Effects of Habitat Type and Group Size on Foraging and Vigilance
Behaviors of the Red Collared Dove Streptopelia tranquebarica
Herng-Chein Li(1), Tzung-Su Ding(2), Chu-Fa Tsai(3) and Fu-Hsiung Hsu(1*)1. Department of Biological Resources, National Chiayi University, 300, University Road, Chiayi 600, Taiwan.
2. School of Forestry and Resource Conservation, National Taiwan University, 1, Sec. 4, Roosevelt Road, Taipei 106, Taiwan. 3. Endemic Species Research Institute, 1 Ming-Shen E. Road, Jiji, Nantou 552, Taiwan.
* Corresponding author. Tel: 886-5-2717828; Fax: 866-5-2717816; Email: richbear@mail.ncyu.edu.tw
(Manuscript received 12 December 2011; accepted 26 March 2012)
ABSTRACT: One of the most beneficial effects of foraging in group for animals is enhancement of predation avoidance. Habitat type and group size are known to affect foraging and vigilance behaviors of the animals. We video-filmed and analyzed foraging, vigilance and moving behaviors of 127 focal Red Collared Doves (Streptopelia tranquebarica) in the western Taiwan to determine the effects of habitat types (open and obstructed) and group sizes (1 to 27 doves) on the behaviors. The results showed that the total foraging duration (sec) and number of pecking increased with the increase in group size at both habitats, while the total vigilance duration (sec), number of scanning bouts and scan duration were higher at the obstructed habitat than those at the open habitat. The group-size effect on vigilance was found only at the obstructed habitat but not at the open habitat. However, the low potential predation threats and possible use of peripheral vision to detect predators might dismiss the group-size effect. Also, the total moving duration (sec) decreased with the increase in group size, an indication of increasing foraging efficiency and anti-predatory benefits. KEY WORDS: Foraging, group size, habitat, Streptopelia tranquebarica, vigilance.
INTRODUCTION
Many birds interrupt their feeding to scan the surroundings for potential predation threats. This anti-predatory vigilance is generally defined as the proportion of time spent by a bird raising its head during the foraging period (Whittingham et al., 2004). It is assumed that predators will be detected more quickly in the ‘head-up’ period (Elgar, 1989; Caro, 2005) than in the period with the head lowered down to search for food (Lima, 1987; Lima and Bednekoff, 1999). For these birds, vigilance and feeding are often mutually exclusive. This is particularly true for ground feeding birds which must look down to search for food.
The trade-off between vigilance and feeding has been studied extensively (Pulliam, 1973). Shift between the two activities during the foraging period has been linked to many environmental and social factors, such as habitat characteristics, predation risk, sex, and group size (Jones et al., 2006; Li et al., 2009). The habitat characteristics include food abundance (Butler et al., 2005), substrate of feeding patches (Whittingham and Markland, 2002; Jones et al., 2006), habitat obstruction that reduces predator detection (Barta et al., 2004; Devereux et al., 2004; Whittingham et al., 2004; Butler et al., 2005; Devereux et al., 2005; Jones et al., 2006), and the presence of refuges that affect individual’s feeding activity and ability to detect predators (Whittingham and Evans, 2004). For birds that rely on sight to detect predators, the risk of predation may be a
function of visibility of the surroundings (Lima and Dill, 1990). An obstructed habitat may increase head-up vigilance with reduction in accessibility of food items (Guillemain et al., 2001, Devereux et al., 2005; Jones et al., 2006). On the other hand, an open environment making birds use peripheral vision more effectively to detect predators may be more advantageous in foraging efficiency (Metcalfe, 1984; Lima et al., 1999; Whittingham et al., 2004; Whittingham and Evans, 2004; Devereux et al., 2005).
A negative relationship between group size and vigilance, referred to as the group-size effect on vigilance, has been documented in many species of birds (Elgar, 1989; Lima and Dill, 1990; Lima, 1995; Caro, 2005; Beauchamp, 2008; Carro and Fernández, 2008). There are two main hypotheses to explain the group-size effect: the many-eyes hypothesis and the dilution effect hypothesis. The latter is also called the safety in numbers hypothesis (Bednekoff and Lima, 1998). The many-eyes hypothesis states that individuals in a group are allowed to reduce their own vigilance by taking advantage of shared vigilance from other members (Pulliam, 1973; Powell, 1974), so that the chance of being attacked by a predator is reduced when group size increases. The dilution effect hypothesis states that increasing group size allows a reduction in vigilance because as predation occurs, the risk of being depredated is shared by all group members, and thus the risk is diluted (Dehn, 1990; Robert, 1996).
35 years to evaluate the negative relationship between vigilance and group size of birds. They found that about one third of the studies did not support the predicted relationship. The lack of negative relationship has been explained in several studies, mainly by the confounding effects of other variables, such as age, sex, food density and quality, distance to refuges, and foraging strategies (Elgar, 1989; Barbosa, 2002).
The Red Collared Dove (Streptopelia
tranquebarica) is a resident bird in Taiwan. It is
abundant in coastal plains and peripheral hills with low elevations. The male is reddish but the female is lighter in color, and both sexes have a black crescent torque on the back of neck. The species usually forage in flocks and peck grains on the ground. These make them an ideal species for investigating the relationship between vigilance and group size. We recorded behaviors of the Red Collared Doves in groups at an obstructed forest and an open farmland to evaluate the effects of habitat types and group sizes on the foraging and vigilance behaviors.
MATERIALS AND METHODS
Study areas
We selected two areas that represented an obstructed habitat and an open habitat in Chiayi County of the western Taiwan. The obstructed habitat area was located at coastal woodland near the Au-go Wetland Reserve (23°30’N, 120°08’E) with an elevation of 1 m. This woodland was created in 2002 by the Taiwan Sugar Corporation in complying with the policy of forestation in the plain area. Individuals of several tree species Trema orientalis, Koelreuteria paniculata,
Bischofia javanica, Terminalia mantaly, Melaleuca leucadendra and Eucalyptus grandis were planted 3 m
apart. Most of them had grown to 3 to 8 meters high. Several gravel roads (ca. 7 m width) crisscrossed the woodland. The open habitat area was located at an open farmland (23°26’N, 120°13’E) with an elevation of 5 m. It was composed mostly of rice paddies and vegetable gardens, such as spinach, mustard, cabbage and sweet corn. There were some houses, dispersed tree patches, a 2-lane high way (County Road 161), and several small local roads. We set up a 5 km survey route in each of the two habitat areas to observe the vigilance and foraging behaviors of the Red Collared Doves from March to May in 2010.
Behavioral observation
The daily searches for the Red Collared Doves were conducted on the survey routes of the two habitat areas during the daylight period. When a dove group was found on the ground, the vehicle was parked at a distance about 20-50 m from the group. Observations
were made with 8×25 binoculars inside the vehicle and recorded the behavior with a video-recorder (Panasonic NV-GS400 camcorder). The doves did not seem to be disturbed by the parked vehicle, perhaps due to the fact that they had been habituated to vehicles that were common in the area.
Data collection
Individual birds in a flock with a separating distance less than 5 m were considered belonging to the same foraging group (Dolman et al., 1996). The group size was determined by counting the number of individuals at the beginning of each observation. When a group size was changed due to moving in or out of its members, the observation was terminated and the data collected was abandoned. The groups observed ranged from 1 to 27 individuals in size. We sampled the behaviors of only one individual in a group as a focal bird to minimize pseudo-replication (Barbosa, 2002; Dias, 2006). Different groups observed on the study routes were separated far apart that we considered they belonged to different groups.
A focal individual was chosen arbitrarily and video-recorded for 3 minutes with the focal-animal sampling method (Martin and Bateson, 2007). The film was later played back on analyzing equipment to quantify the behavior variables. Six response variables of its foraging (total foraging duration and number of pecking), vigilance (total vigilance duration, number of scanning bouts and scan duration) and moving (total moving duration) activities were quantified. The foraging mode occurred when the bird was not moving and its head was down below the horizontal line of its body in pecking food on the ground or vegetation. The vigilance mode occurred when the bird was also not moving and its head was up above the horizontal line of its body longer than a second to scan the surroundings. Moving occurred when the bird was walking or running continuously with the head slightly above the horizontal line of its body (Phelan, 1987; Sadedin and Elgar, 1998; Dias, 2006). The length of each of the foraging, vigilance, and moving periods (seconds) was measured with an electronic audio timer during the 3-minute recording period (Reboreda and Fernández, 1997; Dias, 2006). The number of active pecks on the ground or at the vegetation and the number of scanning bouts during the recording period were also recorded. The scan duration was calculated as the total length of vigilance duration divided by the number of scanning bouts. Statistical analysis
Spearman correlation (rs) test was used to evaluate
the relationships between the group sizes, the numbers of birds actually counted, and each of the behavioral variables. In addition, the generalized linear model
(GLM) was used to analyze the effects of habitat types, group sizes, and their interactions on the response variables. To analyze the group size effect, we pooled the data into six group size categories: solitary bird, 2 birds, 3 birds, 4-5 birds, 6-10 birds, and 11-27 birds. To handle non-normal distribution in the data, we used GLM with appropriate error distribution and linked function: gamma error with logarithmic link for time variables, and Poisson error with logarithmic link for number of pecking and scanning bouts (Catterall et al., 1992; Barta et al., 2004; Fernández-Juricic et al., 2007; Li et al., 2009). Mann-Whitney U test was used to compare the variables between two habitat types. All analyses were conducted in SPSS for Windows version 17.0 (SPSS Inc., Chicago, Illinois, USA). All results are displayed as mean ± SE.
RESULTS
A total of 127 focal Red Collared Doves were video-filmed: 53 from obstructed habitat and 74 from open habitat. When the data of both habitats were combined, the doves spent 132.7 ± 2.93 sec (73.7 ± 1.63%) for foraging and 30.1 ± 2.38 sec (16.7 ± 1.32%) for moving during a 3-minute recording period, but only 13.9 ± 1.61 sec (7.7 ± 0.89%) in vigilance with 3.9 ± 0.27 scanning bouts and 2.9 ± 0.23 sec in each bout of scan (scan duration).
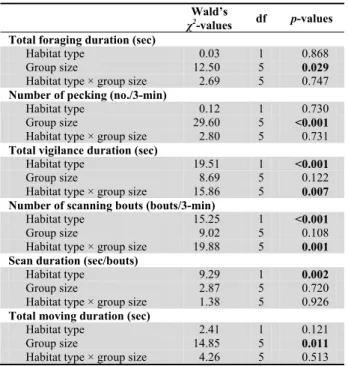
Habitat effects
The differences between the obstructed and open habitats are presented in Table 1. There was no significant difference in the total foraging duration (Wald’s χ2 = 0.03, p = 0.87) and the number of pecking (Wald’s χ2 = 0.12, p = 0.73) between the two habitat types, suggesting that the habitat types did not affect the foraging activity. On the other hand, there were significantly higher values in the vigilance variables at the obstructed habitat as compared to those at the open habitat. For the three vigilance variables, the total vigilance duration was 18.3 ± 2.46 sec, the number of scanning bouts was 4.7 ± 0.38 bouts/3-min, and the scan duration was 3.4 ± 0.33 sec at the obstructed habitat (n = 74). At the open habitat, the total vigilance duration was 7.7 ± 1.39 sec, number of scanning bouts was 2.7 ± 0.32 bouts/3-min, and scan duration was 2.2 ± 0.28 sec (n = 53). The GLMs analyses indicated a significant effect of habitat type on total vigilance duration (Wald’s
χ2 = 19.5, p < 0.001), number of scanning bouts (Wald’s χ2 = 15.3, p < 0.001) and scan duration (Wald’s χ2 = 9.3, p = 0.002) (Table 1). Evidently, the doves became more
vigilant at the obstructed habitat by increasing the total vigilance duration accompanied by increasing both the number of scanning bouts and the scan duration.
Table 1. Results of generalized linear model analysis on effects of habitat type, group size, and their interactions on total foraging duration, number of pecking, total vigilance duration, number of scanning bouts, scan duration and total moving duration of 127 focal Red Collared Doves observed in Chiayi County, Taiwan.
Wald’s
χ2-values df p-values
Total foraging duration (sec)
Habitat type 0.03 1 0.868
Group size 12.50 5 0.029
Habitat type × group size 2.69 5 0.747
Number of pecking (no./3-min)
Habitat type 0.12 1 0.730
Group size 29.60 5 <0.001
Habitat type × group size 2.80 5 0.731
Total vigilance duration (sec)
Habitat type 19.51 1 <0.001
Group size 8.69 5 0.122
Habitat type × group size 15.86 5 0.007
Number of scanning bouts (bouts/3-min)
Habitat type 15.25 1 <0.001
Group size 9.02 5 0.108
Habitat type × group size 19.88 5 0.001
Scan duration (sec/bouts)
Habitat type 9.29 1 0.002
Group size 2.87 5 0.720
Habitat type × group size 1.38 5 0.926
Total moving duration (sec)
Habitat type 2.41 1 0.121
Group size 14.85 5 0.011
Habitat type × group size 4.26 5 0.513
Significant results are marked in bold.
Group-size effects
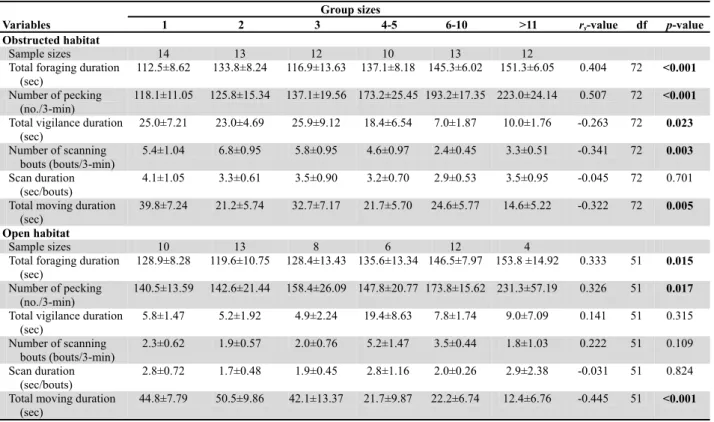
The values of the foraging, vigilance and moving variables for different group sizes from the obstructed habitat and the open habitat, and the results of the Spearman correlation (rs) analyses are shown in Table 2. The total
foraging duration and the number of pecking were significantly and positively correlated with group sizes at both habitats, indicating the beneficial group-size effects on foraging. For the vigilance activities, the total vigilance durations and the number of scanning bouts were significantly but negatively correlated with group sizes at the obstructed habitat but not at the open habitat. The scan durations were not significantly correlated with group sizes at both habitats. The total moving durations were also negatively correlated with the group sizes at both habitats. The GLMs analyses also indicated a significant effect of the six group size categories on total foraging duration (Wald’s
χ2 = 12.5, p = 0.029), number of pecking (Wald’s χ2 = 29.6,
p < 0.001) and total moving duration (Wald’s χ2 = 14.9, p =
0.011) (Table 1).
Interaction between habitat type and group size Based on the GLM analyses (Table 1), the interaction term between habitat types and group size categories was significant on the total vigilance duration (Wald’s χ2 = 15.9, p = 0.007) and the number of scanning bouts (Wald’s
χ2 = 19.9, p = 0.001). They indicated that patterns of the
Table 2. Foraging, vigilance and moving variables (mean ± SE) of 127 focal Red Collared Doves in six group size categories observed in the obstructed habitat and open habitat in Chiayi County, Taiwan.
Group sizes
Variables 1 2 3 4-5 6-10 >11 rs-value df p-value
Obstructed habitat
Sample sizes 14 13 12 10 13 12
Total foraging duration (sec)
112.5±8.62 133.8±8.24 116.9±13.63 137.1±8.18 145.3±6.02 151.3±6.05 0.404 72 <0.001
Number of pecking (no./3-min)
118.1±11.05 125.8±15.34 137.1±19.56 173.2±25.45 193.2±17.35 223.0±24.14 0.507 72 <0.001
Total vigilance duration
(sec) 25.0±7.21 23.0±4.69 25.9±9.12 18.4±6.54 7.0±1.87 10.0±1.76 -0.263 72 0.023 Number of scanning bouts (bouts/3-min) 5.4±1.04 6.8±0.95 5.8±0.95 4.6±0.97 2.4±0.45 3.3±0.51 -0.341 72 0.003 Scan duration (sec/bouts) 4.1±1.05 3.3±0.61 3.5±0.90 3.2±0.70 2.9±0.53 3.5±0.95 -0.045 72 0.701
Total moving duration (sec)
39.8±7.24 21.2±5.74 32.7±7.17 21.7±5.70 24.6±5.77 14.6±5.22 -0.322 72 0.005
Open habitat
Sample sizes 10 13 8 6 12 4
Total foraging duration
(sec) 128.9±8.28 119.6±10.75 128.4±13.43 135.6±13.34 146.5±7.97 153.8 ±14.92 0.333 51 0.015
Number of pecking (no./3-min)
140.5±13.59 142.6±21.44 158.4±26.09 147.8±20.77 173.8±15.62 231.3±57.19 0.326 51 0.017
Total vigilance duration (sec) 5.8±1.47 5.2±1.92 4.9±2.24 19.4±8.63 7.8±1.74 9.0±7.09 0.141 51 0.315 Number of scanning bouts (bouts/3-min) 2.3±0.62 1.9±0.57 2.0±0.76 5.2±1.47 3.5±0.44 1.8±1.03 0.222 51 0.109 Scan duration (sec/bouts) 2.8±0.72 1.7±0.48 1.9±0.45 2.8±1.16 2.0±0.26 2.9±2.38 -0.031 51 0.824
Total moving duration
(sec) 44.8±7.79 50.5±9.86 42.1±13.37 21.7±9.87 22.2±6.74 12.4±6.76 -0.445 51 <0.001
Significant results are marked in bold.
total vigilance duration and the number of scanning bouts between the two habitat types were different across six group size categories. The total vigilance duration and the number of scanning bouts at the obstructed habitat were higher than those at the open habitat when group size categories were smaller than 4-5 (Mann-Whitney U test, p < 0.05 for all comparisons) but not when the size categories were larger (Table 2). No interaction effect was found on the scan duration and the foraging and moving variables.
DISCUSSION
The Red Collared Doves spent approximately three-fourths of their time on the ground at their foraging posture. The total foraging duration and the number of pecking increased with increasing in group size at both open and obstructed habitats. Beauchamp (1998) showed that there are four types of relationships between foraging performance and group size in animals. They are positive relationship, negative relationship, first increases and then decreases with group size, and no correlation, depending on how individuals actually respond to their social companions. However, it is an established fact that birds benefit from foraging in group resulted from reduction in cost and increase in benefit as a consequence
of larger group size (Elgar, 1989; Beauchamp, 2008). Dias (2006) found a positive correlation between group size and the time spent on foraging in Scaled Doves (Columbina squammata). The Greater Rheas (Rhea
Americana) foraging in group allocate more time to
feeding than the solitary ones do (Reboreda and Fernández, 1997; Fernández et al., 2003). The net intake rate and pecking success of White Storks (Ciconia ciconia) increase with aggregation size (Carrascal et al., 1990). In a seminatural experiment, the pecking rate of Brown-headed Cowbirds (Molothrus ater) increases with the group size and decreases with the neighbour distance (Fernández-Juricic et al., 2007).
Two nonexclusive groups of hypotheses have been advanced to explain the benefits of foraging in group: enhanced predator avoidance and increased foraging efficiency (Carrascal et al., 1990; Bednekoff and Lima, 2005; Dias, 2006). In this study, however, the group-size effect on vigilance was supported only at the obstructed habitat but not at the open habitat. The vigilance activities of the Red Collared Doves at the obstructed habitat were more frequent or took longer than those at the open habitat. There were some resident and migratory raptors, such as Elanus
Pandion haliaetus at the obstructed habitat; we never
observed these raptors at the open habitat during the study period. The high predation risk from raptors may increase the level of vigilance of the Red Collared Doves, and the vigilance decreased with the increase in group size at the obstructed habitat.
Many studies have reported the increase in vigilance activities at obstructed habitats (Metcalfe, 1984; Whittingham and Evans, 2004; Whittingham et al., 2004; Devereux et al., 2005). In visually obstructed environments, presence of predators may be hidden behind obstructions, increasing actual predation risk (Arenz and Leger, 1997). Therefore, foraging animals frequently change their pattern of vigilance when they move from a patch with a clear view to another with a relatively obstructed view. In this study the total vigilance durations of the Red Collared Doves were longer at the obstructed habitat, perhaps because poor visibility of the obstructed environment may reduce the ability to detect approaching predator (Whittingham et al., 2004). When feeding at obstructed habitat, animals increase the scan duration to make up for the loss of peripheral information (Bednekoff and Lima, 2005). We also found that the scan durations of the Red Collared Doves at the obstructed habitat were longer than those at the open habitat. However, increase in vigilance behavior can arise just because birds need to raise their head up between foraging periods through the long stubble impeding to access seeds (Whittingham et al., 2004). The doves may enhance their head-up rate at the obstructed habitat to search available food.
The total vigilance duration and number of scanning bouts of the Red Collared Doves at the open habitat were low in the small groups with less than 4 individuals, but the groups with 4-5 individuals had the highest value in all group sizes. We failed to detect the effect of group size on the vigilance by the Spearman correlation analysis at the open habitat. No influence of group size on vigilance has been reported for White-crowned Sparrows (Zonotrichia leucophrys gambelii) (Slotow and Rothstein, 1995). Catterall et al. (1992) found that vigilance levels are unrelated to group sizes in Silvereyes (Zosterops lateralis), though they suggested that this may be due to a lack of predators. The potential predators of adult Red Collared Doves were relatively rare at the open habitat. This low predation pressure might have dismissed the ability of the doves to express their social vigilance tendencies.
An individual bird is usually able to direct vigilance toward potential predators as well as other competitive members in its own group (Waite 1987; Ferriere et al., 1996; Dias, 2006). Vigilance to con-specific members within a group would increase in larger group sizes which may dismiss the tendency of decreasing vigilance with increasing group size. However, the outside stimuli to
induce vigilance activity of individual doves are difficult to be determined under natural conditions; it is still unknown how surrounding environments affect the vigilance activities of the Red Collared Doves.
To forage in group gets anti-predatory benefit and advantages in foraging efficiency (Lazarus, 2003). One of the most important aspects in a foraging trip of a bird is to search food. The search time may be reduced through membership of its group. In this study we recorded the total moving durations to evaluate the searching effort and found that the total moving durations decreased with the group size at both open and obstructed habitats. Larger groups of doves are usually found to associate with better foraging sites, reducing the amount of time to locate food and increasing the effectiveness of foraging. Barnard (1980) found that House Sparrows (Passer domesticus) peck more rapidly in the areas with high seed density and these areas are also attracted the sparrows in larger groups. A laboratory experiment of Canaries (Serinus canaria) also showed that the pecking rate increases with food density (Whittingham and Markland, 2002). Besides, social facilitation of birds in feeding that may lead to a higher pecking rate in large groups, the mere presence of companions is also sufficient to enhance such pecking behavior (Lazarus, 2003).
In conclusion, individual foraging in group may reduce predation risk and improve foraging benefits including finding of a better foraging place. Effects of predators and other factors, such as habitat structure, group size, age, sex, and interference competition (Beauchamp, 1998; Lima et al., 1999), may affect vigilance activities to form a complex vigilance pattern of the Red Collared Doves, but they remain to be studied in the future.
ACKNOWLEDGEMENTS
We thank Dr. Su-Ju Lai for critical reading of the manuscript and Mr. Chun-Hui Lee for assistance in the field work. We are also thankful to Mr. Yaw-Shyang Chen and Mr. Chin-Tai Tsai in Taiwan Sugar Corporation for their cooperation. We thank referees for providing helpful comments and suggestions on the manuscript. This study was partly supported by Forestry Bureau (99-FD-08.2-C-2), Council of Agriculture, Taiwan.
LITERATURE CITED
Arenz, C. L. and D. W. Leger. 1997. Artificial visual obstruction, antipredator vigilance, and predator detection in the thirteen-lined squirrel (Spermophilus
tridecemlineatus). Behaviour 134: 1101-1114.
Barbosa, A. 2002. Does vigilance always covary negatively with group size? Effects of foraging strategy. Acta Ethologica 5: 51-55.
Barnard, C. J. 1980. Factors affecting flock size mean and variance in a winter population of house sparrows (Passer
domesticus L.). Behaviour 74: 114-127.
Barta, Z., A. Liker and F. Mónus. 2004. The effects of predation risk on the use of social foraging tactics. Anim. Behav. 67: 301-308.
Beauchamp, G. 1998. The effect of group size on mean food intake rate in birds. Biol. Rev. 73: 449-472.
Beauchamp, G. 2008. What is the magnitude of the group-size effect on vigilance? Behav. Eco. 19: 1361-1368.
Bednekoff, P. A. and S. L. Lima. 1998. Re-examining safety in numbers: interactions between risk dilution and collective detection depend upon predator targeting behaviour. P. Roy. Soc. Lond. B. Bio. 265: 2021-2026. Bednekoff, P. A. and S. L. Lima. 2005. Testing for peripheral
vigilance: do birds value what they see when not overtly vigilant? Anim. Behavi. 69: 1165-1171.
Butler, S. J., M. J. Whittingham, J. L. Quinn and W. Cresswell. 2005. Quantifying the interaction between food density and habitat structure in determining patch selection. Anim. Behavi. 69: 337-343.
Caro, T. M. 2005. Antipredator defenses in birds and mammals. University of Chicago Press, Chicago, USA. 592pp.
Carro, M. E. and G. J. Fernández. 2009. Scanning pattern of greater rheas, Rhea americana: collective vigilance would increase the probability of detecting a predator. J. Etho. 27: 429-436.
Carrascal, L. M., J. C. Alonso and J. A. Alonso. 1990. Aggregation size and foraging behaviour of white stork
Ciconia cinconia during the breeding season. Ardea 78:
399-404.
Catterall, C. P., M. A. Elgar and J. Kikkawa. 1992. Vigilance does not covary with group size in an island population of sivereyes (Zosterops lateralis). Behav. Ecol. 3: 207-210.
Dehn, M. M. 1990. Vigilance for predators: detection and dilution effects. Behav. Ecol. Sociobiol. 26: 337-342. Devereux, C. L., C. U. McKeever, T. G. Benton and M. J.
Whittingham. 2004. The effect of sward height and drainage on common starlings Sturnus vulgaris and northern lapwings Vanellus vanellus foraging in grassland habitats. Ibis 146: 115-122.
Devereux, C. L., M. J. Whittingham, E. Fernández-Juricic, J. A. Vickery and J. R. Krebs. 2005. Predation detection and avoidance by starlings under differing scenarios of predation risk. Behav. Ecol. 17: 303-309.
Dias, R. I. 2006. Effects of position and flock size on vigilance and foraging behaviour of the scaled dove Columbina
aquammata. Behav. Process. 73: 248-252.
Dolman, C. S., J. Templeton and L. Lefebvre. 1996. Mode of foraging competition is related to tutor preference in Zenaida
aurita. J. Comp. Psychol. 110: 45-54.
Elgar, M. A. 1989. Predator vigilance and group size in mammals and birds: a critical review of the empirical evidence. Biol. Rev. 64: 13-33.
Fernández, G. J., A. F. Capurro and J. C. Reboreda. 2003. Effect of group size on individual and collective vigilance in greater rheas. Ethology 109: 413-425.
Fernández-Juricic, E., G. Beauchamp and B. Bastain. 2007. Group-size and distance-to- neighbour effects on feeding
and vigilance in brown-headed cowbirds. Anim. Behav. 73: 771-778.
Ferriere, R., B. Cazelles, F. Cezilly and J. P. Desportes. 1996. Predictability and chaos in bird vigilant behaviour. Anim. Behav. 52: 457-472.
Guillemain, M., P. Duncan and H. Fritz. 2001. Switching to a feeding method that obstructs vision increases head-up vigilance in dabbling ducks. J. Avian Biol. 32: 345-350. Jones, K. A., J. R. Kreb and M. J. Whittingham. 2006.
Interaction between seed crypsis and habitat structure influence patch choice in a granivorous bird, the chaffinch
Fringilla coelebs. J. Avian Biol. 37: 413-418.
Lazarus, L. 2003. Vigilance and group size: early studies, the edge effect, secondary defences, the double advantage trade-off and the future. Behav. Process. 63: 129-131. Li, Z., Z. Jiang and G. Beauchamp. 2009. Vigilance in
Przewalski’s gazelle: effects of sex, predation risk and group size. J. Zool. 277: 302-308.
Lima, S. L. 1987. Distance to cover, visual obstructions, and vigilance in house sparrows. Behaviour 102: 231-238. Lima, S. L. 1995. Collective detection of predatory attack by
social foragers: fraught with ambiguity? Anim. Behav. 50: 1097-1108.
Lima, S. L. and P. A. Bednekoff. 1999. Back to the basics of antipredatory vigilance: can nonvigilant animal detect attack? Anim. Behav. 58: 537-543.
Lima, S. L. and L. M. Dill. 1990. Behavioral decisions made under the risk of predation: a review and prospectus. Can. J. Zool. 68: 619-640.
Lima, S. L., P. A. Zollner and P. A. Bednekoff. 1999. Predation, scramble competition, and the vigilance group size effect in dark-eyed juncos (Junco hyemalis). Behav. Ecol. Sociobiol. 46: 110-116.
Martin, P. and P. Bateson. 2007. Measuring behaviour. Cambridge University Press, New York, USA. 176pp. Metcalfe, N. B. 1984. The effects of habitat on the vigilance of
shorebirds: is visibility important? Anim. Behav. 32: 981-985.
Phelan, J. P. 1987. Some components of flocking behavior in the rock dove (Columba livia). J. Field Ornithol. 58: 135-143.
Powell, G. V. N. 1974. Experimental analysis of the social value of flocking by starlings (Sturnus vulgaris) in relation to predation and foraging. Anim. Behav. 22: 501-505. Pulliam, H. R. 1973. On the advantages of flocking. J. Theor.
Biol. 38: 419-422.
Reboreda, J. C. and G. J. Fernandez. 1997. Sexual, seasonal and group size difference in the allocation of time between vigilance and feeding in the greater rhea, Rhea americana. Ethology 103: 198-207.
Robert, G. 1996. Why individual vigilance declines as group size increase. Anim. Behav. 51: 1077-1086.
Sadedin, S. R. and M. A. Elgar. 1998. The influence of flock size and geometry on the scanning behaviour of spotted turtle doves, Streptopelia chinensis. Aust. J. Ecol. 23: 177-180.
Slotow, R. and S. I. Rothstein. 1995. Influence of social status, distance from cover, and group size on feeding and vigilance in white-crowned sparrows. Auk 112: 1024-1031.
effects of dominance and sociality. Auk 104: 429-434. Whittingham, M. J., S. J. Butler, J. L. Quinn and W.
Creewell. 2004. The effect of limited visibility on vigilance behaviour and speed of predator detection: implications for the conservation of granivorous passerines. Oikos 106: 377-385.
Whittingham, M. J. and K. L. Evans. 2004. The effects of habitat structure on predation risk of birds in agriculture landscapes. Ibis 146: 210-220.
Whittingham, M. J. and H. M. Markland. 2002. The influence of substrate on the functional response of an avian granivore and its implications for farmland bird conservation. Oecologia 130: 637-644.
棲地類型與群體規模對紅鳩之覓食與警戒行為的影響
李姮蒨(1)、丁宗蘇(2)、蔡住發(3)、許富雄(1*) 1.國立嘉義大學生物資源學系。600 嘉義市鹿寮里學府路 300 號,台灣。 2.國立台灣大學森林環境暨資源學系。106 台北市羅斯福路四段 1 號,台灣。 3.特有生物研究保育中心。552 南投縣集集鎮民生東路 1 號,台灣。*通訊作者。Tel: 886-5-2717828;Email: richbear@mail.ncyu.edu.tw