Elsevier Editorial System(tm) for Immunobiology
Manuscript Draft
Manuscript Number: IMBIO-D-11-00010R1
Title: Kaempferol identified from Semen Cuscutae attenuates the immune function of dendritic cells
Article Type: Research Paper
Keywords: dendritic cell; immunomodulation; Th1 response; antigen presentation; flavonoid
Corresponding Author: Dr. Ching-Liang Chu, Ph.D.
Corresponding Author's Institution: Yeastern Biotech Co.
First Author: Ming-Kuem Lin
Order of Authors: Ming-Kuem Lin; Yen-Ling Yu; Kai-Chieh Chen; Wen-Te Chang; Meng-Chiou Lee;
Meng-Ja Yang; Keng-Liang Ou; Jody Chen; Ching-Liang Chu, Ph.D.
Abstract: Dendritic cells (DCs) are the critical leukocytes in regulating immune responses. Accordingly,
DCs are the major target in the development of immunomodulators. In this study, we examined the
effect of Semen Cuscutae (SC), an important traditional Chinese medicine, on mouse bone
marrow-derived DCs. We found that the n-butanol and methanol extracts of SC significantly suppressed
LPS-stimulated DC activation. Several flavonoids were verified in the extracts using HPLC, and then
kaempferol was identified as the major flavonoid in the methanol fraction of SC. Kaempferol was able
to reduce cytokines and chemokines produced by LPS-stimulated DCs, and this reduction was not due
to its cytotoxicity on DCs. In addition, DC maturation was impaired by kaempferol. Furthermore,
kaempferol abrogated the ability of LPS-stimulated DCs to promote Ag-specific T cell activation, both in
vitro and in vivo. Thus, we show for the first time that SC exhibits an immunosuppressive effect on DCs
and that the active ingredient kaempferol attenuates DC function, which suggests that kaempferol has
potential in the treatment of chronic inflammatory and autoimmune diseases.
Dear editors:
I would like to thank you for your positive decision about our manuscript IMBIO-D-11-00010
“Kaempferol identified from Semen Cuscutae attenuates the immune function of dendritic cells”
for publication in the Immunobiology.
In addition, we have revised the manuscript as reviewer’s suggestions and used Word edit
system. The answers are listed below:
1. Protocol for preparation of DCs has to be provided, since this protocol is highly important for
the paper.
Reply 1: We have added the brief protocol for DC preparation in revised manuscript (page 5).
Thank reviewer’s reminding.
2. Figure 4. It is stated in the figure legend that all experiments are representative of two to four
independent experiments. It is necessary that all experiments are repeated at least 3 times.
Reply 2: The results are representative of three (Fig. 4A), two (Fig. 4B), and four (Fig. 4C)
independent experiments. So, we did the microscopy for Fig. 4B one more time and then got a
similar result. Thus, we modified the description into “All results are representative of three to
four independent experiments” (page 20).
3. In discussion the authors mention that the anti-inflammatory effects of kaempherol were
studied in various cell types (2nd paragraph of discussion). Since macrophages are closely
related to DCs it is important to compare effects of kaempherol on DC observed by the
authors with the effects of kaempherol on macrophages. It is published, for instance, that
kaempherol inhibits LPS induced production of inflammatory cytokines (TNF, IL-1 and
others) in macrophages. Some citations are 1) Harasstani et al Inflamm Res. 2010
Sep;59(9):711-21; 2) Fang et al Bioorg Med Chem. 2005 Apr 1;13(7):2381-8; 3) Kowalski et
al Pharmacol Rep. 2005 May-Jun;57(3):390-4
Reply 3: We have added this comparison and references in revised manuscript (2nd paragraph of
discussion, page 11). Thank reviewer’s comment.
We would like to thank reviewer and editors for the constructive suggestions. Please let me know
if you have any more question. Thank you again for your positive decision.
Best regards,
Ching-Liang Chu, Ph.D.
Kaempferol Identified from Semen Cuscutae Attenuates the Immune
Function of Dendritic Cells
Ming-Kuem Lin1,*, Yen-Ling Yu2, Kai-Chieh Chen2, Wen-Te Chang1, Meng-Chiou Lee1,
Meng-Ja Yang1, Keng-Liang Ou3, Jody D.C. Chen43, and Ching-Liang Chu2,43,*
1School of Chinese Pharmaceutical Sciences and Chinese Medicine Resources, China Medical
University, Taichung, Taiwan
2National Health Research Institutes, Miaoli County, Taiwan
3Research Center for Biomedical Implants and Microsurgery Devices, Taipei Medical University,
Taipei, Taiwan
34Yeastern Biotech Co., New Taipei City County, Taiwan
Running title: Kaempferol attenuates dendritic cell function
Keywords: antigen presentation, dendritic cell, flavonoid, immunomodulation, Th1 response
* Correspondence: Dr. Ching-Liang Chu, Yeastern Biotech Co., F6-3, No 23, Lane 169, Kang-Ning St., Shijr Area, New Taipei CityCounty 221, Taiwan. Tel: +886-911-506872; Fax: +886-2-26953979; E-mail: clchu@yeastern.com.tw or Dr. Ming-Kuem Lin, China Medical University, No.91 Hsueh-Shih Road, Taichung 40402, Taiwan. Tel: 22053366ext5212; Fax: +886-4-22078083; E-mail: linmk@mail.cmu.edu.tw
Formatted: Superscript
Formatted: Superscript Formatted: Font:
Abbreviations: SC, Semen Cuscutae; DC, dendritic cell; OVA, ovalbumin; IFA, incomplete Freund’s adjuvant; LN, lymph node
Abstract
Dendritic cells (DCs) are the critical leukocytes in regulating immune responses. Accordingly, DCs are the major target in the development of immunomodulators. In this study, we examined the effect of Semen Cuscutae (SC), an important traditional Chinese medicine, on mouse bone marrow-derived DCs. We found that the n-butanol and methanol extracts of SC significantly suppressed LPS-stimulated DC activation. Several flavonoids were verified in the extracts using HPLC, and then kaempferol was identified as the major flavonoid in the methanol fraction of SC. Kaempferol was able to reduce cytokines and chemokines produced by LPS-stimulated DCs, and this reduction was not due to its cytotoxicity on DCs. In addition, DC maturation was impaired by kaempferol. Furthermore, kaempferol abrogated the ability of LPS-stimulated DCs to promote Ag-specific T cell activation, both in vitro and in vivo. Thus, we show for the first time that SC exhibits an immunosuppressive effect on DCs and that the active ingredient kaempferol attenuates DC function, which suggests that kaempferol has potential in the treatment of chronic inflammatory and autoimmune diseases.
Introduction
Dendritic cells (DCs) are specialized bone marrow-derived leukocytes which are critical in the initiation of immune responses (Steinman, 2007). They capture antigens in peripheral tissues and then migrate to secondary lymphoid organs where they present processed antigens to T lymphocytes (Buckwalter and Albert, 2009). When pathogen-associated molecular patterns (PAMPs) are recognized by pattern recognition receptors (PRRs) on DCs, the DCs are activated and become mature (Joffre et al., 2009). Toll-like receptors (TLRs) are the major PRRs to detect PAMPs in DCs and TLR ligation induces the release of both cytokines and chemokines and up-regulates the expression of MHC class II and costimulatory molecules (Takeuchi and Akira, 2010). Thus, DCs have been applied in controlling cancer and infectious diseases (Palucka et al., 2010; Schuler, 2010). However, DCs are also involved in the pathogenesis of chronic inflammation and autoimmunity (Fransen et al., 2010). Substances such as natural products (Li and Vederas, 2009) that modulate the function of DCs can potentially be used in the treatment of immune disorders.
Semen Cuscutae (SC), the dry matured seed of Cuscuta chinensis, is an important Chinese medicine and has been included in a herbal formulation (Sohn et al., 2008). The total flavones of SC have been shown to invigorate the reproductive system and to improve defective kidneys in animal models (Qin et al., 2000; Yang et al., 2008). SC also induces neuronal differentiation (Jian-Hui et al., 2003), has hepatoprotective and antioxidant effects against acetaminophen-induced hepatotoxicity (Yen et al., 2007), and promotes osteoblast differentiation (Yang et al., 2009). In addition to these bioactivities, the ethanol extract of SC increases the ovalbumin (OVA)-specific splenocyte proliferation and antibody production in mice (Pan et al., 2005). Furthermore, polysaccharides isolated from SC enhance lymphocyte proliferation both in vitro
and in vivo (Bao et al., 2002; Wang et al., 2000). These reports suggest that SC has potential to modulate immune responses. Although a number of ingredients of SC have been found (Bao et al., 2002; Du et al., 1998; He et al., 2010; Li et al., 1999), the mechanism of and the components associated with any such immunomodulation by SC remain to be elucidated.
In this study, we examined the potential effect of SC on activation and function of mouse bone marrow-derived DC. Our results showed that n-butanol and methanol extracts of SC possessed the ability to inhibit LPS-induced DC activation. Subsequently, we identified that kaempferol was the major flavonoid in SC and contributed to this inhibitory effect. Thus, it is suggested that kaempferol may be a potent immunosuppressant and applied in reducing the harmful immune responses such as chronic inflammation and autoimmunity.
Materials and Methods
Preparation of SC samples
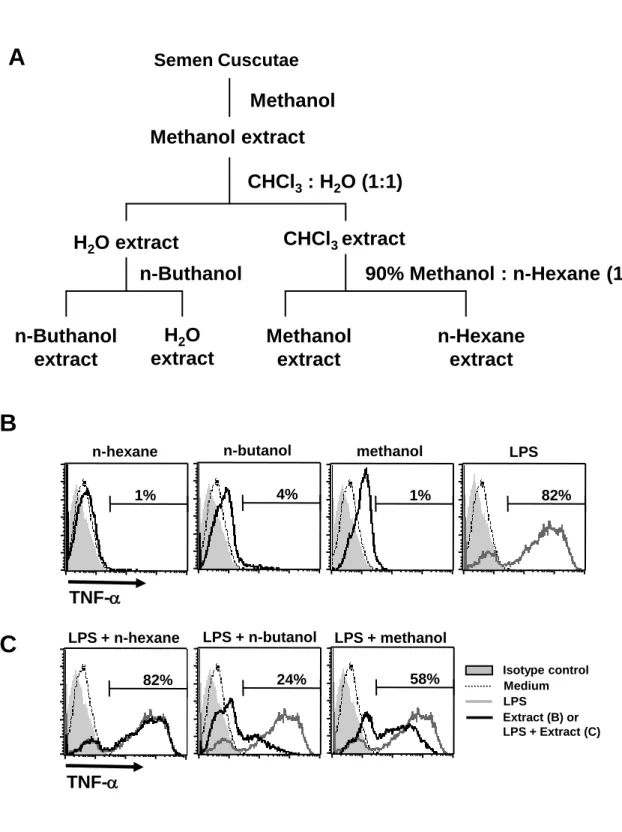
SC was purchased from a Chinese medicine store (Taichung, Taiwan). The procedure for extraction of SC was summarized in Fig. 1A. Briefly, the seeds were pulverized into fine powder by a pulverizer and then passed through a 50-mesh sieve. The powder (8 g) was extracted first with methanol (500 mL) by sonification and centrifuged. The supernatant was collected, concentrated, and then dried with a rotary evaporator. The residue was dissolved in water and then extracted with chloroform. Next, the water layer was extracted with n-butanol and the chloroform layer was dried and partitioned with 90% methanol and hexane. The n-butanol, methanol, and n-hexane extracts were dried and dissolved in DMSO for HPLC analysis. Hyperoside (quercetin 3-D-galactoside), quercetin, and kaempferol were purchased from Sigma-Aldrich Co.
Generation of mouse DCs
C57BL/6 mice were purchased from National Laboratory Animal Center (Taipei, Taiwan). The mouse bone marrow-derived DCs were generated as described previously (Chu and Lowell, 2005). In brief, bone marrow cells were isolated from femurs and tibias and seeded on 24-well culture plates (Corning) in 1 mL/well complete RPMI 1640 medium (Gibco), and 10 ng/mL recombinant mouse GM-CSF (Peprotech). At day 3, fresh medium (1 mL/well) containing 10 ng/ml GM-CSF was added. At day 5, half of the cell-free supernatant was exchanged and fresh medium containing 10 ng/mL GM-CSF was added. The 7-day-cultured DCs (>80% CD11c+ cells) were used for all experiments. In T cell activation experiment, DCs were purified by EasySep Positive Selection Kit (StemCell Technology) according to manufacturer’s instruction. The purity of CD11c+ cells was over 95% (data not shown). The OT-II TCR-transgenic mice were provided by Dr. Clifford Lowell (UCSF, San Francisco, CA). All animals were kept in a specific pathogen free facility (NHRI, Miaoli, Taiwan) and handled according to protocols approved by Institutional Animal Care and Use Committee of NHRI.
Measurement of cytokine and chemokine production
TNF- production was measured by intracellular staining and ELISA, as described previously (Chu et al., 2008). DCs were treated with SC samples (50 g/mL), LPS (100 ng/mL), or SC samples + LPS for 6 hrs. The percentages of CD11c+ TNF-α+ cells were determined by
flow cytometry. For quantification, the production of cytokines (TNF-α, IL-6, and IL-12p70) and chemokines (MCP-1, MIP-1β, and RANTES) was measured using ELISA (eBioscience and R&D systems).
Assay for the cytotoxicity of kaempferol
DCs were treated with kaempferol (dissolved in DMSO at 20 mg/mL as a stock) at indicated concentrations for 24 hrs. The cells were then harvested and stained with Annexin V kit (Invitrogen) according to manufacturer’s instruction. Annexin V+ cells were determined by
flow cytometry. DMSO was only 0.1% (v/v) at the concentration of 20 g/mL kaempferol, so DMSO had no effect on DCs in all experiments (data not shown).
Analysis of DC maturation
DCs were untreated or treated with LPS (100 ng/mL) or LPS + kaempferol (20 g/mL) for 16 hrs. Cell aggregation was examined by microscopy (40X). Maturation was determined by measuring the expression of MHC class II and co-stimulatory molecules as described previously (Liu et al., 2010). Cells were stained with mAbs specific for mouse CD11c, I-Ab, CD40, CD80,
and CD86 (Biolegend), and then analyzed by flow cytometry. The change of mean fluorescence intensity (MFI) from LPS alone to LPS + kaempferol is indicated.
OVA-specific T cell activation
The ability of antigen presentation of DCs was determined by OVA-specific T cell activation as described previously (Yu et al., 2009). Briefly, OVA323-339 peptide (2
g/mL)-pulsed DCs were incubated with LPS (100 ng/mL), LPS + DMSO, or LPS + kaempferol (20
g/mL) for 16 hrs. The OT-II T cells were then added at various DC:T cell ratios as indicated, and T cell proliferation was measured by [3H]thymidine incorporation. For the in vivo assay, C57BL/6 mice were immunized with OVA (10 μg; Sigma-Aldrich) and incomplete Freund’s
adjuvant (IFA; Sigma-Aldrich) mixed with LPS (10 μg), LPS + DMSO, or LPS + kaempferol (50 μg) via footpadinjection. After 10 days, the draining lymph node (LN) cells were cultured with OVA at indicated concentrations and then T cell proliferation was measured by [3H]thymidine incorporation. To measure IFN-γ production, supernatants were collected from
the DC/OT II T cell and LN cell cultures, and the IFN-γproduction was assayed by ELISA (eBioscience).
Data analysis
Significance of the inhibition during LPS and kaempferol co-treatment in comparison with LPS treatment alone was determined using a Student’s t-test with 2-sample equal variance with a 2-tailed distribution. P < 0.05 was considered significant.
Results
The n-butanol and methanol extracts of SC inhibited LPS-induced DC activation
It is suggested that SC has potential to modulate immune responses; however, the mechanism for this immunomodulation has not been studied. Therefore, we prepared various SC samples for testing the effect of SC on DCs, the critical leukocytes in initiating immune responses. Fractions were obtained by extracting SC with three organic solvents, including n-butanol, 90% methanol, and n-hexane (Fig. 1A). Next, we examined the effect of the three SC extracts on TNF-α production by mouse bone marrow-derived DCs, which is a hallmark for DC activation. All extracts did not induce TNF-α production, suggesting that these SC extracts do not have stimulatory activity on DCs (Fig. 1B). On the other hand, we checked the suppressive effect of these SC extracts on activated DCs. Surprisingly, we found that the TNF-α secreted
from LPS-stimulated DCs was reduced by co-treatment with the n-butanol or methanol extract of SC (Fig. 1C), indicating that these two SC extracts possess the ability to inhibit DC activation. Our results thus reveal that SC contains immunomudulatory substances which weaken the activity of DCs.
Kaempferol was identified in methanol and n-butanol extracts of SC by HPLC
The constituents within these three SC extracts were further analyzed by HPLC. As shown in Fig. 2, the n-butanol extract contained many ingredients, but only a major peak was found in methanol extract. This component seems to contribute to the inhibitory activity of methanol extract of SC on DCs. Since the flavonoids hyperoside, querctin, and kaempferol have been reported as the major contents of SC, we used these three flavonoids as standards of the HPLC analysis for these extracts. Therefore, we identified that kaempferol was very likely the major component in the methanol extract of SC. The map showed that kaempferol was also present in the n-butanol extract of SC (Fig. 2).
Kaempferol reduced cytokine and chemokine production by LPS-stimulated DCs
To confirm that the inhibitory effect of methanol extract of SC on DCs was mainly contributed by kaempferol, we used commercial kaempferol to treat DCs. We found that kaempferol reduced the production of TNF-α by LPS-stimulated DCs in the same way as the methanol extract of SC did (Fig. 3A). In addition to TNF-α, the generation of other cytokines (IL-6, and IL-12 p70) and chemokines (MCP-1, MIP-1β, and RANTES) by activated DCs was also diminished by kaempferol (Fig. 3). These results suggest that kaempferol is the major
component in the methanol extract of SC to inhibit DC activation and is a potent immunosuppressor.
LPS-induced DC aggregation and maturation were impaired by kaempferol at non-cytotoxic dosage
Although the suppressive effect of kaempferol on DCs was obvious, this could be due to the cytotoxic effect of kaempferol on the cells. To rule out this possibility, we determined the cytotoxicity of kaempferol on DCs by measuring apoptosis. As shown in Fig. 4A, kaempferol induced significant DC apoptosis at concentrations ≧ 40 μg/mL. Thus, we selected the concentration 20 g/mL of kaempferol for further tests on DCs and then excluded the cytotoxic effect of kaempferol in all experiments. Next, we study the effect of kaempferol on DC maturation. We first examined DC aggregation, which is a major phenotype of DC maturation, by microscopy. Upon LPS stimulation, a lot of DC clusters were observed; however, this aggregation disappeared in the presence of kaempferol (Fig. 4B). In addition, LPS stimulation enhanced the expression of MHC class II and the costimulatory molecules CD40, CD80, and CD86 in DCs, but kaempferol treatment significantly decreased the expression levels of these proteins (Fig. 4C). These data indicate that kaempferol impairs LPS-induced DC maturation, thus compromising the immunostimulation of the activated DCs.
Kaempferol attenuated T cell activation induce by LPS-stimulated DCs
The most important function of mature DC is to activate naive T cell, so we examined whether kaempferol affects the ability of DCs to induce T cell activation. OVA323-339
with OT-II T cells. We found that LPS-stimulated DCs enhanced T cell proliferation, but this proliferation was reduced by kaempferol (Fig. 5A). In addition to this in vitro experiment, we also performed a recall assay in vivo. Mice were immunized with OVA mixed with IFA plus LPS + DMSO, LPS, or LPS + kaempferol, and then draining LN cells were collected and stimulated with OVA after 10 days. Consistent with the in vitro results, kaempferol significantly decreased OVA-specific T cell proliferation (Fig. 5B). Moreover, kaempferol also lowered the amount of IFN-γ produced by the activated T cells both in vitro and in vivo (Figs. 5C and 5D). These results illustrate that kaempferol attenuates the ability of DCs to activate Ag-specific T cells. The attenuation of the recall response by kaempferol is in agreement with the reduction of DC activation and maturation after kaempferol treatment (Figs. 3 and 4C).
Discussion
In this study, we examined the activity of SC on the immune function of DCs. We found the inhibitory effect of the n-butanol and methanol extracts of SC on DC activation. Furthermore, we identified that kaempferol, which is a flavonoid in SC, was able to suppress DC activation. This is the first study to report that SC has immunomodulatory activity on DCs and that kaempferol is a potent immunosuppressant.
Kaempferol is a common flavonoid in human diet and has various biological activities including antioxidant, anticancer, and anti-inflammatory effects (Kang et al., 2008; Mahat et al., 2010). A number of reports have shown the immunomodulatory effect of kaempferol on T lymphocytes (Okamoto et al., 2002), B cells (Zunino and Storms, 2009), macrophages (Comalada et al., 2006; Hamalainen et al., 2007; Harasstani et al., 2010; Kim et al., 2005; Liang et al., 2001), neutrophils (Moreira et al., 2007; Selloum et al., 2001; Wang et al., 2006),
basophils (Shim et al., 2009), and mast cells (Kempuraj et al., 2005; Lee et al., 2010). Here we reported that kaempferol attenuates DC activation for the first time. The similar inhibition of inflammatory cytokines (TNF-, IL-12, and IL-1) by kaempferol is also observed in LPS-stimulated macrophages (Fang et al., 2005; Harasstani et al., 2010; Kowalski et al., 2005). This novel function may enhance the potential of kaempferol in medical applications, such as treatment of airway inflammation (Lee et al., 2010). In addition, kaempferol may become a major content for quality control of SC (Ye et al., 2002).
A large number of evidences for the effects of flavonoids in inflammation and immunity have been summarized (Gonzalez-Gallego et al., 2010), but the studies related to DC function are not much. Some flavonoids with the immunomodulatory activity on DCs have been investigated, including luteolin, apigenin, silibinin, taxifolin glycoside, epigallocatechin gallate, and quercetin (summarized in (Huang et al., 2010a)). Kaempferol as a new modulator on DCs can now be added to this list according to this study. Since kaempferol disturbs the NF-κB and mitogen-activated protein kinase (MAPK) pathways (Hamalainen et al., 2007; Lee et al., 2010; Lee et al., 2009) and is a suppressant of calcineurin (Wang et al., 2008), that may be the mechanism by which it inhibits DC function. Another possible mechanism is the involvement of peroxisome proliferator-activated receptor (PPAR) a transcription factor which has been implicated in anti-inflammatory response, because kaempferol significantly stimulates PPAR transcriptional activity (Liang et al., 2001).
In addition to kaempferol, several other flavonoids were also found in SC, including quercetin and hyperoside in the n-butanol extract (Fig. 2). Quercetin has recently been demonstrated to exhibit an immunosuppressive effect on DC activation (Huang et al., 2010a). The presence of both kaempferol and quercetin may has synergistic effect (Harasstani et al.,
Formatted: Font: Symbol Formatted: Font: Symbol Field Code Changed
2010) and is probably the reason that the n-butanol extract of SC has stronger inhibitory activity than the methanol extract of SC, which mainly contains kaempferol alone (Fig. 1C). Another flavonoid hyperoside (quercetin 3-D-galactoside) was the major component in the n-butanol extract of SC (Fig. 2), but it did not have any activity on DCs (data not shown). It seems that the addition of a galactoside may destroy the suppressive effect of quercetin on DCs. At the present, we are exploring more active ingredients in the n-butanol extract of SC. Together, our data may be helpful in designing a powerful immunosuppressant based on the structure of flavonol (Kim et al., 2005; Kim et al., 2006; Li et al., 2008).
Although there are insufficient data to provide conclusive evidence on the health effects of most flavonoids (Kay, 2010), the in vivo effects of kaempferol have been shown in several animal models, such as allergic lung disease (Medeiros et al., 2009), carrageenan induced rat air pouch model (Mahat et al., 2010), thermal burn-induced skin injuries (Park et al., 2010), and mouse model of osteosarcoma (Huang et al., 2010b). These reports imply that kaempferol may have clinical application, especially the safety issue of kaempferol has been discussed (Lee et al., 2009; Li et al., 2008).
In conclusion, SC has immunoregulatory activity on DCs. Our results point out that SC can be described as a novel “biofactory” because it contains various substances that modulate DC function with both promotion and inhibition. Importantly, we provide the evidence that kaempferol may have potential to be developed into immunosuppressant in pharmacology. Recently, the nanoparticle system has been applied to overcome the poor water solubility of flavonoids in SC and then to reduce the treatment dosage (Yen et al., 2008). This technique may enhance the uptake of kaempferol by human and then promote the suppressive effect of kaempferol on the harmful immune responses such as chronic inflammation and autoimmunity.
Acknowledgments
We thank Dr. Ren-Yeong Huang and Chun-Nan OuYang for technical help. This work was supported by China Medical University grant CMU98N102, National Science Council grant NSC982311B039001MY3 (for M.K.L), Small Business Innovation Research grant IZ970524, and Southern Taiwan Science Park grant EY13151099 (for J.D.C.C and C.L.C.) of Taiwan. The authors declare no financial or commercial conflict of interest.
References
Bao, X., Wang, Z., Fang, J., and Li, X., 2002. Structural features of an immunostimulating and antioxidant acidic polysaccharide from the seeds of Cuscuta chinensis. Planta Med. 68, 237-243. Buckwalter, M.R., and Albert, M.L., 2009. Orchestration of the immune response by dendritic cells. Curr. Biol. 19, R355-361.
Chu, C.L., and Lowell, C.A., 2005. The Lyn tyrosine kinase differentially regulates dendritic cell generation and maturation. J. Immunol. 175, 2880-2889.
Chu, C.L., Yu, Y.L., Shen, K.Y., Lowell, C.A., Lanier, L.L., and Hamerman, J.A., 2008. Increased TLR responses in dendritic cells lacking the ITAM-containing adapters DAP12 and FcRgamma. Eur. J. Immunol. 38, 166-173.
Comalada, M., Ballester, I., Bailon, E., Sierra, S., Xaus, J., Galvez, J., de Medina, F.S., and Zarzuelo, A., 2006. Inhibition of pro-inflammatory markers in primary bone marrow-derived mouse macrophages by naturally occurring flavonoids: analysis of the structure-activity relationship. Biochem. Pharmacol. 72, 1010-1021.
Du, X.M., Kohinata, K., Kawasaki, T., Guo, Y.T., and Miyahara, K., 1998. Components of the ether-insoluble resin glycoside-like fraction from Cuscuta chinensis. Phytochemistry 48, 843-850. Fang, S.H., Rao, Y.K., and Tzeng, Y.M., 2005. Inhibitory effects of flavonol glycosides from Cinnamomum osmophloeum on inflammatory mediators in LPS/IFN-gamma-activated murine macrophages. Bioorg. Med. Chem. 13, 2381-2388.
Fransen, J.H., van der Vlag, J., Ruben, J., Adema, G.J., Berden, J.H., and Hilbrands, L.B., 2010. The role of dendritic cells in the pathogenesis of systemic lupus erythematosus. Arthritis Res. Ther. 12, 207.
Gonzalez-Gallego, J., Garcia-Mediavilla, M.V., Sanchez-Campos, S., and Tunon, M.J., 2010. Fruit polyphenols, immunity and inflammation. Br. J. Nutr. 104 Suppl 3, S15-27.
Hamalainen, M., Nieminen, R., Vuorela, P., Heinonen, M., and Moilanen, E., 2007. Anti-inflammatory effects of flavonoids: genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-kappaB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-kappaB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediators Inflamm. 2007, 45673.
Harasstani, O.A., Moin, S., Tham, C.L., Liew, C.Y., Ismail, N., Rajajendram, R., Harith, H.H., Zakaria, Z.A., Mohamad, A.S., Sulaiman, M.R., and Israf, D.A., 2010. Flavonoid combinations cause synergistic inhibition of proinflammatory mediator secretion from lipopolysaccharide-induced RAW 264.7 cells. Inflamm. Res. 59, 711-721.
He, X.H., Yang, W.Z., Meng, A.H., He, W.N., Guo, D.A., and Ye, M., 2010. Two new lignan glycosides from the seeds of Cuscuta chinensis. J. Asian Nat. Prod. Res. 12, 934-939.
Huang, R.Y., Yu, Y.L., Cheng, W.C., Ouyang, C.N., Fu, E., and Chu, C.L., 2010a.
Immunosuppressive Effect of Quercetin on Dendritic Cell Activation and Function. J. Immunol. 184, 6815-6821.
Huang, W.W., Chiu, Y.J., Fan, M.J., Lu, H.F., Yeh, H.F., Li, K.H., Chen, P.Y., Chung, J.G., and Yang, J.S., 2010b. Kaempferol induced apoptosis via endoplasmic reticulum stress and
mitochondria-dependent pathway in human osteosarcoma U-2 OS cells. Mol. Nutr. Food Res. 54, 1585-1595.
Jian-Hui, L., Bo, J., Yong-Ming, B., and Li-Jia, A., 2003. Effect of Cuscuta chinensis glycoside on the neuronal differentiation of rat pheochromocytoma PC12 cells. Int. J. Dev. Neurosci. 21, 277-281.
Joffre, O., Nolte, M.A., Sporri, R., and Reis e Sousa, C., 2009. Inflammatory signals in dendritic cell activation and the induction of adaptive immunity. Immunol. Rev. 227, 234-247.
Kang, B.Y., Kim, S., Lee, K.H., Lee, Y.S., Hong, I., Lee, M.O., Min, D., Chang, I., Hwang, J.S., Park, J.S., et al., 2008. Transcriptional profiling in human HaCaT keratinocytes in response to kaempferol and identification of potential transcription factors for regulating differential gene expression. Exp. Mol. Med. 40, 208-219.
Kay, C.D., 2010. The future of flavonoid research. Br. J. Nutr. 104 Suppl 3, S91-95. Kempuraj, D., Madhappan, B., Christodoulou, S., Boucher, W., Cao, J., Papadopoulou, N., Cetrulo, C.L., and Theoharides, T.C., 2005. Flavonols inhibit proinflammatory mediator release, intracellular calcium ion levels and protein kinase C theta phosphorylation in human mast cells. Br. J. Pharmacol. 145, 934-944.
Kim, A.R., Cho, J.Y., Zou, Y., Choi, J.S., and Chung, H.Y., 2005. Flavonoids differentially modulate nitric oxide production pathways in lipopolysaccharide-activated RAW264.7 cells. Arch. Pharm. Res. 28, 297-304.
Kim, J.D., Liu, L., Guo, W., and Meydani, M., 2006. Chemical structure of flavonols in relation to modulation of angiogenesis and immune-endothelial cell adhesion. J. Nutr. Biochem. 17, 165-176.
Kowalski, J., Samojedny, A., Paul, M., Pietsz, G., and Wilczok, T., 2005. Effect of apigenin, kaempferol and resveratrol on the expression of interleukin-1beta and tumor necrosis factor-alpha genes in J774.2 macrophages. Pharmacol. Rep. 57, 390-394.
Lee, E.J., Ji, G.E., and Sung, M.K., 2010. Quercetin and kaempferol suppress immunoglobulin E-mediated allergic inflammation in RBL-2H3 and Caco-2 cells. Inflamm. Res. 59, 847-854. Lee, S., Kim, Y.J., Kwon, S., Lee, Y., Choi, S.Y., Park, J., and Kwon, H.J., 2009. Inhibitory effects of flavonoids on TNF-alpha-induced IL-8 gene expression in HEK 293 cells. BMB Rep. 42, 265-270.
Li, J.W., and Vederas, J.C., 2009. Drug discovery and natural products: end of an era or an endless frontier? Science 325, 161-165.
Li, N., Liu, J.H., Zhang, J., and Yu, B.Y., 2008. Comparative evaluation of cytotoxicity and antioxidative activity of 20 flavonoids. J. Agric. Food Chem. 56, 3876-3883.
Li, Y., Ye, M., Liu, H., Ji, X., and Yan, Y., 1999. Analysis of flavonoids in Semen Cuscutae by micellar electrokinetic chromatography and high-performance liquid chromatography. J. Capill. Electrophor. Microchip Technol. 6, 157-162.
Liang, Y.C., Tsai, S.H., Tsai, D.C., Lin-Shiau, S.Y., and Lin, J.K., 2001. Suppression of inducible cyclooxygenase and nitric oxide synthase through activation of peroxisome proliferator-activated receptor-gamma by flavonoids in mouse macrophages. FEBS Lett. 496, 12-18.
Liu, W.C., Lin, S.C., Yu, Y.L., Chu, C.L., and Wu, S.C., 2010. Dendritic Cell Activation by Recombinant Hemagglutinin Proteins of H1N1 and H5N1 Influenza A Viruses. J. Virol. 84, 12011-12017.
Mahat, M.Y., Kulkarni, N.M., Vishwakarma, S.L., Khan, F.R., Thippeswamy, B.S., Hebballi, V., Adhyapak, A.A., Benade, V.S., Ashfaque, S.M., Tubachi, S., and Patil, B.M., 2010. Modulation of the cyclooxygenase pathway via inhibition of nitric oxide production contributes to the anti-inflammatory activity of kaempferol. Eur. J. Pharmacol. 642, 169-176.
Medeiros, K.C., Faustino, L., Borduchi, E., Nascimento, R.J., Silva, T.M., Gomes, E., Piuvezam, M.R., and Russo, M., 2009. Preventive and curative glycoside kaempferol treatments attenuate the TH2-driven allergic airway disease. Int. Immunopharmacol. 9, 1540-1548.
Moreira, M.R., Kanashiro, A., Kabeya, L.M., Polizello, A.C., Azzolini, A.E., Curti, C., Oliveira, C.A., A, T.d.A., and Lucisano-Valim, Y.M., 2007. Neutrophil effector functions triggered by Fc-gamma and/or complement receptors are dependent on B-ring hydroxylation pattern and physicochemical properties of flavonols. Life Sci. 81, 317-326.
Okamoto, I., Iwaki, K., Koya-Miyata, S., Tanimoto, T., Kohno, K., Ikeda, M., and Kurimoto, M., 2002. The flavonoid Kaempferol suppresses the graft-versus-host reaction by inhibiting type 1 cytokine production and CD8+ T cell engraftment. Clin. Immunol. 103, 132-144.
Palucka, K., Banchereau, J., and Mellman, I., 2010. Designing vaccines based on biology of human dendritic cell subsets. Immunity 33, 464-478.
Pan, H.J., Sun, H.X., and Pan, Y.J., 2005. Adjuvant effect of ethanol extract of Semen Cuscutae on the immune responses to ovalbumin in mice. J. Ethnopharmacol. 99, 99-103.
Park, B.K., Lee, S., Seo, J.N., Rhee, J.W., Park, J.B., Kim, Y.S., Choi, I.G., Kim, Y.E., Lee, Y., and Kwon, H.J., 2010. Protection of burn-induced skin injuries by the flavonoid kaempferol. BMB Rep. 43, 46-51.
Qin, D.N., She, B.R., She, Y.C., and Wang, J.H., 2000. Effects of flavonoids from Semen Cuscutae on the reproductive system in male rats. Asian J. Androl. 2, 99-102.
Schuler, G., 2010. Dendritic cells in cancer immunotherapy. Eur. J. Immunol. 40, 2123-2130. Selloum, L., Reichl, S., Muller, M., Sebihi, L., and Arnhold, J., 2001. Effects of flavonols on the generation of superoxide anion radicals by xanthine oxidase and stimulated neutrophils. Arch. Biochem. Biophys. 395, 49-56.
Shim, S.Y., Choi, J.S., and Byun, D.S., 2009. Kaempferol isolated from Nelumbo nucifera stamens negatively regulates FcepsilonRI expression in human basophilic KU812F cells. J. Microbiol. Biotechnol. 19, 155-160.
Sohn, D.W., Kim, H.Y., Kim, S.D., Lee, E.J., Kim, H.S., Kim, J.K., Hwang, S.Y., Cho, Y.H., and Kim, S.W., 2008. Elevation of intracavernous pressure and NO-cGMP activity by a new herbal formula in penile tissues of spontaneous hypertensive male rats. J. Ethnopharmacol. 120, 176-180.
Steinman, R.M., 2007. Dendritic cells: versatile controllers of the immune system. Nat. Med. 13, 1155-1159.
Takeuchi, O., and Akira, S., 2010. Pattern recognition receptors and inflammation. Cell 140, 805-820.
Wang, H., Zhou, C.L., Lei, H., Zhang, S.D., Zheng, J., and Wei, Q., 2008. Kaempferol: a new immunosuppressant of calcineurin. IUBMB Life 60, 549-554.
Wang, L., Tu, Y.C., Lian, T.W., Hung, J.T., Yen, J.H., and Wu, M.J., 2006. Distinctive antioxidant and antiinflammatory effects of flavonols. J. Agric. Food. Chem. 54, 9798-9804. Wang, Z., Fang, J.N., Ge, D.L., and Li, X.Y., 2000. Chemical characterization and
immunological activities of an acidic polysaccharide isolated from the seeds of Cuscuta chinensis Lam. Acta Pharmacol. Sin. 21, 1136-1140.
Yang, H.M., Shin, H.K., Kang, Y.H., and Kim, J.K., 2009. Cuscuta chinensis extract promotes osteoblast differentiation and mineralization in human osteoblast-like MG-63 cells. J. Med. Food 12, 85-92.
Yang, J., Wang, Y., Bao, Y., and Guo, J., 2008. The total flavones from Semen cuscutae reverse the reduction of testosterone level and the expression of androgen receptor gene in kidney-yang deficient mice. J. Ethnopharmacol. 119, 166-171.
Ye, M., Li, Y., Yan, Y., Liu, H., and Ji, X., 2002. Determination of flavonoids in Semen Cuscutae by RP-HPLC. J. Pharm. Biomed. Anal. 28, 621-628.
Yen, F.L., Wu, T.H., Lin, L.T., Cham, T.M., and Lin, C.C., 2008. Nanoparticles formulation of Cuscuta chinensis prevents acetaminophen-induced hepatotoxicity in rats. Food Chem. Toxicol. 46, 1771-1777.
Yen, F.L., Wu, T.H., Lin, L.T., and Lin, C.C., 2007. Hepatoprotective and antioxidant effects of Cuscuta chinensis against acetaminophen-induced hepatotoxicity in rats. J. Ethnopharmacol. 111, 123-128.
Yu, Y.L., Chen, I.H., Shen, K.Y., Huang, R.Y., Wang, W.R., Chou, C.J., Chang, T.T., and Chu, C.L., 2009. A triterpenoid methyl antcinate K isolated from Antrodia cinnamomea promotes dendritic cell activation and Th2 differentiation. Eur. J. Immunol. 39, 2482-2491.
Zunino, S.J., and Storms, D.H., 2009. Resveratrol alters proliferative responses and apoptosis in human activated B lymphocytes in vitro. J. Nutr. 139, 1603-1608.
Figure legends
Figure 1. Effect of the three SC samples extracted by organic solvents on DC activation. (A) Sample preparation from SC. Briefly, the powder of the seeds was extracted with methanol first. Then, the methanol extract was further partially separated by chloroform, n-butanol, 90% methanol, and hexane extraction. The four extracts in water, butanol, methanol, and n-hexane were obtained. (B-C) DCs were collected after treatment for 6 hrs and TNF-α production was measured by intracellular staining and flow cytometry. The percentage of TNF-α cells is indicated. (B) For the stimulation assay, DCs were untreated (dotted line) or treated with LPS (100 ng/mL, gray line) or various extracts (50 μg/mL, black line) as indicated. (C) For the inhibition assay, DCs were untreated (dotted line) or treated with LPS (gray line) or LPS + various extracts (black line) as indicated. All results shown were gated on CD11c+ cells. The
gray-filled area represents staining with an isotype-matched control antibody. All data are representative of three independent experiments.
Figure 2. HPLC chromatograms of the three SC extracts and flavonoid standards. The n-butanol, methanol, and n-hexane extracts of SC were injected into the HPLC and the maps were displayed as indicated. The maps and structures of three flavonoid standards (hyperoside, quercetin, and kaempferol) were also shown. Obviously, kaempferol is the major component in methanol extract and all three flavonoids are found in n-butanol extract. There is no significant signal in n-hexane extract.
Figure 3. Cytokines and chemokines released from LPS-stimulated DCs were impaired by Kaempferol. DCs were untreated or treated with kaempferol (40 μg/mL), LPS (100 ng/mL), or
LPS + various doses of kaempferol as indicated. Supernatants were collected after 24 hrs (6 hrs for TNF-α and RANTES). The amounts of cytokines (A) and chemokines (B) were determined by ELISA. Data shown are the mean + SD of three samples. NSp>0.05; *p<0.05; **p<0.01
(Student's t-test) are comparisons between kaempferol-treated and non-treated LPS-stimulated DCs. All results are representative of three independent experiments.
Figure 4. Kaempferol attenuated the LPS-induced DC maturation at non-cytotoxic dosage. (A) DCs were treated with various doses of kaempferol as indicated for 24 hrs. The cytotoxicity of kaempferol on DCs was analyzed by Annexin V staining. The percentage of Annexin V+ cells was determined by flow cytometry. Data shown are the mean + SD of three samples. NSp>0.05; **p<0.01 (Student's t-test) are comparisons between kaempferol-treated and non-treated DCs. (B)
DCs were untreated or treated with kaempferol (20 g/mL), LPS (100 ng/mL), or LPS + kaempferol for 16 hrs. DC aggregation was examined by microscopy (40X). (C) DCs were untreated (dotted line) or treated with LPS (thin line) or LPS + kaempferol (thick line) for 16 hrs. The expressions of MHC class II, CD40, CD80, and CD86 were determined by staining and flow cytometry. All data shown were gated on CD11c+ cells. The gray-filled area represents staining
with an isotype-matched control antibody. The change of mean fluorescence intensity (MFI) from LPS alone to LPS + kaempferol is indicated. All results are representative of two three to four independent experiments.
Figure 5. Kaempferol abrogated Ag-specific T cell activation induced by LPS-stimulated DCs. (A) For in vitro experiments, OT-II CD4+ T cells were co-cultured with untreated (white
DCs pulsed with OVA323-339 peptide at the indicated ratio of DC:T cell. T cell proliferation was
determined by [3H]thymidine incorporation after 3 days. (B) For in vivo experiments, C57BL/6
mice were immunized with OVA mixed with IFA (white bar), IFA + LPS + DMSO (hatch bar), IFA + LPS (gray bar), or IFA + LPS + kaempferol (black bar) via footpad injection. After 10 days, the draining LN cells were incubated with OVA at indicated concentrations. T cell proliferation was measured by [3H]thymidine incorporation after 3 days. Supernatants in (C) and (D) were collected from cultures in (A) and (B) after 4 days, respectively. IFN- production was measured by ELISA. Data shown are mean + SD of three samples. NSp>0.05; *p<0.05; **p<0.01 (Student's t-test) are comparisons between kaempferol-treated and -untreated
Fig. 1
Semen Cuscutae
Methanol extract
Methanol
CHCl
3: H
2O (1:1)
CHCl
3extract
n-Buthanol
extract
90% Methanol : n-Hexane (1:1)
Methanol
extract
n-Hexane
extract
H
2O extract
H
2O
extract
n-Buthanol
82% 58% 24% 82%TNF-
a
Isotype control Medium LPS Extract (B) or LPS + Extract (C) LPSLPS + n-hexane LPS + n-butanol LPS + methanol
1% 4% 1%
n-hexane n-butanol methanol
A
B
C
Fig. 2
Hyperoside Quercetin Kaempferol
n-Butanol extract Methanol extract n-Hexane extract Hyperoside Quercetin Kaempferol