5.0 871 17.1
Occupation
<0.0001White collar
11,647 56.9 2,325 45.5Blue collar
6,908 33.7 2,228 43.6Others
1,918 9.4 556 10.9Premium based income <0.0001
<15,840 8,509 41.6 1,675 32.8 15,840-19,200 6,821 33.3 2,235 43.8 >19,200 5,143 25.1 1,199 23.5
Urbanization
† <0.0001Low
2,353 11.6 744 14.7Moderate
4,258 20.9 1,078 21.3High
13,725 67.5 3,247 64.1 Stroke 1,061 5.2 943 18.5 <0.0001 Hypertension 1,976 9.7 2,686 52.6 <0.0001 Hyperlipldemia 754 3.7 1,930 37.8 <0.0001 Obesity 36 0.2 73 1.4 <0.0001 COPD 190 0.9 223 4.4 <0.0001†
There are 137 and 40 observations of non-diabetes and diabetes patients missing,
respectively, in urbanization and regional classification.
Table 2. Numbers and incidence densities of AC in patients with diabetes and a comparison cohort of non-diabetic patients by sociodemographic characteristics and baseline co-morbidity.
Diabetes
No Yes
Case Person-year Rate† Case Person-year Rate† Ratio (95% C.I.)
All 768 161,012 47.7 553 37,640 146.9 3.08 (2.76,3.44) Gender Female 456 79,106 57.6 318 18,326 173.5 3.01 (2.61,3.47) Male 312 81,905 38.1 235 19,314 121.7 3.19 (2.70,3.78) Age, years 20-29 57 49,918 11.4 2 1,038 19.3 1.69 (0.41,6.91) 30-39 117 45,398 25.8 31 4,131 75.0 2.91 (1.96,4.33) 40-49 275 35,659 77.1 133 9,162 145.2 1.88 (1.53,2.32) 50-59 147 14,344 102.5 171 9,612 177.9 1.74 (1.39,2.16) 60-69 111 9,381 118.3 121 8,332 145.2 1.23 (0.95,1.59) ≥70 61 6,312 96.6 95 5,365 177.1 1.83 (1.33,2.53) Occupation White collar
411
91,758 44.8
270
17,415 155.0
3.46 (2.97,4.04)
Blue collar295
54,421 54.2
216
16,423 131.5
2.43 (2.04,2.89)
Others62
14,833 41.8
67
3,801 176.3
4.22 (2.99,5.96)
Premium based income
<15,840 220 68,385 32.2 189 11,806 160.1 4.98 (4.10,6.04) 15,840-19,200 288 51,440 56.0 227 16,617 136.6 2.44 (2.05,2.90) >19,200 260 41,187 63.1 137 9,217 148.6 2.35 (1.91,2.90)
Urbanization
Low
80
18,285 43.8
69
5,395 127.9
2.92 (2.12,4.03)
Moderate
163
33,088 49.3
116
7,946 146.0
2.96 (2.34,3.76)
High
523
108,507 48.2
362
24,002 150.8
3.13 (2.74,3.58)
Stroke No 695 153,478 45.3 443 31,233 141.8 3.13 (2.78,3.53) Yes 73 7,533 96.9 110 6,407 171.7 1.77 (1.32,2.38) Hypertension No 608 146,556 41.5 239 18,260 130.9 3.16 (2.72,3.66) Yes 160 14,456 110.7 314 19,380 162.0 1.46 (1.21,1.77) Hyperlipldemia No 693 155,111 44.7 315 22,846 137.9 3.09 (2.70,3.53) Yes 75 5,901 127.1 238 14,794 160.9 1.27 (0.98,1.64)Obesity No 765 160,734 47.6 543 37,082 146.4 3.09 (2.76-3.45) Yes 3 278 107.9 10 558 179.2 1.65 (0.46-6.00) COPD No 753 159,817 47.1 524 36,210 144.7 3.08 (2.76-3.44) Yes 15 1,195 125.5 29 1,430 202.8 1.61 (0.86-3.00) †per 10,000 person-years
Table 3. Hazard ratios of AC in association with DM in univariate and multivariate Cox hazard proportional analyses
Variables Univariate Multivariate
† HR (95% CI) HR (95% CI) DM vs. no DM 3.09 (2.77-3.45)*** 1.67 (1.46-1.91)*** Female vs. male 1.47 (1.32-1.64)*** 1.57 (1.41-1.76)*** Age 20-29 1.00 (reference) 1.00 (reference) 30-39 2.58 (1.91-3.49)*** 2.32 (1.71-3.15)*** 40-49 7.87 (5.99-10.3)*** 6.43 (4.85-8.53)*** 50-59 11.5 (8.70-15.2)*** 8.09 (6.03-10.8)*** 60-69 11.4 (8.54-15.1)*** 7.71 (5.69-10.4)*** ≥70 11.7 (8.65-15.8)*** 8.06 (5.86-11.1)*** Occupation White collar
0.90 (0.75-1.09)
--Blue collar1.04 (0.86-1.26)
--Others
1.00 (reference)
1.00 (reference)Premium based income
<15,840 1.00 (reference) 1.00 (reference)
15,840-19,200 1.48 (1.30-1.69)*** 1.18 (1.03-1.34)*
>19,200 1.54 (1.35-1.77)*** 1.69 (1.47-1.95)***
Urbanization
Low
1.00 (reference) 1.00 (reference)Moderate
1.08 (0.87-1.32)High
1.06 (0.89-1.26) -Stroke vs. no stroke 2.14 (1.83-2.50)*** 1.03 (0.87-1.22) Hypertension vs. no hypertension 2.74 (2.45-3.06)*** 1.04 (0.90-1.20) Hyperlipldemia vs. no hyperlipedemia 2.67 (2.36-3.04)*** 1.29 (1.11-1.49)*** Obesity vs. no obesity 2.35 (1.36-4.06)*** 1.42 (0.82-2.46) COPD vs. no COPD 2.59 (1.92-3.50)*** 1.17 (0.86-1.59)Table 4. Gender- and age-specific risk for AC in association with DM
Variables Diabetes
No Yes
Gender† HR (95% CI) HR (95% CI)
Female 1.64 (1.42-1.90)*** 2.62 (2.20-3.13)*** Male 1.00 (reference) 1.77 (1.47-2.12)*** Age, years‡ 20-29 1.00 (reference) 1.49 (0.36-6.10) 30-39 2.11 (1.53-2.91)*** 5.74 (3.68-8.97)*** 40-49 6.23 (4.65-8.35)*** 10.8 (7.80-15.0)*** 50-59 8.11 (5.92-11.1)*** 12.6 (9.17-17.4)*** 60-69 9.40 (6.77-13.1)*** 10.4 (7.40-14.5)*** ≥70 7.87 (5.43-11.4)*** 12.8 (8.97-18.2)***
†Adjusted for age, income, stroke, hypertension, hyperlipidemia, obesity and COPD
‡Adjusted for sex, income, stroke, hypertension, hyperlipidemia, obesity and COPD
Figure 1. Proportion of subjects free from adhesive capsulitis in the follow-up period in diabetes patients and the comparison cohort