Incidence of End Stage Renal Disease among Type 1 Diabetes: A
Nationwide
Cohort Study in Taiwan
Wei-Hung Lin, MD, Chung-Yi Li, PhD, Wei-Ming Wang, MSc , Deng-Chi Yang, MD, Te-Hui Kuo, MD, Ming-Cheng Wang, MD
From the Institute of Clinical Medicine, College of Medicine, National Cheng Kung University(WHL), Division of Nephrology, Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University (WHL, DCY, THK, MCW), Department and Graduate Institute of Public Health, College of Medicine, National Cheng Kung University (CYL, THK), Biostatistics Consulting Center, National Cheng Kung University Hospital, College of Medicine (WMW), Institute of Gerontology, College of Medicine, National Cheng Kung
University (DCY), Institute of Clinical Pharmacy and Pharmaceutical Sciences, College of Medicine, National Cheng Kung University (MCW) Tainan; and Department of Public Health, College of Public Health, China Medical University (CYL) Taichung, Taiwan
WHL and CYL contribute equally to this work. Requests for reprints should be addressed to: Ming-Cheng Wang, MD (corresponding author)
Mailing address: Division of Nephrology, Department of Internal Medicine, National Cheng Kung University Hospital, 138 Sheng-Li Road, 70428 Tainan, Taiwan. Phone: 886-6-2353535 ext. 2594. Fax: 886-6-3028036.
*E-mail: wangmc@mail.ncku.edu.tw Running title:
Type of manuscript: Research article
Declaration of interest None.
ABSTRACT
Our study aims to estimate the risk of end-stage renal disease (ESRD) in type 1 diabetes mellitus (T1DM) in Taiwanese people, and to assess how age and year at registration of T1DM and sex may affect the risk.
A nationwide cohort study of 7203 Taiwanese with T1DM registered in 1999–2010 was followed up until ESRD, death, or end of follow-up on December 31, 2010. Annual age, sex, and calendar year specific incidence rates of ESRD of general population were used to calculate the standardized incidence ratio (SIR) of ESRD in relation to T1DM.
The SIR of ESRD for male and female patients with T1DM was significantly increased at 25.85 (95% CI 23.40-28.29) and 28.08 (95% 25.45-30.71), respectively; and peaked at age 15-29 years for both genders. The cumulative incidence of ESRD was similar in male and female patients, but was significantly higher in age 30 years than in that <30 years (10.25% v.s. 3.57%, P < 0.001). The patients aged <15 years had a significantly lower risk of ESRD, as compared to those aged 15-29 years but patients aged 30-44 (adjusted HR, 1.491) and 45-60 years (adjusted HR, 2.111) showed significantly increased hazards. Our data also demonstrated a lower risk of ESRD in patients registered in later years than in earlier years.
Risk of ESRD is substantially increased in T1DM in ethnic Chinese population. The continuously declining risk of ESRD in T1DM might suggest advancement of multidisciplinary chronic kidney disease care system in Taiwan.
Key words: Epidemiology, End stage renal disease, Population-based, Type 1 diabetes, standardized incidence ratio
Abbreviations: ESRD = end-stage renal disease, T1DM = type 1 diabetes mellitus, SIR = standardized incidence ratio, NHI = National Health Insurance, NHIRD = National Health Insurance Research Database, CICs = catastrophic illness certificates, HR = hazard ratio, CKD = chronic kidney disease
Introduction
Type 1 diabetes mellitus (T1DM) is identified to be associated with a higher age-adjusted mortality risk of death compared with the general population.1,2T1DM can
cause hyperglycemia, which is linked to several acute (e.g., diabetic ketoacidosis) and chronic (e.g., diabetic nephropathy and cardiovascular disease) complications.3 T1DM
has been recognized as an important etiology of diabetic nephropathy which is one of the most overwhelming complications and major predictors of premature death.4
Ethnic differences are likely to cause different incidence rates of diabetes and
accompanying vascular complications.5,6 It has been well known that the incidence of
T1DM is greater among Caucasoid populations than among Mongoloids, Blacks and Chinese, and such ethnic diversity tends to show the importance of differential genetic susceptibility among populations.7
The count of new cases of T1DM in European children less than 5 years is predicted to double between 2005 and 2020, and the prevalent cases less than 15 year will increase by 70%8. Our previous study also found a significant increasing trend for
T1DM in males and female aged <15 years.7 Moreover, Taiwan has been a country
with a very high prevalence of end stage renal disease (ESRD), and diabetes is believed to be the leading cause.9 Particularly, subjects with a low socio-economic
and educational status have a lower awareness of chronic kidney disease (CKD)10 and
reflected the difficulty and importance of early diagnosis. Although the cumulative incidence of ESRD ranged from 4% to 17% at 20 years after T1DM diagnosis4,11,12
noted in different populations, very few studies on ethnic Chinese population have ever been reported. It is well-known that African-American patients and Asian (Taiwanese and Japanese) people have greater risk than Caucasians for developing ESRD in all cohorts.9,13Asians comprise a large portion of non-Black groups in the
U.S. and other developed countries, but there is limited data on nationwide study focusing on ESRD from T1DM in Asian populations.
Using a large population-based cohort with T1DM in Taiwan registered between 1999 and 2010, we reported the long-term mortality trends in patients with T1DM of all ages.7 We now further investigate the long-term risk of ESRD in patients with
T1DM and examine how age at registration of diabetes, time period of registration, and sex affect these risks.
Research Design and Methods Data Sources
The National Health Insurance (NHI) program was launched in Taiwan on 1 March 1995. National Health Insurance Research Database (NHIRD), a large scale
computerized database supervised by Bureau of NHI, Department of Health and maintained by the National Health Research Institutes, is provided to local scientists in Taiwan for research purposes. The NHIRD includes all inpatient and ambulatory medical claims for about 99% of Taiwanese people and information attained from the NHIRD is considered to be complete and accurate.14 Data of NHIRD that can be used
to identify patients or care providers, including medical institutions and physicians, are scrambled before being sent to the National Health Research Institutes for database construction. Data are further scrambled before being released to each researcher. Therefore, individual patient or health care provider cannot be identified from the database.
In Taiwan, the certification of various catastrophic illnesses is subject to evaluation and review by the Bureau of NHI. Patients with catastrophic illness certificates (CICs) are eligible for exemption from insurance premiums and co-payments. Therefore, the CICs data are highly accurate and reliable.15 The CICs will be terminated once the
patients died. Access of this study to the NHIRD has been approved by the Review Committee of the NHRI, and conduct of this study was approved by the Institutional Review Board of National Cheng Kung University Hospital.
Identification of Newly-registered Type 1 Diabetes Mellitus and End Stage Renal Disease
We searched the Taiwan NHIRD for the T1DM population from 1 January 1999 to 31 December 2010 in our nationwide cohort study. (as per the International
Classification of Diseases, 9th Revision, Clinical Modification, ICD-9-CM code 250). We considered the first ever registered CICs for T1DM and ESRD as the patients with newly-registered T1DM or newly-diagnosed ESRD, respectively, and excluded all CICs registered before 1999. T1DM and ESRD are recognized as a catastrophic illness by the Taiwan NHI. Due to the fact that no co-payment is required for
admission, emergency, and outpatient services, this certification is only applied when detailed and specific clinical data are met. This CICs registration is a valid and reliable source of data for the T1DM and ESRD retrieval.15 The term "diagnosed" in
our article means that the determination of which disease or condition is causing a person's signs and symptoms by a medical examination. The term "registered" in our article means that the diagnosed patients with T1DM or ESRD can apply for
catastrophic illness registration cards from the Bureau of National Health Insurance (BNHI) and do not need to make co-payments when seeking health care for
catastrophic illness. The patients with ESRD needing regular dialysis must apply for CIC when they are newly-diagnosed of ESRD due to the huge cost in our medical system. The patients with T1DM may have a time gap between "diagnosed" and "registered" due to minor condition or slowly progressive disease.
We also validated the ICD-9-CM code for the identification of T1DM by analyzing the chart records as previously described.7 In brief, T1DM was ascertained by the
patients who had 3 or more outpatient diabetes diagnoses with insulin prescriptions, and a history of diabetic ketoacidosis, a positive glucagon test, or the presence of glutamic-acid-decarboxylase (GAD) antibodies.16 Among the randomly selected 60
patients coded with T1DM in National Cheng Kung University Hospital, 59 were confirmed by chart review, yielding a positive predictive value of 98.3%.
Statistical Analysis
Patients were followed up from registration of T1DM to the occurrence of ESRD, death, or end of follow-up on December 31, 2010.We first calculated bi-annual incidence rates of ESRD (cases per 1,000 inhabitants) in patients with T1DM according to sex and age (< 15, 15–29, 30–44, 45-59 and 60 years). The bi-annual incident rate of ESRD among T1DM was calculated by dividing the number of incident ESRD cases by the averaged mid-year population of T1DM for every two years. To examine the secular trend of ESRD incidence rate across the study period, we treated the calendar year as a continuous variable and testing the statistical significance of the regression coefficient derived from the multivariable Poisson regression model that simultaneously included age, sex, and calendar year in the model. We also employed the 2000 WHO standard population as the reference to calculate the age- and sex-standardized incidence rate of overall ESRD bi-annually. To estimate the relative risk, indicated by the age, sex, and calendar year (1999-2010) standardized incidence ratio (SIR) of ESRD in patients with T1DM, we compared the risk of ESRD in T1DM with that of the Taiwan’s general population with the same age and sex distributions retrieved from Department of Statistics, Ministry of the Interior, Executive Yuan, Taiwan. The 95% confidence intervals (CIs) of SIR were estimated using the Poisson distribution. Kaplan-Meier estimates of ESRD were plotted, and differences between the two groups were examined by the log-rank test. Cumulative incidence of ESRD was also calculated by the Kaplan-Meier method. Patients were censored on December 31, 2010.
We performed multiple proportional sub-distribution hazards regression model that takes death into account as a competing risk event to estimate the adjusted hazard ratios (HRs) of ESRD associated with sex, age at registration, and time period of
registration. The sub-HR of competing risk regression was computed using the “stcrreg” command in STATA 12.0 (StataCorp LP, College Station, TX), which is based on Fine and Gray’s proportional subhazards model.17 Data analysis was
conducted using professional statistical packages, SPSS for Windows, version 17.0 (SPSS, Inc., Chicago, IL), and STATA for Windows, version 12.0. A two-tailed P value below 0.05 was considered statistically significant.
Results
Incidence of End Stage Renal Disease among Patients with Type 1 Diabetes Mellitus
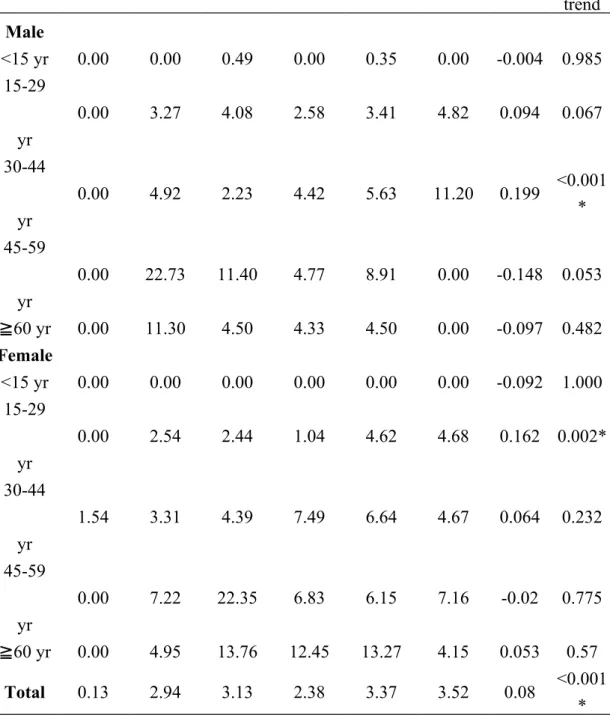
Table 1 shows the bi-annual overall as well as age- and sex-specific incidence rates of ESRD among registered T1DM throughout 1999 to 2010. The standardized bi-annual overall incidence rate rose from 1999-00 (0.13 per 1,000) to 2003-04 (3.13 per 1,000) and then mildly declined thereafter rose to 2009-10 (3.52 per 1,000). Over the study period, the β value was 0.08 with a significant secular trend (P < 0.001). Regardless of gender, the higher age-specific ESRD incidence rate was noted in the older groups (30 years) and especially highest at 45-59 years. Additionally, the incidence rate was zero in female subjects aged <15 years during 1999 to 2010. The mostly notable increase in age specific incidence rate for male and female subjects was 30-44 years (β = 0.199) and 15-29 years (β = 0.162), respectively. (see Table 1, Supplemental Content, which illustrates annual sex- and age-specific prevalent case number of type 1 diabetes in Taiwan, 1999-2010.) (see Table 2, Supplemental Content, which illustrates annual sex- and age-specific incident case of end stage renal disease among type 1 diabetes in Taiwan, 1999-2010.)
Standardized Incidence Ratio of End Stage Renal Disease among Patients with Type 1 Diabetes Mellitus
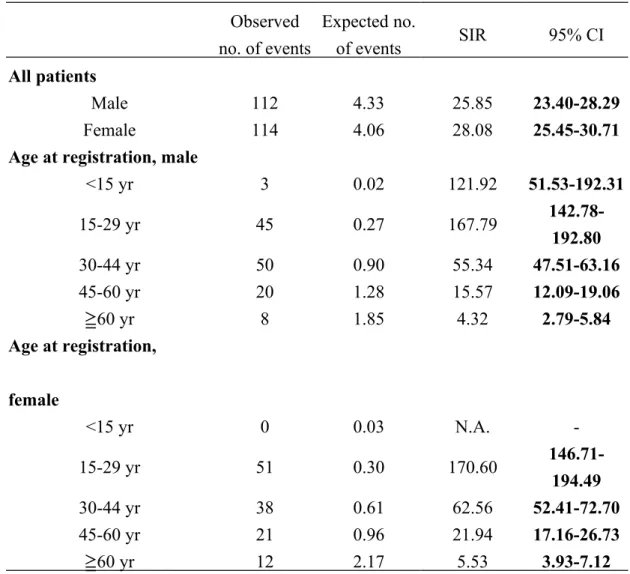
The sex-specific ESRD SIR for male and female patients with registered T1DM was substantially and significantly increased at 25.85 (95% CI 23.40-28.29) and 28.08 (95% 25.45-30.71), respectively(Table 2). Among the age stratifications, the SIR peaked for patients whose age at registration was 15-29 years (males:167.79 ( 95% CI 142.78-192.80); females:170.60 ( 95% CI 146.71-194.49)). In fact, the SIRs were
significantly increased at all age stratifications for both genders, and the SIR tended to decrease with advancing age, except male patients aged <15 years.
Adjusted Hazard Ratios and Cumulative Incidence of End Stage Renal Disease after Adjustment for Competing Risk of Death among Patients with Type 1 Diabetes Mellitus
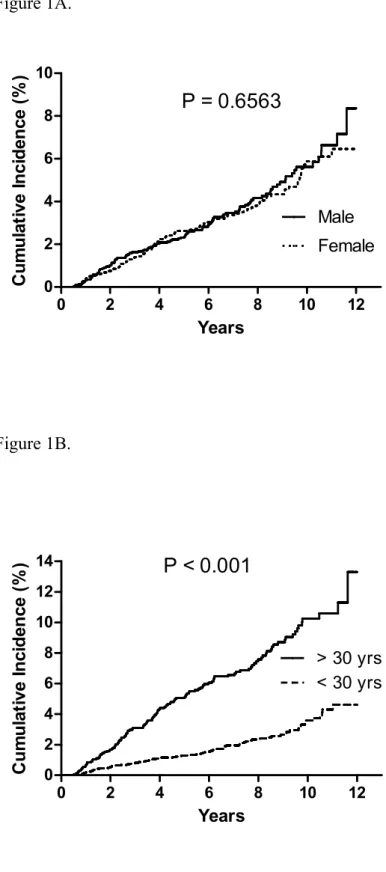
The 10-year cumulative incidence of ESRD among patients with registered T1DM in Taiwan was 5.62% in male and 5.88%, but such difference was statistically
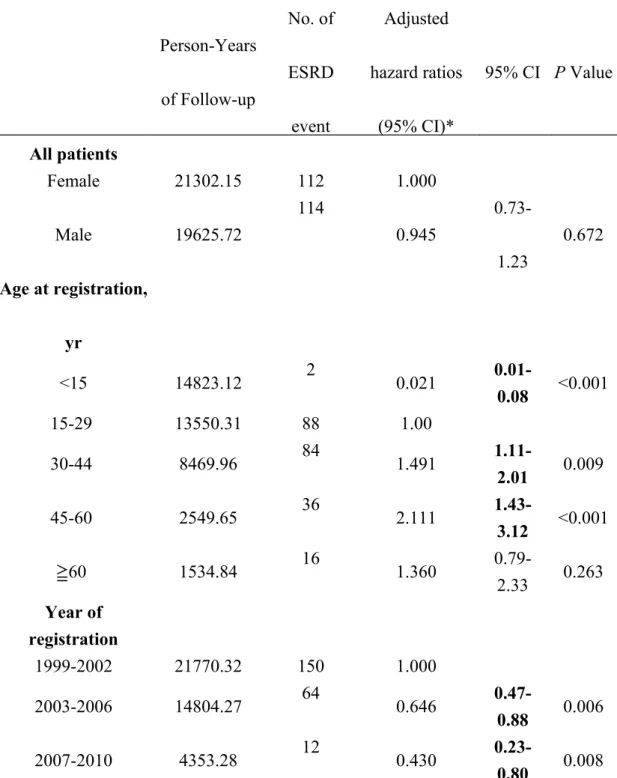
insignificant (P = 0.6563) (Figure 1A). However, the 10-year cumulative incidence was significantly higher in age at registration 30 years when compared with <30 years (10.25% v.s. 3.57%, P < 0.0001) (Figure 1B). The multivariate analysis suggested that age at registration and time period of registration were significantly associated with the risk of developing ESRD, whereas sex was not (Table 3.) Patients aged <15 years had a significantly lower risk than patients in other age groups; and the risk of ESRD increased with age up to 60 years old. Compared to those with T1DM registered in 1999-2002, patients with T1DM who registered in 2003-2006 (adjusted HR, 0.646) and 2007-2010 (adjusted HR, 0.430) experienced significantly reduced risk of ESRD, suggesting a continuous improvement in prognosis of patients with T1DM in Taiwan.
Discussion
This is believed to be the largest population-based T1DM cohort study focusing on ESRD incidence with a follow-up period up to 12 years in ethnic Chinese population. We noticed that the incidence rates of ESRD among patients with T1DM increased significantly throughout 1999 to 2010, with the higher age-specific ESRD incidence rate noted in the age at registration 30 years and highest at 45-59 years. The SIRs of ESRD for male and female patients were both significantly increased and peaked in those with age at registration of 15-29 years in both genders. The cumulative incidence of ESRD among patients with T1DM in Taiwan was similar in both
genders, but was significantly higher in patients registered at 30 years in comparison with those aged <30 years. We also noted that both age at registration and time period of registration were associated with the risk of developing ESRD suggesting a
continuously improved care of T1DM in Taiwan.
Our study included more than 7,000 patients with T1DM, of whom 226 cases developed ESRD, and the study is, to date, the largest study estimating risk of ESRD in Asian societies. One Finnish population-based study, which was the largest trial so far, ever estimated cumulative risk of ESRD in patients with T1DM. In Finland, T1DM accounted for one third of diabetic ESRD cases.18 The Finnish study found that
the cumulative incidence among patients with T1DM for development of ESRD within 30 years was approximately 7.8% and in patients whose diagnosis of diabetes occurred before age 5 years, the risk was found to be significantly lower. The risk of ESRD was also lower for patients whose diagnosis occurred in more recent years.19
Noticeably, the study population stood for a country with a fairly homogeneous health care system and almost all patients are white. With different ethnic groups and health
care systems, our study found similar conclusions that patients with registered age under 15 years had a lower risk for ESRD and there was a continuously improved renal outcome over time. This may have resulted from the success of advanced CKD care system under nephrologists-based multidisciplinary care20 leading to the decrease
of ESRD incidence.9 The trend of ESRD cases caused by T1DM worldwide toward an
older age at onset is clearly shown in the Joslin cohort.21 They found that the majority
of ESRD occurred between ages 36 and 52 years with durations of diabetes of 21 to 37 years. Besides, the ESRD incidence rate declined slightly in ages between 20 to 39 years, but almost doubled for ages 40 to 49 years. We found a higher ESRD risk between 30-60 years, especially highest during 45-60 years and supported the previous finding.
Male sex was a traditional risk factor for ESRD incidence in T1DM from earlier reports.4,5 Although the presence of diabetes also reduces the protection in women, the
development of kidney disease in patients with T1DM has still been thought to be more prevalent in men. This male dominance seemed to shift toward a balance in the proportion of incident cases of ESRD in patients with T1DM in recent studies.19,22 The
higher risk of ESRD in men noted in the earlier cohort was possibly due to discrepancy in HbA1c levels, hypertension, and lipid levels between males and females and the improved management of these risk factors might explain the absence of the male predominance in cases of kidney disease in recent cohort studies
conducted in Western societies.22 Our study showed similar hazard ratios of ESRD for
both genders in patients with T1DM, but indeed a slightly higher SIR noted in female patients.
progression and outcomes on ESRD. Ethnic differences in CKD progression are not well characterized but are of interest across and within countries. Most of the literature compared African-American outcomes to Caucasians in U.S. and found African-American have a significantly higher ESRD incidence, suggesting a faster rate of progression through CKD.23,24 Besides, African-American developed diabetic
ESRD resulting from T2DM at rates far exceeding Caucasians.25 Compared to
Caucasians, American Asians had a higher incidence of ESRD26and had faster CKD
progression.27 Among diabetic ESRD, Asians also had significantly higher incidence
of ESRD than Caucasians. Although types of diabetes were not mentioned or defined in these studies, the results mostly represent those of patients with T2DM.28 There was
higher incidence of microalbuminuria and faster progression of renal failure from a cohort study in South-Asian and Dutch-European patients with T2DM. This
discrepancy could be explained by either a higher incidence of nephropathy in the Asian diabetic patients and/or faster progression to ESRD.29 The racial difference
(excess incidence of ESRD treatment among non-White North Americans) is
particularly striking for presumed nephrosclerosis from nephropathy from T2DM, but is not yet clarified for ESRD attributed to T1DM. There might be some differences between ethnic groups including genetics, social and environmental factors (such as diet,exercise, alcohol, smoking and other exposures).27It is difficult to understand the
impact of these environmental factors on the outcome due to the lack of detailed information in our database, but we believe our study will largely contribute for the ethnic comparison in type 1 diabetic ESRD.
Because death occurring before ESRD is a potential competing risk, the reduction in the risk of ESRD in the patients with T1DM especially in older patients could have been an artifact of earlier deaths before ESRD including various causes of premature
mortality such as cardiovascular disease or complications of diabetes. Individuals with worst renal function are more likely to develop ESRD than to encounter
mortality, whereas those with more preserved renal function were more likely to die than to reach ESRD.30 In this study, we performed the competing risk regression
analysis to account for the above-mentioned potential bias arising competing risk problem. Our data presented relative risk estimates of ESRD associated with sex, age, and year of registration in T1DM based on the nationwide database. These data are practical for advising individualized patients about the appropriate targets and strategies for preventing premature mortality and ESRD.
The strengths of our study included its nationwide population-based cohort design, relatively long duration of follow-up (up to 12 years), and the large number of incident cases of T1DM (>7,000), which allowed us to calculate the incidence and mortality of ESRD according to various sex and age stratifications. In addition, the study used the registration system of catastrophic diseases in Taiwan, which provides reassurance of validity of disease diagnosis. Besides, based on the universal coverage system, all patients with T1DM were enrolled in the study and a nationally
representative sample can be assumed in our study. Findings from our study may provide the updated information on ESRD incidence of T1DM in Asian populations. Despite the above strengths, some limitations involved in our study should be
considered. First, the claim data do not cover laboratory data and clinical details specific to T1DM, which made it difficult to determine the severity of T1DM and predicting factor for ESRD. Second, there is no information on the true underlying cause of ESRD in the NHI claims, thus, we were unable to estimate the cause-specific risk of ESRD in T1DM. Third, we included only patients with a CIC for T1DM; some
in the cohort. Finally, it is based on prevalence cohort instead of incidence cohort and the patients were not followed up from the time of diagnosis; therefore, the
cumulative risk of ESRD after diagnosis of T1DM could not be estimated concisely.
Conclusion
We found that patients with T1DM were at a substantially increased risk of ESRD compared with the general population in Taiwan. Such ethnic information regarding risks for progression to ESRD will serve to focus ongoing and future research efforts. Although there is continuously improved renal outcome over time, the slightly higher SIR in female patients, especially the young group, recommends more aggressive risk factor management to avoid the onset of ESRD in these high-risk segments of
population of Taiwan.
ACKNOWLEDGMENTS
Data from the National Health Insurance Research Database was provided by the Taiwan Bureau of National Health Insurance, Department of Health. The
interpretation and conclusions contained herein do not represent those of Bureau of National Health Insurance, Department of Health or National Health Research Institutes.
References
1. Asao K, Sarti C, Forsen T, et al. Long-term mortality in nationwide cohorts of childhood-onset type 1 diabetes in Japan and Finland. Diabetes Care.
2003;26:2037-2042.
2. Nishimura R, LaPorte RE, Dorman JS, Tajima N, Becker D, Orchard TJ. Mortality trends in type 1 diabetes. The Allegheny County (Pennsylvania) Registry 1965-1999. Diabetes care. 2001;24:823-827.
3. Shankar A, Klein R, Klein BE, Moss SE. Association between glycosylated hemoglobin level and cardiovascular and all-cause mortality in type 1 diabetes. Am J Epidemiol. 2007;166:393-402.
4. Krolewski AS, Warram JH, Christlieb AR, Busick EJ, Kahn CR. The changing natural history of nephropathy in type I diabetes. Am J Med. 1985;78:785-794. 5. Cowie CC, Port FK, Wolfe RA, Savage PJ, Moll PP, Hawthorne VM. Disparities
in incidence of diabetic end-stage renal disease according to race and type of diabetes. N Engl J Med. 1989;321:1074-1079.
6. Stephens GW, Gillaspy JA, Clyne D, Mejia A, Pollak VE. Racial differences in the incidence of end-stage renal disease in types I and II diabetes mellitus. Am J Kidney Dis. 1990;15:562-567.
7. Lin WH, Wang MC, Wang WM, et al. Incidence of and Mortality from Type I Diabetes in Taiwan From 1999 through 2010: A Nationwide Cohort Study. PloS one. 2014;9:e86172.
8. Patterson CC, Gyurus E, Rosenbauer J, et al. Trends in childhood type 1 diabetes incidence in Europe during 1989-2008: evidence of non-uniformity over time in rates of increase. Diabetologia. 2012;55:2142-2147.
Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD.
10. Wen CP, Cheng TY, Tsai MK, et al. All-cause mortality attributable to chronic kidney disease: a prospective cohort study based on 462 293 adults in Taiwan. Lancet. 2008;371:2173-2182.
11. Matsushima M, Tajima N, LaPorte RE, et al. Markedly increased renal disease mortality and incidence of renal replacement therapy among IDDM patients in Japan in contrast to Allegheny County, Pennsylvania, USA. Diabetes
Epidemiology Research International (DERI) U.S.-Japan Mortality Study Group. Diabetologia. 1995;38:236-243.
12. Krolewski M, Eggers PW, Warram JH. Magnitude of end-stage renal disease in IDDM: a 35 year follow-up study. Kidney Int. 1996;50:2041-2046.
13.Xue JL, Eggers PW, Agodoa LY, Foley RN, Collins AJ. Longitudinal study of racial and ethnic differences in developing end-stage renal disease among aged medicare beneficiaries. J Am Soc Nephrol. 2007; 18:1299-1306.
14. Sun Y, Chang YH, Chen HF, Su YH, Su HF, Li CY. Risk of Parkinson disease onset in patients with diabetes: a 9-year population-based cohort study with age and sex stratifications. Diabetes Care. 2012;35:1047-1049.
15. Lin WH, Guo CY, Wang WM, et al. Incidence of progression from newly diagnosed systemic lupus erythematosus to end stage renal disease and all-cause mortality: a nationwide cohort study in Taiwan. Int J Rheum Dis. 2013;16:747-753.
16. Lawrence JM, Black MH, Zhang JL, et al. Validation of pediatric diabetes case identification approaches for diagnosed cases by using information in the electronic health records of a large integrated managed health care organization. Am J Epidemiol. 2014;179:27-38.
17. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of competing risk. J Am Stat Assoc. 1999;94:496–509.
18. Finnish Registry for Kidney Diseases. Report 2012. Helsinki, Finland: Finnish Registry for Kidney Diseases; 2012. Available at:
http://www.musili.fi/smtr/english.
19. Finne P, Reunanen A, Stenman S, Groop PH, Gronhagen-Riska C. Incidence of end-stage renal disease in patients with type 1 diabetes. JAMA. 2005;294:1782-1787.
20. Chen YR, Yang Y, Wang SC, et al. Effectiveness of multidisciplinary care for chronic kidney disease in Taiwan: a 3-year prospective cohort study. Nephrol Dial Transplant. 2013;28:671-682.
21. Rosolowsky ET, Skupien J, Smiles AM, et al. Risk for ESRD in type 1 diabetes remains high despite renoprotection. J Am Soc Nephrol. 2011;22:545-553. 22. Costacou T, Fried L, Ellis D, Orchard TJ. Sex differences in the development of
kidney disease in individuals with type 1 diabetes mellitus: a contemporary analysis. Am J Kidney Dis. 2011;58:565-573.
23. Peralta CA, Shlipak MG, Fan D, et al. Risks for end-stage renal disease, cardiovascular events and death in Hispanic versus non-Hispanic white adults with chronic kidney disease. J Am Soc Nephrol. 2006;17:2892–2899.
24. Hsu CY, Lin F, Vittinghoff E, Shlipak MG. Racial differences in the progression from chronic renal insufficiency to end-stage renal disease in the United States. J Am Soc Nephrol. 2003;14:2902–2907.
25.Brancati FL, Whittle JC, Whelton PK, Seidler AJ, Klag MJ. The excess incidence of diabetic end-stage renal disease among blacks. A population-based study of potential explanatory factors. JAMA. 1992; 268:3079-3084.
conundrum of increased burden of end-stage renal disease in Asians. Kidney Int. 2005;68:2310–2316.
27.Barbour SJ, Er L, Djurdjev O, Karim M, Levin A. Differences in progression of CKD and mortality amongst Caucasian, Oriental Asian and South Asian CKD patients. Nephrol Dial Transplant. 2010; 25:3663-3672.
28.Karter AJ, Ferrara A, Liu JY, Moffet HH, Ackerson LM, Selby JV. Ethnic disparities in diabetic complications in an insured population. JAMA. 2002; 287:2519-2527.
29.Chandie Shaw PK, Baboe F, van Es LA, et al. South-Asian type 2 diabetic patients have higher incidence and faster progression of renal disease compared with Dutch-European diabetic patients. Diabetes Care. 2006; 29:1383-1385. 30. Forsblom C, Harjutsalo V, Thorn LM, et al. Competing-risk analysis of ESRD and
death among patients with type 1 diabetes and macroalbuminuria. J Am Soc Nephrol. 2011;22:537-544.
Table 1. Bi-annual sex- and age-specific incidence rate (per 1,000 inhabitants) of end
stage renal disease among type 1 diabetes in Taiwan, 1999-2010.
1999-00 2001-02 2003-04 2005-06 2007-08 2009-10 β P for trend Male <15 yr 0.00 0.00 0.49 0.00 0.35 0.00 -0.004 0.985 15-29 yr 0.00 3.27 4.08 2.58 3.41 4.82 0.094 0.067 30-44 yr 0.00 4.92 2.23 4.42 5.63 11.20 0.199 <0.001 * 45-59 yr 0.00 22.73 11.40 4.77 8.91 0.00 -0.148 0.053 ≧60 yr 0.00 11.30 4.50 4.33 4.50 0.00 -0.097 0.482 Female <15 yr 0.00 0.00 0.00 0.00 0.00 0.00 -0.092 1.000 15-29 yr 0.00 2.54 2.44 1.04 4.62 4.68 0.162 0.002* 30-44 yr 1.54 3.31 4.39 7.49 6.64 4.67 0.064 0.232 45-59 yr 0.00 7.22 22.35 6.83 6.15 7.16 -0.02 0.775 ≧60 yr 0.00 4.95 13.76 12.45 13.27 4.15 0.053 0.57 Total 0.13 2.94 3.13 2.38 3.37 3.52 0.08 <0.001 *
Table 2. Standardized incidence ratio of end stage renal disease among type 1 diabetes
in Taiwan according to sex and age at registration, 1999-2010.
Observed no. of events Expected no. of events SIR 95% CI All patients Male 112 4.33 25.85 23.40-28.29 Female 114 4.06 28.08 25.45-30.71
Age at registration, male
<15 yr 3 0.02 121.92 51.53-192.31 15-29 yr 45 0.27 167.79 142.78-192.80 30-44 yr 50 0.90 55.34 47.51-63.16 45-60 yr 20 1.28 15.57 12.09-19.06 60 yr ≧ 8 1.85 4.32 2.79-5.84 Age at registration, female <15 yr 0 0.03 N.A. -15-29 yr 51 0.30 170.60 146.71-194.49 30-44 yr 38 0.61 62.56 52.41-72.70 45-60 yr 21 0.96 21.94 17.16-26.73 60 yr ≧ 12 2.17 5.53 3.93-7.12
Table 3. Hazard ratios of end stage renal disease associated with sex, age, and year of
registration in type 1 diabetes, 1999-2010, in competing risk analysis.
Person-Years of Follow-up No. of ESRD event Adjusted hazard ratios (95% CI)* 95% CI P Value All patients Female 21302.15 112 1.000 Male 19625.72 114 0.945 0.73-1.23 0.672 Age at registration, yr <15 14823.12 2 0.021 0.01-0.08 <0.001 15-29 13550.31 88 1.00 30-44 8469.96 84 1.491 1.11-2.01 0.009 45-60 2549.65 36 2.111 1.43-3.12 <0.001 60 ≧ 1534.84 16 1.360 0.79-2.33 0.263 Year of registration 1999-2002 21770.32 150 1.000 2003-2006 14804.27 64 0.646 0.47-0.88 0.006 2007-2010 4353.28 12 0.430 0.23-0.80 0.008 Abbreviations: CI, confidence interval; SIR, standardized incidence rate.
Figure Legends:
Figure 1. Cumulative incidence of end stage renal disease among type 1 diabetes in
Taiwan, 1999-2010. A. According to patient’s gender B. According to age at
Figure 1A. 0 2 4 6 8 10 12 0 2 4 6 8 10 Male Female